Evidence for Sustained Attention and Working Memory in Schizophrenia Sharing a Common Mechanism
Abstract
Sustained attention and working memory (WM) are closely related functions that may share common mechanisms. The authors assessed sustained attention in schizophrenia patients and healthy comparison subjects using visuomotor tracking tasks under varying distractor loads. The attentional effort expended to deal with distractors was lower in patients and showed significant association with WM, executive function and negative symptoms in that group. It is proposed that the anterior attention system of sustained attention models and the executive function of the WM model describe the same or a closely related mechanism that is located in the prefrontal cortex (PFC) and is impaired in schizophrenia.
Studies of information processing and attention in schizophrenia have identified various abnormalities and led to the hypothesis that these may be central to the disorder and may influence clinical and functional outcomes in patients.1
The ability to focus on a target and resist distraction over time, sustained attention, may underlie higher aspects of attention and cognitive capacity in general.2 A model of sustained attention includes three interactive hierarchically organized components: an anterior attention system, posterior attention system, and sensory areas interacting with the basal forebrain.2 The anterior attention system3 involves right mediofrontal and dorsolateral prefrontal cortical regions and modulates (top down), the function of the posterior cortical (parietal) regions. Activation of the basal forebrain cholinergic projections (bottom up) is necessary for sustained attention performance and cortical cholinergic inputs may contribute to activation of frontoparietal regions.2
Evidence from imaging and other studies that prefrontal cortex (PFC) function is disturbed in schizophrenia4–6 suggests that the disturbance in attention may be directly linked to the pathology of the illness.
Another PFC function impaired in schizophrenia is working memory (WM). This online storage system is postulated to include a central executive that allocates attention between relevant tasks and domain specific storage slave systems.7 It has also been shown that executive (manipulation) functions8 are located in the dorsal aspects of the dorsolateral prefrontal cortex (DLPFC).
The apparent similarity in function, and common localization in the DLPFC of sustained attention7 and of WM8 raises the question whether or not they use the same neural mechanism. Desimone9 proposed such a notion based on evidence from animal studies but it has not yet been adequately examined in humans.
To provide this data and test the hypothesis that sustained attention and WM share common mechanisms, we studied the relationship between the ability to track a moving target and performance on a range of WM and WM related cognitive tests.
We chose to study a basic component of sustained attention, the ability to resist distraction, using a computerized visuomotor tracking task, which allowed the amount of distraction to be varied. Visuomotor performance is impaired in schizophrenia independent of motor function10 and the simplicity of the test makes it less vulnerable to interference from nonspecific factors such as lack of motivation or uncooperativeness.
To reduce variability we studied clinically stable chronically ill patients treated with atypical antipsychotics.
We administered similar tests to well functioning healthy subjects and compared the results.
METHOD
Participants
The patient group consisted of 37 individuals (six women, 31 men) suffering from chronic schizophrenia, diagnosed by consensus between treating and research clinicians, all senior psychiatrists, using DSM–IV criteria based on clinical interview, observational and case note information. All were treated with atypical antipsychotics (risperidone [N=16], mean = 5.6 mg/day; SD = 1.9; olanzapine [N=15], mean = 15.2 mg/day, SD = 4.9; and clozapine [N=6], mean = 256.2 mg/day, SD = 74.9) and received the same medication for at least 3 months. Medication dosages were unchanged for at least 4 weeks prior to the study.
Mean age of participants was 40.0 years (SD = 12.6). They had 10.5 years (SD = 3.7) of education. Mean duration of illness was 15.9 years (SD = 10.1) with 4.0 admissions (SD = 5.4).
The comparison group consisted of 21 women and 17 men. The mean age was 42.4 years (SD = 11.2). They had 14.3 years (SD = 1.2) of education This was significantly different from the comparison groups (F = 36.7, df = 1,73, p<0.001).
Visuo-Motor Coordination Testing and Sustained Attention
The ability to coordinate visual and motor functions in the absence of direct eye-hand contact was assessed using a computerized system consisting of a digitizing tablet and a PC computer previously described.10
The tablet was placed at lower chest level and hidden from the subject’s view by an overlying board, positioned 16 cm above it and supporting a computer monitor on which paths for tracing and for tracking were displayed. A screen cursor represented the location of an unseen dome-shaped manipulandum, containing the digitizer’s stylus, which could be moved freely over the surface of the digitizing tablet. The location of the manipulandum was read every 10 msec, with a resolution of 0.05 mm. A one to one correspondence between movements of the manipulandum and movements of the screen cursor was maintained.
A 1 cm target circle was programmed to move along the path at a predetermined speed of 19 mm/sec (minimum=16 mm/sec at the curved peaks, maximum=22 mm/sec at the straight middles) along the sine-wave path. The subject needed to maintain the cursor within the target in order to keep it moving. Whenever the cursor left the target the latter stopped moving (tracking interruption) until the cursor was returned. The time spent outside the cursor was measured. The procedures were performed under three conditions: one target and one cursor (Baseline), two targets and two cursors, and three targets and three cursors. The correct cursor and target were indicated by color. Three trials were performed under each of the three distraction conditions and the mean score for each condition was the performance measure. The number of tracking interruptions, a measure of accuracy, was chosen as the outcome variable. Since this variable measures the number of times the subject loses the target, higher values indicate poorer performance.
To measure the amount of attentional effort generated to cope with distraction, a variable (DEFF) was computed by subtracting the number of tracking interruptions in the baseline (no distractor) condition from the number of tracking interruptions in the presence of three distractors, and dividing this difference by the sum of the scores in the baseline plus three distractor conditions ((A-B)/(A + B)). This procedure gives a value between 0 (no effort expanded to cope with distraction) and 1 (full effort is expanded to cope with distraction) and indicates the attentional resources recruited to cope with distractors as a proportion of the total attention resources expended.
There were no significant differences between men and women in DEFF (healthy group: F = 2.24, df = 1,32, p=0.145; patients: F = 1.54, df = 1,34, p=0.223) or other visuomotor coordination variables.
Clinical Assessments
Negative and positive symptoms were assessed using the Scale for the Assessment of Negative (SANS),11 and Positive (SAPS)12 Symptoms. Mean total scores were SANS 43.8 (SD = 22.1), SAPS 20.4 (SD = 14.7).
Neuropsychological Tests
Digit Span Verbal WM.
The backward digit span test from the WAIS version 113 was used to measure verbal WM.
Dot Test.14 Modified15 Spatial WM
The subject is presented with a card bearing a mark and asked immediately to reproduce the mark on a blank card. The sum (in millimeters) of the distance between the target mark and that recalled by the subject was the outcome measure.
Finger Tapping Test (FT).16
Patients were asked to tap with the index finger on 2 points set 30 cm apart as rapidly as possible. The outcome measure was the number of taps per minute with the dominant hand.
Computerized Neuropsychological Tests.
Selected tests from the computerized Penn Neuropsychological Battery, previously described15 were used:
Executive Function: Abstraction, Inhibition, and WM Task (AIM).17
This is designed as a measure of abstraction and concept formation with and without additional working memory loads. Total number correct on the trial without additional memory load was the performance measure.
Penn Face Memory Test (PFMT).18
The PFMT consists of 20 target faces and 40 foils. Stimuli are black-and-white photographs of faces balanced for gender and age. Total number of true positive responses for short delay periods was the performance measure.
Visual Object Learning Test (VOLT).19
This was designed as a spatial analogue of the California Verbal Learning Test.20 Total number of true positive responses for short delay was the performance measure.
Computerized Judgment of Line Orientation (CJOLO).21
In this computerized adaptation of the original paper and pencil task, participants are shown two lines at an angle and are asked to indicate the corresponding lines on a simultaneously presented array. The number of correct responses was the performance measure.
Identification of Facial Emotions (PEAT).22
The PEAT contains pictures depicting happy, sad and neutral facial emotional expressions. Mean number of correct responses (within one point of standard) was the performance measure.
Penn Continuous Performance Test (PCPT).23
In this test of sustained attention the participant is asked to respond to a set of vertical and horizontal lines whenever they form a digit. Total number of true positive responses was the performance measure.
Mini Mental State Examination (MMSE)24 was administered to obtain a measure of general cognitive function. Mean scores for the patient and comparison groups were 25.66 (SD = 3.96) and 29.05 (SD = 1.27), respectively.
Data Analysis
Analysis of variance was used to compare groups. Correlations used Spearman’s r.
The testing procedure was extensive and not all participants completed all tests so the sample sizes differ slightly.
Two-tailed significance tests with the significance level set at 5% was used.
RESULTS
Visuomotor Function
Patients showed poorer visuomotor coordination than healthy individuals in baseline and distractor conditions (Figure 1). The differences between the groups were significant (p<0.001) under all conditions.
The DEFF was significantly higher (F = 14.5, df = 1,68, p<0.001) in healthy individuals (mean 0.74, SD = 0.43) than patients (mean 0.38, SD = 0.36) indicating that patients had fewer resources to devote to dealing with distractors (a saturation effect with higher load).
Cognitive Function
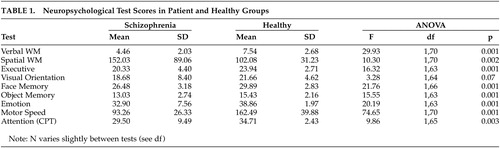
Test scores for cognitive tests are shown in Table 1. Patients scored significantly worse on all tests except for visual orientation where the difference failed to reach statistical significance.
Correlation With WM and Other Cognitive Tests
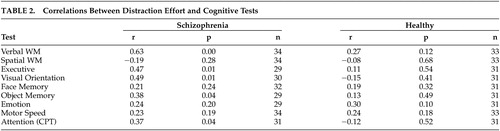
The relationship between DEFF and other cognitive tests are shown in Table 2.
In the patient group significant correlations were found between DEFF and verbal WM, executive function visual orientation and object memory. No correlation was found with spatial WM, memory for faces or identification of facial emotions.
There was no significant correlation between DEFF and motor speed (finger tap) but a significant correlation with Continuous Performance Test was found (rs= 0.37 p=0.04).
In the healthy group no significant correlations were found between DEFF and any of the test variables.
Correlation With Sociodemographic Variables
There was significant correlation between DEFF and age in the patient (r= −0.45, p<0.01) and healthy (r= −0.43, p<0.01) groups.
There was no correlation between DEFF and education in either group.
Correlation With Clinical Symptoms and Illness Variables
There was no significant correlation between tracking performance in the baseline (no distractor) condition and negative or positive symptoms.
There was a significant correlation between DEFF and Negative symptoms (SANS r=−0.47, p=0.02) but not positive symptoms (SAPS r=0.09, p=0.66).
No significant correlation was found between DEFF and the length of illness, number of hospitalizations or dosage of medication.
DISCUSSION
Our main finding was that the attentional effort needed to track a moving target in the presence of distractors, a measure of sustained attention, showed a strong relationship with working memory and executive tests in schizophrenia patients (Figure 2). This is consistent with findings of Barch and Carter25 who found correlation between measures of selective attention on the Stroop Test and Speaking Span performance.
The poorer performance of patients compared to healthy individuals on the visuomotor task in the baseline, no distractor condition, is consistent with our previous report that visuomotor function is impaired in schizophrenia.10
Patients could recruit fewer attention resources to cope with distraction, (as measured by DEFF) than healthy individuals, indicating that attention resources were saturated in the presence of a distractor load. This is consistent with abundant evidence of impaired attention in schizophrenia.1
Our measure of sustained attention, coping with distraction, was designed to reflect activity of the anterior attention system, which is related to the right mediofrontal and dorsolateral prefrontal cortical regions.8 Frontal lobe lesion patients are particularly sensitive to the detrimental effect of distractors on sustained attention tasks.26 Furthermore, imaging studies are consistent in showing activation of anterior cingulate and dorsolateral prefrontal as well as parietal regions in subjects performing sustained attention tasks irrespective of the modality used for those tasks,27–30 located primarily but not exclusively in the right hemisphere. The close association between sustained attention and WM and executive functions in our patients is consistent with a PFC location for attention.
Model
We interpret our findings as supporting the hypothesis that the mechanism that supports sustained attention in visuomotor function is the same as (or closely related to) the supervisory attention system supporting working memory functions.
In this model the “attention supervisory system” that maintains target schemata that correspond with the actual detection requirements31 and is responsible for top down facilitation of perceptual components of attention processes is also responsible for allocation of attention resources in manipulation of material in working memory. This system is located in the PFC (or PFC circuit) and its impairment is part of the core pathology of schizophrenia. In schizophrenia, the reduced attention capacity becomes rate limiting at relatively low task demands.
Our proposition is supported by considerable experimental evidence that attention selection and working memory share common neural mechanisms and circuits. Single cell studies show that both attention and working memory can induce top-down bias in visual cortex in the absence of sensory input.32,33
Increased activity during delay periods in working memory tasks have been found in the inferior temporal (IT) cortex in both single-cell recording34 and functional brain imaging studies.35 The biasing signals found in IT cortex during working memory tasks are similar to the biasing signals found in extrastriate areas during directed attention in the absence of visual stimulation.36,37
Evidence from animal lesion38,39 and brain cooling40 experiments indicates that top-down signals related to both attention and working memory processes may be generated from common sources in the PFC.
The response properties of many PFC neurons, which show stimulus-specific delay activity41,42 that reflects behaviorally relevant information43 support a crucial role of prefrontal cortex in working memory.
Correlation With Clinical Symptoms
The findings of a significant association between DEFF and negative symptoms is consistent with similar reports of a relationship between attention measures and negative symptoms.44–46
Interestingly, we found no relationship between negative symptoms and tracking performance under baseline conditions, suggesting that the negative symptoms may be related to the limited ability to recruit additional attention resources on demand rather than to baseline attention performance. This is consistent with findings of Cornblatt et al.47 who assessed the relationship between positive and negative symptoms and measures of distractibility and the ability to process auditory information under conditions of information overload. They observed that positive symptoms, but not negative symptoms were related to measures of auditory distractibility. In contrast, negative symptoms were uniquely and inversely related to the ability to process information under conditions of information overload.
Relation to Other Tests of Attention and Motor Speed
DEFF and performance on the CPT, a standard measure of sustained attention (vigilance) correlated significantly, indicating that the two tasks measure common attention systems although since the strength of correlation was only moderate the overlap may be incomplete. There is evidence that the CPT measures a range of attention-related functions.48
The lack of a relationship to motor speed (Finger tap) indicates that the impaired distractor effort was not due to impairment in motor activity.
Verbal and Spatial WM
It was of interest that DEFF showed no correlation with spatial WM, in contrast to verbal WM. This is consistent with our findings that the two types of WM can be differentiated and may have different mechanisms.15
Comparison With Healthy Subjects
The lack of correlation between attention and other neuropsychological functions in healthy subjects can be explained by postulating that the test demands were well within their attention capacity and hence not rate limiting.
This was also reflected in the lower score variability in that group, which may have contributed to the lack of correlations between tests.
Since sustained attention is a limited resource and demands on working memory tax sustained attention performance,2 it can be predicted that increasing task demands to threshold capacity will result in correlation patterns in normals similar to those observed in patients.
Limitations
A strength of this study is the relatively homogenous nature of the patient group, the simple measure of sustained attention and the extensive range of neuropsychological functions tested simultaneously.
Being a correlation analysis, this study cannot establish a causal relationship between attention and other functions, and the existence of a common mediating factor cannot be excluded. However since we used a very basic measure of sustained attention, such potential factors would be limited to less complex, perceptual processes. It is unlikely that the findings could be explained by motor impairment, since there was no relationship with motor speed, nor by nonspecific factors such as lack of motivation or cooperation, since patient cooperation was good and their motivation and performance was similar for the simpler and more demanding tests.
The effect of medication is unlikely to be a significant confound as all patients received atypical antipsychotics, which minimally affect and may even improve cognitive function in schizophrenia.49
The selected nature of the study group, however, limits generalization to other schizophrenia patients.
CONCLUSIONS
In conclusion, our findings support the hypothesis that the anterior attention system supporting sustained attention and the central executive of WM share the same neural mechanism and may indeed represent the same construct. A common “attention supervisory system”30 located in the PFC, may be responsible for top down facilitation of perceptual components of attention and for allocation of resources in working memory. In schizophrenia the limited ability of this attention system to cope with information load makes it rate limiting for other cognitive functions including WM.
Further studies are needed to examine whether these relationships are also present in less severely ill patients and in healthy individuals tested under more demanding conditions.
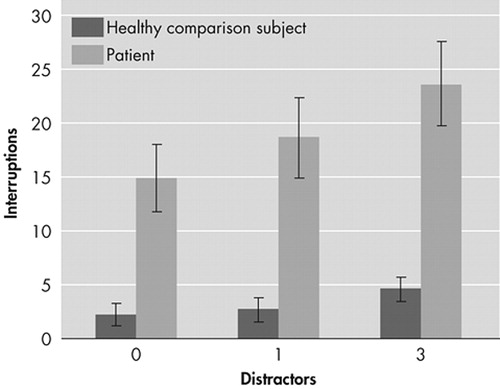
FIGURE 1. Performance of Patients and Healthy Individuals on Visuomotor Tracking Task Without and With Distractors
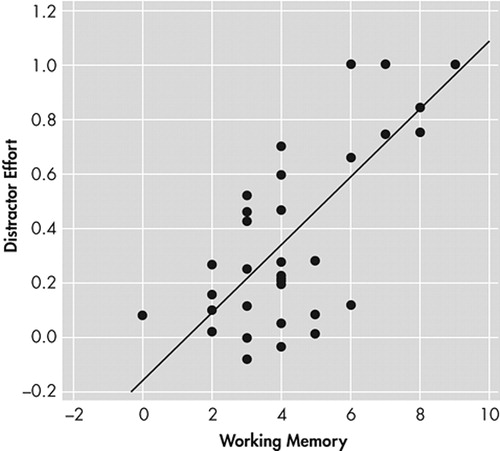
FIGURE 2. Relationship Between Distractor Effort and Working Memory in Schizophrenia Patients
 |
 |
1 Cadenhead KS, Perry W, Braff DL: The relationship of information-processing deficits and clinical symptoms in schizotypal personality disorder. Biol Psychiatry 1996; 40:853–858Crossref, Medline, Google Scholar
2 Sarter M, Givens B, Bruno JP: The cognitive neuroscience of sustained attention: where top-down meets bottom-up. Brain Res Brain Res Rev 2001; 35:146–160Crossref, Medline, Google Scholar
3 Posner MI, Petersen SE: The attention system of the human brain. Annu Rev Neurosci 1990; 13:25–42Crossref, Medline, Google Scholar
4 Saykin AJ, Gur RC, Gur RE, et al: Neuropsychological function in schizophrenia: selective impairment in memory and learning. Arch Gen Psychiatry 1991; 48:141–162Crossref, Google Scholar
5 Goldberg TE, Ragland DR, Gold J, et al: Neuropsychological assessment of monozygotic twins discordant for schizophrenia. Arch Gen Psychiatry 1990; 147:1066–1072Crossref, Google Scholar
6 Weinberger DR, Berman KF, Zec RF: Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence Arch Gen Psychiatry 1986; 43:114–124Google Scholar
7 Baddeley A: Working memory. Science 1992; 255(5044):556–559Crossref, Medline, Google Scholar
8 Petrides M: The role of the mid-dorsolateral prefrontal cortex in working memory. Exp Brain Res 2000l; 133:44–54Google Scholar
9 Desimone R: Visual attention mediated by biased competition in extrastriate visual cortex. Philos Trans R Soc Lond B Biol Sci 1998; 353:1245–1255Crossref, Medline, Google Scholar
10 Silver H, Shlomo N, Schwartz M, et al: Impaired visuomotor function in schizophrenic patients compared with control subjects. J Neuropsychiatry Clin Neurosci 2002; 14:72–76Link, Google Scholar
11 Andreasen, NC: Scale for the assessment of positive symptoms (SAPS). The University of Iowa, Iowa 1984Google Scholar
12 Andreasen, NC: Scale for the assessment of negative symptoms (SANS). Iowa City, University of Iowa, 1983Google Scholar
13 Wechsler D: Wechsler Adult intelligence Manual. New York, Psychological Corporation, 1955Google Scholar
14 Keefe RSE, Lees-Roitman SE, Dupre RL: Performance of patients with schizophrenia on a pen and paper visuospatial working memory task with short delay. Schizophr Res 1997; 26:9–14Crossref, Medline, Google Scholar
15 Silver H, Feldman P, Bilker W, et al: Working memory deficit is a core neuropsychological dysfunction in schizophrenia. Am J Psychaitry 2003 (in press).Google Scholar
16 Reitan RM, Davison LA: Clinical neuropsychology: current status and applications. New York: Hemisphere, 1974Google Scholar
17 Glahn DC, Cannon TD, Gur RE, et al: Working memory constrains abstraction in schizophrenia. Biol Psychiatry 2000; 47:34–42Crossref, Medline, Google Scholar
18 Gur RC, Jaggi JL, Ragland JD: Effects of memory processing on regional brain activation: cerebral blood flow in normal subjects. Int J Neurosci 1993; 72:31–44Crossref, Medline, Google Scholar
19 Glahn DC, Gur RC, Ragland JD, et al: Reliability, performance characteristics, construct validity, and an initial clinical application of a visual object learning test (VOLT). Neuropsychology 1997; 11:602–612Crossref, Medline, Google Scholar
20 Delis DC, Kramer JH, Kaplan E, et al: California Verbal Learning Test: Adult Version Manual. San Antonio TX: The Psychological Corporation 1987Google Scholar
21 Benton AL, Hannay HJ, Varney NR: Visual perception of line direction in patients with unilateral brain disease. Neurol 1975; 25:907–910Crossref, Medline, Google Scholar
22 Erwin RJ, Gur RC, Gur RE, et al: Facial emotion discrimination: I. task construction and behavioral findings in normal subjects. Psychiatry Res 1992; 42:231–240Crossref, Medline, Google Scholar
23 Kurtz MM, Ragland JD, Bilker W, et al: Comparison of the continuous performance test with and without working memory demands in healthy controls and patients with schizophrenia. Schizophr Res 2001; 48:307–316Crossref, Medline, Google Scholar
24 Folstein MF, Folstein SE, McHugh PR: ”Mini-mental state“. J Psychiatr Res 1975; 12:189–198Crossref, Medline, Google Scholar
25 Barch DM, Carter CS: Selective attention in schizophrenia: relationship to verbal working memory. Schizophr Res 1998; 33:53–61Crossref, Medline, Google Scholar
26 Rueckert L, Grafman J: Sustained attention deficits in patients with right frontal lesions. Neuropsychologia 1996; 34:953–963Crossref, Medline, Google Scholar
27 Cohen RM, Semple WE, Gross M, et al: Metabolic brain pattern of sustained auditory discrimination. Exp Brain Res 1992; 92:165–172Crossref, Medline, Google Scholar
28 Coull JT: Neural correlates of attention and arousal: insights from electrophysiology, functional neuroimaging and psychopharmacology. Prog Neurobiol 1998; 55:343–361Crossref, Medline, Google Scholar
29 Fink GR, Halligan PW, Marshall JC, et al: Neural mechanisms involved in the processing of global and local aspects of hierarchically organized visual stimuli. Brain 120 (Pt 10):1779–1791,1997Medline, Google Scholar
30 Pardo JV, Fox PT, Raichle ME: Localization of a human system for sustained attention by positron emission tomography. Nature 1991; 349:61–64Crossref, Medline, Google Scholar
31 Stuss DT, Shallice T, Alexander MP, et al: A multidisciplinary approach to anterior attentional functions. Ann N Y Acad Sci 1995; 769:191–211Crossref, Medline, Google Scholar
32 Chelazzi L, Miller EK, Duncan J, et al: A neural basis for visual search in inferior temporal cortex. Nature 1993; 363:345–347Crossref, Medline, Google Scholar
33 Chelazzi L, Duncan J, Miller EK, et al: Responses of neurons in inferior temporal cortex during memory-guided visual search. J Neurophysiol 1998; 80:2918–2940Crossref, Medline, Google Scholar
34 Miller EK, Li L, Desimone R: Activity of neurons in anterior inferior temporal cortex during a short-term memory task. J Neurosci 1993; 13:1460–1478Crossref, Medline, Google Scholar
35 Courtney SM, Ungerleider LG, Keil K, et al: Transient and sustained activity in a distributed neural system for human working memory. Nature 1997; 386:608–611Crossref, Medline, Google Scholar
36 Luck SJ, Chelazzi L, Hillyard SA, et al: Neural mechanisms of spatial selective attention in areas V1, V2, and V4 of macaque visual cortex. J Neurophysiol 1997; 77:24–42Crossref, Medline, Google Scholar
37 Kastner S, Pinsk MA, De Weerd P, et al: Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron 1999; 22:751–761Crossref, Medline, Google Scholar
38 Mishkin M: : effects of small frontal lesions on delayed alternation in monkeys. J Neurophysiol 1957; 20:615–622Crossref, Medline, Google Scholar
39 Funahashi S, Bruce CJ, Goldman-Rakic PS: Dorsolateral prefrontal lesions and oculomotor delayed-response performance: evidence for mnemonic ”scotomas“. J Neurosci 1993; 13:1479–1497Crossref, Medline, Google Scholar
40 Fuster JM, Bauer RH, Jervey JP: Functional interactions between nferotemporal and prefrontal cortex in a cognitive task. Brain Res 1985; 330:299–307Crossref, Medline, Google Scholar
41 Goldman-Rakic PS: Architecture of the prefrontal cortex and the central executive. Ann N Y Acad Sci 1995; 769:71–83Crossref, Medline, Google Scholar
42 Fuster JM: Temporal processing. Ann N Y Acad Sci 1995; 769:173–181Crossref, Medline, Google Scholar
43 Rainer G, Asaad WF, Miller EK: Selective representation of relevant information by neurons in the primate prefrontal cortex. Nature 1998; 393:577–579Crossref, Medline, Google Scholar
44 Walker E, Lewine RJ: The positive/negative symptom distinction in schizophrenia. validity and etiological relevance. Schizophr Res 1988; 5:315–328Crossref, Google Scholar
45 Strauss ME: Relations of symptoms to cognitive deficits in schizophrenia. Schizophr Bull 1993; 19:215–231Crossref, Medline, Google Scholar
46 Green M, Walker E: Symptom correlates of vulnerability to backward masking in schizophrenia. Am J Psychiatry 1986; 143:181–186Crossref, Medline, Google Scholar
47 Cornblatt BA, Lenzenweger MF, Dworkin RH, et al: Positive and negative schizophrenic symptoms, attention, and information processing. Schizophr Bull 1985; 11:397–408Crossref, Medline, Google Scholar
48 Elvevag B, Weinberger DR, Suter JC, et al: Continuous performance test and schizophrenia: a test of stimulus-response compatibility, working memory, response readiness, or none of the above? Am J Psychiatry 2000; 157:772–780Crossref, Medline, Google Scholar
49 Keefe RS, Silva SG, Perkins DO, et al: The effects of atypical antipsychotic drugs on neurocognitive impairment in schizophrenia: a review and meta-analysis. Schizophr Bull 1999; 25:201–222Crossref, Medline, Google Scholar



