Donepezil Effects on Cerebral Blood Flow in Older Adults With Mild Cognitive Deficits
Previous imaging studies have used largely the technique of single photon emission computed tomography (SPECT) to assess changes in cerebral blood flow (CBF) among subjects who have dementia and are undergoing treatment studies. For example, Staff et al. 6 evaluated 12 patients with Alzheimer’s disease who previously had undergone 99m Tc-hexamethylpropylene-amine-oxime ( 99m Tc-HMPAO) SPECT imaging and proceeded clinically to open-label donepezil treatment. This study reported a mean increase in global CBF, with the frontal lobes showing the most significant increase. 6 In another SPECT imaging study using 123 iodoamphetamine (IMP), Hanyu et al. found a greater decrement in regional CBF (rCBF) in the medial and lateral frontal lobes in donepezil nonresponders, compared with 61 responders with Alzheimer’s disease who were classified according to response, based on the Mini-Mental State Examination. 7 In a 99m Tc-ethyl cysteinate dimer SPECT study of 35 patients with Alzheimer’s disease, the effect of donepezil compared to placebo treatment was evaluated over a 1-year period. The anterior cingulate gyri, right middle temporal gyrus, right inferior parietal lobules, and prefrontal cortex demonstrated decrements in rCBF in placebo-treated patients, compared with patients receiving donepezil. 8 In another controlled study, Nobili et al. 9 used 99m Tc-HMPAO SPECT imaging to compare 13 untreated patients with Alzheimer’s disease to 25 patients receiving donepezil over a 1-year period. At intake the untreated group demonstrated higher CBF than the treated group in left frontal and left parietal cortex. However, over the treatment period, there was a greater reduction in the left temporal and occipital-temporal cortex in the untreated group, with no changes in the treated group. Nobili et al. concluded that cerebral perfusion may be preserved in patients who have Alzheimer’s disease and are undergoing chronic donepezil therapy. 9
A number of positron emission tomography (PET) studies have examined CBF in the context of dementia (for a review, see Wolf et al., 2003 10 ). To our knowledge, however, no previous studies have used [ 15 O]water positron emission tomography to determine donepezil effects in patients with mild cognitive deficits. One study used 18 F-fluorodeoxyglucose PET imaging in a 26-week trial that compared 27 patients with Alzheimer’s disease being treated with the cholinesterase inhibitor rivastigmine with patients undergoing placebo treatment. Subjects who responded to rivastigmine demonstrated a significant increase in metabolic activity in the hippocampus, compared to a relative decrease in hippocampal metabolism across the same time interval in patients who did not respond to treatment. 11
The above investigations involving Alzheimer’s disease patients have suggested a beneficial effect of the cholinesterase inhibitor donepezil. This study aimed to evaluate the effect of donepezil in a sample of persons with mild cognitive deficits (as opposed to overt dementia). Treatment effects were evaluated in terms of global cerebral blood flow (gCBF) and regional cerebral blood flow (rCBF) as measured by quantitative [ 15 O]water PET imaging and cognitive testing. The goal of this work was to determine whether donepezil exerted detectable effects in this early state of cognitive decline that could provide information on the potential clinical utility of donepezil for mild cognitive impairment. Subjects for this study both were recruited from the community and volunteered because of a self-perception of memory loss. Given this self-awareness, this sample may represent a treatable population that is accessible if clear benefits can be demonstrated from the treatment intervention.
METHOD
Fifteen older adults with mild cognitive deficits were studied after written informed consent was obtained in accordance with the University of Iowa Institutional Review Board. The study involved a double-blind, placebo-controlled randomization to donepezil titrated to 10 mg daily over the first 6 weeks and then continued for a 6-month period with imaging sessions (described below) at baseline and at 6 months. Cognitive impairment was objectively assessed with several neuropsychological measures, including the Mini-Mental State Examination (MMSE) 12 and the Hopkins Verbal Learning Test–Revised (HVLT-R). 13 The MMSE is a widely used screening instrument that evaluates global cognitive functioning, and the HVLT-R is a list-learning test that assesses verbal learning and memory. Consistent with existing criteria for mild cognitive impairment (MCI), 14 all participants complained of memory problems and demonstrated objective memory deficits on at least one test of learning and memory (Mattis Dementia Rating Scale: Memory subscale, 15 Wechsler Memory Scale–III: Logical Memory, 16 Brief Visuospatial Memory Test–Revised), 13 which were defined as one or more standard deviations below age-adjusted expectations based on estimates of premorbid functioning. 17 Global cognition (MMSE, Mattis Dementia Rating Scale total score), however, was within normal limits for all participants. No subjects reported significant difficulties with activities of daily living.
Subjects were excluded if they had a history of major neurological, metabolic, or psychiatric disorders, cardiovascular disease, cerebrovascular events, substance abuse or other significant medical problems. Medical conditions were evaluated through a review of all available medical records, current medications, and patient examination. Any acute or unstable condition was exclusionary in the event that a medical condition was present (e.g., hypothyroidism with stable replacement therapy) or a consensus was achieved by the primary investigators that the condition and its treatment would not incur a variation in CBF. An independent clinical neuroradiologist also reviewed each subject’s structural MRI so that any evidence of a complicating disease state could be excluded (e.g., stroke). Not all subjects had taken donepezil prior to enrollment. At intake, all 15 subjects were imaged using both magnetic resonance (MR) and positron emission tomography (PET) imaging. Seven patients (two men, five women) were selected at random to receive donepezil (titrated to 10 mg/day), whereas eight patients (three men, five women) received placebo. Three subjects in the donepezil group and one subject in the placebo group chose not to continue participation through the second imaging session. Of these, two subjects withdrew because of noncompliance with study medication, one chose to participate in a competing medication study, and another withdrew because of a developing respiratory illness. These subjects were comparable to those who did continue the study in terms of age, education, and global cognition. However, on one memory measure, the Mattis DRS Memory Scale, the subjects who withdrew scored lower than continuing subjects. The remaining 11 subjects were reevaluated with PET and cognitive testing after 6 months of donepezil or placebo treatment. All procedures were conducted in a double-blind fashion such that the raters and PET imaging facility staff were not aware of the medication group status of each subject.
The structural MR images were obtained using a GE Signa 1.5T MR scanner. These images were used to localize regions of interest (ROI) on PET images and to exclude overt structural abnormalities (e.g., stroke). Two sequences were acquired for each subject. The T1 sequence was obtained as a 3 Da volume in the coronal plane using a spoiled GRASS sequence with the following parameters: TE = 6 msec, TR = 20 msec, flip angle = 30°, FOV = 160×160×192 mm, matrix = 256×256×124, NEX = 2. The T2 images were acquired using a 2 Da fast spin-echo sequence in the coronal plane with the following parameters: TE = 85 msec, TR = 4800 msec, slice thickness/gap = 1.8/0.0 mm, FOV = 160×160 mm, matrix = 256×256, NEX = 3, number of echoes = 8, number of slices = 124. The MRI data were transferred to the Department of Radiology Image Processing Laboratory for standard analysis by locally developed software (BRAINS2). The T1 weighted images were aligned spatially along the interhemispheric fissure and the AC-PC line was resampled to 1.0 mm 3 voxels. The T2 weighted scans were coregistered with the resampled T1 weighted images using the automated image registration (AIR) program. 18 The Talairach bounding box and the AC and PC points were defined to allow warping of the Talairach grid onto each subject in the study. The T1 and T2 weighted scans were used to perform a multispectral tissue classification, generating a continuous (fuzzy) classification of the brain tissue. 19 The resulting image contained the percentage of gray matter, white matter, and cerebro-spinal fluid (CSF) at every voxel. These continuous tissue classification images were entered into the neural network to define the brain regions. 20 – 22 The Talairach atlas system defined brain lobes including frontal, temporal, parietal, and occipital lobes. 23 The lobar and brain regions were limited to tissue only (e.g., CSF voxels were excluded).
Quantitative [ 15 O]water PET images were acquired on a GE 4096 whole-body PET scanner (GE Medical Systems, Milwaukee) at intake and 6 months after the start of a placebo-controlled donepezil trial. The imaging technical methodology employed was identical to methods previously reported for our institution, 24 – 27 including the insertion of radial artery catheters and the sampling and analysis of arterial blood via an online sampler. 27 Global CBF and rCBF were assessed during a verbal production task (counting) and a memory task (word list recall). The counting task served as a control for the aspects of verbal production associated with articulating words so that the memory functions of interest in the verbal recall memory task could be isolated. Subjects counted aloud from 1 to 3 repetitively during the image acquisition period. For the verbal recall memory task, the subjects were given a word list to learn prior to the imaging session. During the imaging session they were asked to name as many of the words as they were able to remember. During the two tasks, the subjects performed at a rate of approximately one word/second and continued for the 100 seconds of image acquisition. The 40 seconds immediately postbolus transit were used to create a static image. This static image and the arterial input function calculated the corresponding parametric (i.e., flow) image (scaled in mL/min/100g). The cerebral blood flow images were coregistered with their corresponding MR scan using AIR. 18 All analyses were performed on the flow, not on the activity images.
Data Analysis
Imaging data were analyzed using both region of interest (ROI) and function of interest (FOI) approaches. ROIs were defined on the anatomical MR images. (See Tables 1 and 2 for the specific ROIs evaluated.) Blood flow values within these regions were compared using SPSS 11.0 software. All results are expressed as means (standard deviation). A probability level of less than 0.05 was considered significant. The donepezil-treated group and the placebo group were compared in terms of age and cognitive testing (independent-samples t test) and gender prevalence (chi-square test). The rCBF values in the counting task and the memory task were compared between the two groups according to the following methods: a comparison of each group between intake and 6-months was carried out using the paired-samples t test. Comparisons between the donepezil and placebo groups at intake and 6-months were performed using independent-sample t tests without correction.
 |
 |
Function of interest (FOI) analyses between the two groups were performed using statistical parametric mapping (SPM2) (Wellcome Department of Imaging Neuroscience, London, England) running on Matlab 7.0 (Mathworks Inc). In the preprocessing step, percutaneous ethanol injection therapy (PEIT) images were coregistered to MR images that were AC-PC aligned. The T1 weighted image for each subject was spatially normalized to the MNI-305 template T1 using the SPM2 spatial normalization procedure. The final voxel size was 1.0 mm 3 . The normalized data were then smoothed using a Gaussian kernel at 10 mm full width at half maximum (FWHM). Global voxel intensity normalization was performed using proportional scaling with the value of 100. The statistical threshold of SPM{t} map was set at p = 0.001. For the FOI analysis the significance level was raised to 0.001 uncorrected to help control for the multiple comparisons. In addition, regions were considered significant in the FOI analysis only if a cluster of at least 100 contiguous voxels exceeded the 0.001 level, which helped lower the possibility of spurious positive findings. The changes in CBF were compared between intake and 6 months using the “Population main effect: two conditions, one scan/condition (paired t test)” in SPM. This was done independently for the two groups. The “two sample t test” in PET models was selected to compare the CBF between the two groups at intake and 6 months, as well as to compare the CBF between the placebo and donepezil groups. The differences between 6 months and intake were also analyzed to determine areas of blood flow decrease (CBF at 6 months subtracted from intake CBF) and blood flow increase (CBF at intake subtracted from CBF at 6 months) as required by SPM2.
RESULTS
The group receiving donepezil consisted of four subjects (one man, three women) with a mean age of 74.8 (SD = 7.4) years and mean baseline MMSE score of 29.8 (SD = 0.50). The placebo group was composed of seven subjects (three men, four women); the mean age was 68.4 (SD = 4.0) years and the mean baseline MMSE score was 29.6 (SD = 0.8). The two groups were not significantly different in age (p = 0.1), education (p = 0.4), or gender (p = 0.7). At the intake visit, global cognition, as assessed by the MMSE, Mattis Dementia Rating Scale Total score, and Barona IQ estimate, was comparable between the two groups (p = 0.9, p = 0.6, p = 0.6, respectively). Despite random assignment, the two groups were not equivalent for performance on a list-learning test (HVLT Total Learning) at baseline, with the placebo group performing significantly better than the donepezil group (23.4 words versus 18.0 words; t (9) = 2.37, p = 0.042). Performance on the same measure at the 6-month follow-up visit, however, was similar for the two groups (25.3 words versus 23.2 words, p = 0.42), suggesting that the placebo group remained stable across time, whereas the participants taking donepezil improved in list-learning performance across time.
Intake Data
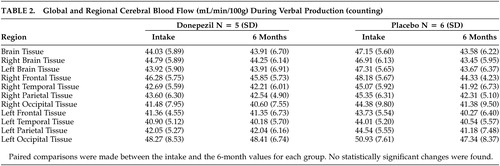
The rCBF in the defined ROIs did not show significant differences between the donepezil and placebo groups during either the verbal production (counting) task or the verbal recall memory task (see the “Intake” columns in Tables 1 and 2 for donepezil and placebo groups). However, the FOI analysis of intake data using SPM2 showed higher relative rCBF in several brain regions in the placebo group at intake compared with the donepezil group (see Table 3 ). These differences were evident in the regional blood flow measures acquired during the verbal recall memory task. These regions included the superior frontal, temporal and occipital gyrus, the inferior parietal gyrus and postcentral gyrus of the left hemisphere and the right precentral gyrus.
 |
Six-Month Data
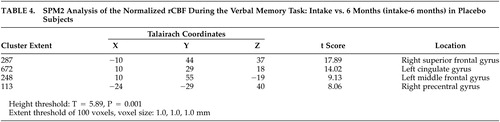
Using the ROI method, the donepezil group did not vary in rCBF between intake and 6 months during either the verbal production (counting) or verbal recall task, as noted in the “Donepezil” columns in Tables 1 and 2 . However, the placebo group did show a reduction in blood flow during the verbal recall task at 6 months compared to baseline in several brain regions, including total brain tissue, left hemisphere brain tissue, and the left frontal and temporal lobes ( Table 1 ), although the results should be interpreted with caution, given the uncorrected probability level of 0.05. Performance on the counting task between intake and the 6-month follow-up for the placebo group was comparable. Using the FOI analysis of data at the 6-month time point, CBF in the right superior parietal lobule was higher in the placebo group compared to the donepezil group during the verbal recall task ( Table 3 ). In the placebo group, an rCBF reduction was observed over the treatment period in the left cingulate gyrus, the left middle frontal gyrus, the right superior frontal gyrus, and the right precentral gyrus by SPM2 analysis ( Table 4 ). In both the memory and counting tasks, the change in CBF over the 6-month period in the donepezil group did not reach statistical significance when directly compared with the CBF change in the placebo group.
 |
DISCUSSION
This study utilized [ 15 O]water PET imaging as a functional neuroimaging technique to assess the effects of donepezil in a group of older adults with mild cognitive deficits by monitoring changes in CBF as a measure of neuronal function. The region of interest (ROI) results revealed decreases in CBF during the verbal memory task in the left frontal and temporal lobes of the placebo group across a 6-month period. In contrast, no changes were observed across the same time frame for the donepezil group. These results resemble the findings from the SPECT study by Nobili et al. 9 (2002) which used 99m Tc-HMPAO SPECT to evaluate the effect of donepezil in a group of patients with mild to moderate Alzheimer’s disease. Nobili et al. also found significant reductions in the CBF in the left temporal lobe and occipital-temporal cortex in the untreated group after 12 months, but no changes in the donepezil group across time. 9 Furthermore, one fluorodeoxyglucose positron emisson tomography (FDG-PET) study showed a similar pattern in brain glucose metabolism in 28 patients with Alzheimer’s disease participating in a placebo-controlled treatment study of donepezil 10 mg/day for 6 months. 28 Striatal glucose metabolism was maintained in donepezil-treated patients but demonstrated a mean decline of 10.4% in placebo-treated patients over the study period.
The cognitive changes reported here over the 6-month period demonstrated a similar pattern to the findings observed in cerebral blood flow. During the baseline evaluation, the placebo group performed better than the donepezil group on a list-learning test. At follow-up, however, the groups were comparable on the same measure. One interpretation of these findings is that the donepezil group improved across time, whereas the placebo group remained stable. With repeated assessments, some improvements in performance are expected (i.e., practice effects); an absence of the expected practice effects could indicate cognitive decline. 29 Therefore, an alternative interpretation of these findings is that the placebo group declined cognitively, as evident in their inability to benefit from practice, whereas the donepezil group remained stable. This latter interpretation of the cognitive data also is consistent with the rCBF data reported here. Despite reasons for caution in interpreting these results in this small sample, all four of the rCBF findings in the placebo group reflected lower flow at 6 months compared to intake. Although the donepezil group showed the same pattern, none of the ROIs showed a significant change in rCBF. This is consistent with the FOI analysis which also showed several regions of decreased rCBF at 6 months in the placebo group and no differences in the donepezil group.
The finding that the donepezil and placebo groups showed different patterns over time in CBF measures obtained during a verbal memory task is consistent with the neuroimaging literature regarding the utility of a cognitive stimulation, in contrast to resting [ 18 F]fluorodeoxyglucose (FDG) PET imaging for the prediction of the severity of dementia. 30 , 31 Just as with other physiological processes, decrements in performance ability may become evident only under the exertion of an activation task. The use of activation tasks may be particularly important in the context of treatment studies as well as in milder syndromes such as mild cognitive impairment where the severity of neurobiologic deficits may be more subtle and variable among individuals.
In conclusion, these data suggest that the CBF in certain regions appeared stable during a 6-month treatment period in subjects receiving donepezil. However, given that cerebral blood flow measures may be affected by age, gender and the methods used in their calculation, 32 one must be cautious in inferring abnormalities in CBF without accounting for the composition of each sample and the methods employed. The conclusions that may be derived from these findings are clearly limited by several factors, including the small number of cases and heterogeneity in cognition reflecting possible variations in cholinergic deficit, as well as variations in effort in performing the verbal recall task. While these limitations may reduce the applicability of these findings, this type of exploratory work may aid in the development of methods to detect treatment effects of cognitive agents. These findings convey the possibility that there may be an effect of cholinesterase inhibitors in early cognitive decline which may be further explored in larger studies.
1. Tsukada H, Nishiyama S, Fukumoto D, et al: Effects of acute acetylcholinesterase inhibition on the cerebral cholinergic neuronal system and cognitive function: functional imaging of the conscious monkey brain using animal PET in combination with microdialysis. Synapse 2004; 52:1–10Google Scholar
2. Erkinjuntti T, Roman G, Gauthier S, et al: Emerging therapies for vascular dementia and vascular cognitive impairment. Stroke 2004; 35:1010–1017Google Scholar
3. Honer WG, Prohovnik I, Smith G, et al: Cortical effects of scopolamine compared to Alzheimer’s disease. J Cerebr Blood Flow Metab 1987; 7(Suppl 1):S387Google Scholar
4. Sitaram N, Weingartner H, Gillin JC: Human serial learning: enhancement with arecholine and choline impairment with scopolamine. Science 1978; 201:274–276Google Scholar
5. Katzman R: Alzheimer’s disease. N Engl J Med 1986; 314:964–973Google Scholar
6. Staff RT, Gemmell HG, Shanks MF, et al: Changes in the RCBF images of patients with Alzheimer’s disease receiving donepezil therapy. Nucl Med Commun 2000; 21:37–41Google Scholar
7. Hanyu H, Shimizu T, Tanaka Y, et al: Regional cerebral blood flow patterns and response to donepezil treatment in patients with Alzheimer’s disease. Dement Geriatr Cogn Disord 2003; 15:177–182Google Scholar
8. Nakano S, Asada T, Matsuda H, et al: Donepezil hydrochloride preserves regional cerebral blood flow in patients with Alzheimer’s disease. J Nucl Med 2001; 42:1441–1445Google Scholar
9. Nobili F, Vitali P, Canfora M, et al: Effects of long-term donepezil therapy on rcbf of Alzheimer’s patients. Clin Neurophysiol 2002; 113:1241–1248Google Scholar
10. Wolf HJV, Gertz HJ, Nordberg A, et al: A critical discussion of the role of neuroimaging in mild cognitive impairment. Acta Neurol Scand Suppl 2003; 179:52–76Google Scholar
11. Potkin S, Anand R, Fleming K, et al: Brain metabolic effects of rivastigmine in Alzheimer’s disease. Int J Neuropsychopharmacol 2001; 4:223–230Google Scholar
12. Folstein M, Folstein S, McHugh P: Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198Google Scholar
13. Benedict RHB, Schretlen D, Groninger L, et al: Hopkins Verbal Learning Test–Revised: normative data and analysis of inter-form and test-retest reliability. Clin Neuropsychol 1998; 12:43–55Google Scholar
14. Petersen RC, Smith GE, Waring SC, et al: Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999; 56:303–308Google Scholar
15. Lucas JA, Ivnik RJ, Smith GE, et al: Normative data for the Mattis Dementia Rating Scale. J Clin Exp Neuropsychol 1998; 20:536–547Google Scholar
16. Wechsler D: Wechsler Memory Scale–III. San Antonio, TX, Psychological Corp, 1997Google Scholar
17. Barona A, Reynolds CR, and Chastain R: A demographically based index of premorbid intelligence for the WAIS-R. J Consult Clin Psychol 1984; 52:885–887Google Scholar
18. Woods RP, Cherry SR, Mazziotta JC: Rapid automated algorithm for aligning and reslicing PET images. J Comput Assist Tomogr 1992; 16:620–633Google Scholar
19. Harris G, Andreasen NC, Cizadlo T, et al: Improving tissue classification in MRI: a three-dimensional multispectral discriminant analysis method with automated training class selection. J Comput Assist Tomogr 1999; 23:144–154Google Scholar
20. Magnotta VA, Heckel D, Andreasen NC, et al: Measurement of brain structures with artificial neural networks: two- and three-dimensional applications. Radiology 1999; 211:781–790Google Scholar
21. Pierson R, Corson PW, Sears LL, et al: Manual and semiautomated measurement of cerebellar subregions on MR images. Neuroimage 2002; 17:61–76Google Scholar
22. Spinks R, Magnotta VA, Andreasen NC, et al: Manual and automated measurement of the whole thalamus and mediodorsal nucleus using magnetic resonance imaging. Neuroimage 2002; 17:631–642Google Scholar
23. Andreasen NC, Rajarethinam R, Cizadlo T, et al: Automatic atlas-based volume estimation of human brain regions from MR images. J Comput Assist Tomogr 1996; 20:98–106Google Scholar
24. Hichwa RD, Ponto LLB, Watkins GL: Clinical blood flow measurement with [15–0] water and positron emission tomography, in Chemists’ Views of Imaging Centers. Edited by Emran AM New York, Plenum Press, 1995, pp 401–417Google Scholar
25. Hurtig RR, Hichwa RD, O’Leary DS, et al: Effects of timing and duration of cognitive activation in [15O]water PET studies. J Cereb Blood Flow Metab 1994; 14:423–430Google Scholar
26. Ponto LLB, Schultz SK, Watkins GL, et al: Technical issues in the determination of cerebrovascular reserve in elderly subjects using 15-O-water PET imaging. Neuroimage 2004; 21:201–210Google Scholar
27. Wollenweber SD, Hichwa RD, Ponto LLB: A simple on-line arterial time-activity curve detector for [O-15] water PET studies. IEEE Trans Nuclear Science 1997; 44:1613–1617Google Scholar
28. Tune L, Tiseo P, Ieni J, et al: Donepezil HCl (E2020) maintains functional brain activity in patients with Alzheimer disease results of a 24-week, double-blind, placebo-controlled study. Am J Geriatr Psychiatry 2003; 169–177:169–177Google Scholar
29. McCaffrey RJ, Duff K, Westervelt HJ: Practitioner’s Guide to Evaluating Change with Neuropsychological Assessment Instruments. New York, Kluwer/Plenum, 2000Google Scholar
30. Pietrini P, Alexander GE, Furey ML, et al: Cerebral metabolic response to passive audiovisual stimulation in patients with Alzheimers disease and healthy volunteers assessed by PET. J Nucl Med 2000; 41:575–583Google Scholar
31. Pietrini PFM, Alexander GE, Mentis MJ, et al: Association between brain functional failure and dementia severity in Alzheimer’s disease: resting versus stimulation PET study. Am J Psychiatry 1999; 156:470–473Google Scholar
32. Ponto LL, Watkins GL, Hichwa RD, et al: [15O]water pharmacokinetics: influence of age and gender in normal subjects. Mol Imaging Biol 2002; 4:129–137Google Scholar



