Functional Neuroanatomy of Sleep and Sleep Deprivation
Chronic sleep restriction is pervasive in society, with 40% of people in the U.S. reporting less than 7 hours of sleep most nights.3 Sleep is undervalued, perhaps because the functions of sleep are not yet well understood.4 The biological importance of sleep, on the other hand, is quite clear:
Sleep has survived ubiquitously throughout all of mammalian evolution; some experiments have shown that animals cannot survive without sleep; and animals have made numerous behavioral and physiological accommodations to permit the survival of sleep in different habitats and life styles. Sleep persists in predators and prey; in carnivores and vegetarians; on the land and in the water (marine mammals); in most mammals as they lie down relaxed, in ruminants while they stand, in birds while they perch, and in dolphins which constantly swim; in hot and cold climates; in elephants and shrews; in sloths that hardly move and mice that hardly sit still; in the smartest and the dumbest of all mammalian species. These facts suggest a primary, essential, functional core to sleep….”5
If sufficiently prolonged, sleep deprivation is deadly, indicating the critical role it plays in maintaining health.5 Sleep appears to affect many processes in the body including energy metabolism, immune system function, learning/memory, appetite regulation, and gene expression.6–16 Recent work suggests that sleep may enhance creativity by facilitating the mental restructuring that is critical to insight.17 Sleep abnormalities are frequently associated with both primary psychiatric disorders and traumatic brain injuries.
Regulation of the sleep-waking cycle is complex.18–21 Onset of sleep is governed by the interacting forces of the sleep drive, which steadily increases with duration of wakefulness, and circadian fluctuations in arousal level. The ascending arousal system is comprised of multiple ascending projections from brainstem, hypothalamus, thalamus and basal forebrain (Figure 1A). There is interplay among many neurotransmitter systems to maintain the waking state, as recently reviewed.18–21 Wakefulness-promoting actions of acetylcholine (midbrain, pons and basal forebrain areas, Figure 1 light orange), dopamine (substantia nigra and ventral tegmental area, Figure 1 green), and norepinephrine (locus coeruleus nucleus, Figure 1 purple) are well known. Recent work indicates that serotonin (dorsal raphe nucleus, Figure 1 yellow), histamine (tuberomammillary nucleus, Figure 1 red), and orexin (also called hypocretin, lateral hypothalamic area, Figure 1 blue) also promote wakefulness. Sleep-promoting regions in the anterior hypothalamus (principally the ventrolateral preoptic area, Figure 1B pink) utilize the neurotransmitters GABA and galanin to inhibit wake-promoting regions in the hypothalamus and brainstem during slow wave sleep (SWS, also called non REM (NREM) sleep) (Figure 1B). Brainstem regions inhibited during wakefulness and NREM sleep become active during REM sleep (Figure 1C). Ascending projections from cholinergic neurons in the brainstem (laterodorsal tegmental and pendunculopontine areas, Figure 1C light orange) activate the thalamus which in turn activates the cortex. Descending projections from this area, utilizing other neurotransmitters in addition to acetylcholine, inhibit motor neurons, producing atonia (Figure 1C light orange). Further complexity has been introduced by the recognition that sleep-promoting substances (somnogens) accumulate during wakefulness. Synthesis of adenosine (which appears to directly inhibit wake-promoting neurons), for example, increases during periods of high metabolic demand (e.g., prolonged wakefulness, seizures, ischemia). The wake-promoting effect of caffeine is probably due to its ability to block adenosine receptors.
In the waking state the EEG is characterized by low amplitude fast activity (13–30 Hz, beta frequency band). Functional imaging studies indicate that during waking (as compared to sleeping) cerebral blood flow is high in the prefrontal and parietal cortices (Figure 2A).1 With relaxation, particularly when the eyes are closed, slower activity (8–12 Hz, alpha frequency band) dominates the EEG. During the transition to sleep, EEG activity gradually decreases further. In deep (stage 3 and 4) sleep (SWS), the EEG is characterized by large amplitude slow activity (<2 Hz, delta frequency band) due to widespread synchronous rhythmic thalamocortical activity. Animal studies indicate that thalamic neurons become hyperpolarized and change their firing pattern to a delta rhythm because tonic activation by brainstem centers decreases. The heart and respiratory rates also slow and blood pressure decreases (increased parasympathetic tone). Functional imaging studies indicate that cerebral blood flow decreases compared to wakefulness, particularly in core subcortical structures such as the thalamus, basal forebrain, basal ganglia, and brainstem (Figure 2B).1 Decreases have also been reported for the cerebellum, and ventromedial prefrontal (orbitofrontal and anterior cingulate), parietal and mesiotemporal cortices. Several times during a normal night’s sleep both the metabolic rate of the brain and EEG activity increase to levels similar to the waking state, accompanied by deep relaxation of the muscles. This state (paradoxical sleep, PS) is associated with vivid dreaming and rapid eye movements (REM). Functional imaging indicates that thalamus, pons, limbic/paralimbic areas and occipital cortex (visual association cortex) are very active (high blood flow and/or metabolic rate), while frontal and parietal cortices are suppressed (Figure 2C).1,22
Decreases in both global and regional cerebral metabolism have been found following periods of sleep deprivation compared to the baseline rested state.2,23,24 Only regional decreases in cerebral metabolic rate were found in the earliest study.24 Following sleep deprivation, cerebral metabolism in thalamus, basal ganglia, cerebellum, frontal and temporal cortex was decreased, whereas parietal cortex was increased (a visual vigilance task was performed during uptake in both conditions to standardize brain state). Decreased performance was positively correlated with decreased cerebral metabolic rate for thalamus and basal ganglia. More recent work has found globally decreased metabolism (∼6%) with relative regional decreases (2–11%) in multiple areas of cortex (a serial addition/subtraction task was performed during uptake).2,23 During a period of 72 hours of sleep deprivation, the most consistent regional decreases (compared to the awake rested state) were in prefrontal, posterior parietal, and temporal cortices as well as thalamus and cerebellum (Figure 2 D). Decreased performance was positively correlated with decreased metabolic rate for several prefrontal cortex regions and thalamus. These studies support the vulnerability of the thalamocortical circuits that are so critical for higher order cognitive functions to the effects of sleep deprivation.
The cognitive and emotional effects of sleep loss (generally quantified as sleep debt) have been studied using a variety of interventions including total sleep deprivation, sleep restriction (<8 hours of sleep allowed per night) and sleep disruption (frequent awakenings during the sleep period). Comparison across studies is hampered by many factors, including differences in the method and duration of sleep disturbance employed and in the cognitive tasks used to evaluate performance deficits.25 In addition, the common practice of averaging performance measures across groups may obscure differences, as there is a substantial range of sensitivity among individuals to the negative effects of sleep deprivation (trait-like differential vulnerability).26
Both sleep deprivation and sleep restriction have adverse effects on mood, cognitive performance, and motor function.25,27 The most commonly used tasks in this research area are simple vigilance and continuous performance tasks, which are quite sensitive to sleep deprivation. Studies utilizing more complex tasks indicate that convergent, logical thinking is relatively insensitive to sleep deprivation, whereas divergent, flexible thinking is adversely affected. Although controversy exists, executive functions (working memory, divided attention, self-monitoring, risk assessment) seem to be particularly vulnerable, leading to the suggestion that the prefrontal areas of the brain are more sensitive to the effects of sleep disturbances.
Recent studies have shown that both acute sleep deprivation (∼24 hour) and short-term chronic sleep restriction (<6 hours sleep per night for week) cause as much impairment on a simulated driving test as moderate alcohol consumption.28–30 Similar results have been found in more naturalistic studies. Research utilizing medical residents has shown decrements in several tests of psychomotor performance following a 4 week period of heavy duty (mean work time ∼90 hours per week) compared to following a similar period of normal duty (mean work time ∼44 hours per week).31 When alcohol was administered to residents following a period of normal duty, performance was impaired to an extent similar to following 4 weeks of heavy duty.31
Performance impairment is greater with total sleep deprivation than sleep restriction for a set number of hours of total sleep debt.32–34 Thus, a single night of total sleep deprivation (8 hours of sleep debt) results in a greater impairment than 4 nights of mild (2 hours of sleep debt per night) or 2 nights of moderate (4 hours of sleep debt per night) sleep restriction.33 One interpretation of this finding is that adaptation occurs to chronic sleep deprivation.32 Another is that the critical factor is the number of hours of wakefulness beyond what is normal for the individual (cumulative wake extension time).33 When analyzed from this perspective, the behavioral impairments due to total and partial sleep deprivation approximated a single near-linear model.33,34 These results support the view that even mild chronic sleep restriction gradually but inexorably erodes performance. Interestingly, sleep deprivation studies indicate that subjects are often not aware of their level of sleep debt, as they do not necessarily experience daytime sleepiness.33,35 Lack of insight increases the risk of accidents and sleep-related errors.36 These results are worrisome, given the prevalence of chronic sleep restriction in the U.S.3
The impact of sleep loss is potentially more devastating for the elderly, the medically compromised, and for individuals with psychiatric disorders. Sleep disturbances are extremely common in this vulnerable population (estimates for insomnia range from 50–80% in psychiatric illness), and are part of the diagnostic criteria for many psychiatric disorders.37–39 Commonly related disorders include the sleep disorders themselves such as narcolepsy, mood, anxiety, and substance use disorders. Of note is the recent evidence that sleep architecture can remain abnormal in patients with treated depressive episodes and is also found in nondepressed relatives of these patients.37 Many patients who have achieved sobriety after significant substance abuse continue to have abnormalities in sleep (slow wave) for years after recovery.37 Insomnia is also frequently present following traumatic brain injury (present in 30–50% of patients with traumatic brain injury), and studies indicate that these symptoms may persist for many years.39
All major classes of psychotropic medications affect the neurotransmitters that modulate the sleep-wake cycle. For example, the selective serotonin reuptake inhibitors and tricyclic antidepressants decrease REM sleep and benzodiazepines increase the affinity of GABA receptors for GABA, thus increasing sleep. Clinicians often consider too much or too little “sleep” when choosing a psychopharmacologic intervention for a mood or other disorder. However, the secondary effects of these medications on neurotransmitter balance in sleep-waking functional anatomy and ultimately in the quality of resulting sleep has not, to date, been incorporated into standard treatment guidelines. The complexities of sleep-arousal neuroanatomy/neurochemistry and the relationship with stress, anxiety, and psychiatric disease has been an area of study for decades. Yet, there is still much more required to fully understand this complex system in order to design pharmacologic interventions for illness that will produce symptom relief, natural sleep, and have minimal side effects.
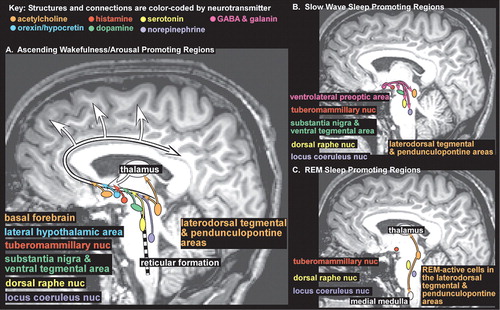
FIGURE 1. The brain regions that govern arousal and sleep are illustrated on a midline sagittal magnetic resonance image. Structures and pathways are color-coded by neurotransmitter.
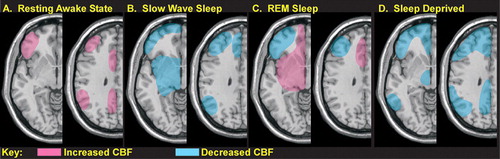
FIGURE 2. The activity level of the brain, as indicated by regional cerebral blood flow(CBF) imaging, varies with the state of consciousness and rest.1, 2 The areas most active in the ‘control’ resting waking state (lying still with the eyes closed) compared to sleeping are in prefrontal and parietal cortex (A). During slow wave sleep (SWS) several areas consistently exhibit decreased activity compared to wakefulness (B). When REM sleep is compared to SWS there are areas of increased activity (C, pink). There are areas of decreased activity when REM sleep is compared to the awake state (C, blue). Sleep deprivation is associated with decreases in several areas compared to the rested awake state (D).
1 Maquet P: Functional neuroimaging of normal human sleep by positron emission tomography. J Sleep Res 2000; 9:207–231Crossref, Medline, Google Scholar
2 Thomas M, Sing M, Belenky G , et al: Neural basis of alertness and cognitive performance impairments during sleepiness. I. effects of 24 hour of sleep deprivation on waking human regional brain activity. J Sleep Res 2000; 9:335–352Crossref, Medline, Google Scholar
3 National Sleep Foundation: 2005 Sleep in America Poll. National Sleep Foundation; http://www.sleepfoundation.org/_content/hottopics/2005_summary_of_findings.pdf (accessed 12-9-2005)Google Scholar
4 Foster RG, Wulff K: The rhythm of rest and excess. Nat Rev Neurosci 2005; 6:407–414Crossref, Medline, Google Scholar
5 Rechtschaffen A: Current perspectives on the function of sleep. Perspect Bio Med 1998; 41:359–390Crossref, Medline, Google Scholar
6 Marshall L, Born J: Brain-immune interactions in sleep. Int Rev Neurobiol 2002; 52:93–131Crossref, Medline, Google Scholar
7 Vgontzas AN, Chrousos GP: Sleep, the hypothalamic-pituitary-adrenal axis, and cytokines: multiple interactions and disturbances in sleep disorders. Endocrinol Metab Clin North Am 2002; 31:15–36Crossref, Medline, Google Scholar
8 Spiegel K, Leproult R, L’hermite-Baleriaux M, et al: Leptin levels are dependent on sleep duration: relationship with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab 2004; 89:5762–5771Crossref, Medline, Google Scholar
9 Spiegel K, Tasali E, Penev P, et al: Brief communication: sleep curtailment in healthy young men is assocated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med 2004; 141:846–850Crossref, Medline, Google Scholar
10 Bryant PA, Trinder J, Curtis N: Sick and tired: Does sleep have a vital role in the immune system? Nat Rev Immunol 2004; 4:457–467Crossref, Medline, Google Scholar
11 Cirelli C: A molecular window on sleep: changes in gene expression between sleep and wakefulness. Neuroscientist 2005; 11:63–74Crossref, Medline, Google Scholar
12 Cirelli C: How sleep deprivation affects gene expression in the brain: a review of recent findings. Appl Physiol 2002; 92:394–400Crossref, Medline, Google Scholar
13 Greene R, Siegel J: Sleep: a functional enigima. Neuromolecular Med 2004; 5: 59-68Google Scholar
14 Rauchs G, Desgranges B, Foret J, et al: The relationship between memory systems and sleep stages. J Sleep Res 2005; 14:123–140Crossref, Medline, Google Scholar
15 Stickgold R, Walker MP: Memory consolidation and reconsolidation: what is the role of sleep? Trends Neurosci 2005; 28:408–415Crossref, Medline, Google Scholar
16 Gais B, Born J: Declarative memory consolidation: mechanisms acting during human sleep. Learn Mem 2004; 11:679–685Crossref, Medline, Google Scholar
17 Wagner U, Gais S, Haider H, et al: Sleep inspires insight. Nature 2004; 427:352–355Crossref, Medline, Google Scholar
18 España R.A., Scammell TE: Sleep neurobiology for the clinician. Sleep 2004; 27:811–820Medline, Google Scholar
19 Stiller JW, Postolache TT: Sleep-wake and other biological rhythms: functional neuroanatomy. Clin Sports Med 2005; 24:205–235Crossref, Medline, Google Scholar
20 Staunton H: Mammalian sleep. Naturwissenschaften 2005; 92:203–220Crossref, Medline, Google Scholar
21 Zeman A, Reading P: The science of sleep. Clin Med 2005; 5:97–101Crossref, Medline, Google Scholar
22 Nofzinger EA: Functional neuroimaging of sleep. Semin Neurol 2005; 25 9-18Google Scholar
23 Thomas ML, Sing HC, Belenky G, et al: Neural basis of alertness and cognitive performance impairments during sleepiness II. Effects of 48 and 72 hour of sleep deprivation on waking human brain activity. Thalamus Relat Syst 2003; 2: 199-229Google Scholar
24 Wu JC, Gillin JC, Buchsbaum MS, et al: The effect of sleep deprivation on cerebral glucose metabolic rate in normal humans assessed with positron emission tomography. Sleep 1991; 14:155–162Medline, Google Scholar
25 Durmer JS, Dinges DF: Neurocognitive consequences of sleep deprivation. Semin Neurol 2005; 25:117–129Crossref, Medline, Google Scholar
26 Van Dongen HP, Baynard MD, Maislin G et al: Systematic interindividual differences in neurobehavioral impairment from sleep loss: evidence of trait-like differential vulnerability. Sleep 2004; 27: 423-433Google Scholar
27 Harrison Y, Horne JA: The impact of sleep deprivation on decision making: a review. J Exp Psychol Appl. 2000; 6:236-249Google Scholar
28 Arnedt JT, Wilde GJ, Munt PW, et al: How do prolonged wakefulness and alcohol compare in the decrements they produce on a simulated driving task? Accid Anal Prev 2001; 33: 337-344Google Scholar
29 Powell NB, Schechtman KB, Riley RW, et al: The road to danger: the comparative risks of driving while sleepy. Laryngoscope 2001; 111: 887-893Google Scholar
30 Maruff P, Falleti MG, Collie A et al: Fatigue-related impairment in the speed, accuracy and variability of psychomotor performance: comparison with blood alcohol levels. J Sleep Res 2005; 14:21–27Crossref, Medline, Google Scholar
31 Arnedt JT, Owens J, Crouch M, et al: Neurobehavioral performance of residents after heavy night call versus after alcohol ingestion. JAMA 2005; 294: 1025-1033Google Scholar
32 Drake CL, Roehrs TA, Burduvali E, et al: Effects of rapid versus slow accumulation of 8 hours of sleep loss. Psychophysiology 2001; 38:979–987Crossref, Medline, Google Scholar
33 Van Dongen HP, Maislin G, Mullington JM, et al: The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep 2003; 26: 117-126Google Scholar
34 Van Dongen HP, Dinges DF: Sleep debt and cumulative excess wakefulness (letter). Sleep 2003; 26: 249Google Scholar
35 Dement WC: Sleep extension: getting as much extra sleep as possible. Clin Sports Med 2005; 24: 251-268Google Scholar
36 Carskadon MA: Sleep deprivation: health consequences and societal inpact. Med Clin North Amer 2004; 88: 767-776Google Scholar
37 Krahn LE: Psychiatric disorders associated with disturbed sleep. Semin Neurol 2005; 25: 90-96Google Scholar
38 Spira AP, Friedman L, Flint A, et al: Interaction of sleep disturbances and anxiety in later life: perspectives and recommendations for future research. J Geriatr Psychiatry Neurol 2005; 18: 109-115Google Scholar
39 Ouellet MC, Savard J, Morin CM: Insomnia following traumatic brain injury. Neurorehabil Neural Repair 2004; 18:187–198Crossref, Medline, Google Scholar



