Brain Neuroimaging in Cannabis Use: A Review
Given the distribution of cannabinoid receptors in associational and limbic areas, there is a wide range of neurobehavioral and cognitive effects seen in acute and chronic cannabis use. 5 – 9 Acute cannabis intoxication, especially in experienced users, is generally associated with feelings of euphoria, relaxation, and depersonalization. 5 , 10 , 11 Anxiety, panic, and psychotic phenomena can also occur, but particularly in inexperienced users or in high dosages. 10 Cognitively, individuals experience decreased attention, memory, and psychomotor speed. 9 There remains considerable debate on potential residual psychological and cognitive effects of chronic, heavy marijuana use. Short-term cognitive deficits have been reliably identified, 7 – 9 , 12 , 13 but deficits or changes identified during sustained abstinence 14 either have not been consistently found 7 , 12 , 15 , 16 or may relate to differences predating use. 15 , 17
Neuroimaging studies complement the biological and psychological research being done in substance use disorders, potentially providing further insight into the pharmacological effects of cannabis and its related cognitive, behavioral, and psychiatric sequelae, including addiction. We review the current empirical findings as they pertain to the structural and functional neuroimaging of cannabis use, with the aim of correlating the findings with neurobiological and neuropsychological data, as well as putative theories of cannabis’s mechanisms of action on brain function.
METHOD
We performed a search of the MedLine database from 1966 to February 2005 for studies published in English, using the key words “cannabis,” “marijuana,” or “tetrahydrocannabinol” (THC), combined with “computed tomography” (CT), “MRI,” “functional MRI” (fMRI), “single photon emission computed tomography” (SPECT), “positron emission tomography” (PET), “cerebral blood flow,” or “neuroimaging.” The initial search yielded 112 citations. Animal studies and single case reports were excluded. Selected studies were required to be published in peer-reviewed journals, focus on users who were directly exposed to cannabis, and employ anatomical structural or functional neuroimaging techniques rather than receptor binding or pharmacologically-oriented studies. Thirty articles met these selection criteria. The references of selected papers were also screened for relevant articles, yielding one further article.
Findings
Structural Studies
Ten structural imaging studies of chronic cannabis users have been published ( Table 1 ). An initial study using pneumoencephalography 18 suggested the presence of enlarged ventricles compared to neurologically healthy comparison subjects; however, multiple subject variables confounded the results, of particular relevance that patients were referred for heterogeneous neurological complaints. Subsequent studies employing CT 19 – 22 did not identify structural differences in chronic cannabis smokers, although limitations in study designs also existed and included a lack of comparison subjects, heavy comorbid substance use, and a wide variability in marijuana consumption.
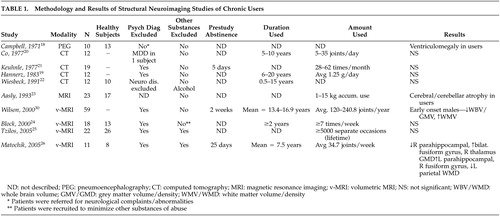 |
Though one structural MRI study of cannabis users by Aasly et al. 23 reported abnormalities, volumetric measurements were not performed and the findings could not be attributed to cannabis use alone. Results were more consistent with the neurotoxic effects of alcohol (i.e., atrophy of the cerebellar vermis) as subjects all had significant alcohol (and other substance) use histories. Three methodologically rigorous volumetric MRI studies have been done to date with two reporting no significant differences between subjects and comparison subjects 24 , 25 and one reporting significant differences. 26 The negative studies evaluated all brain regions, including analyses of regional and whole brain volumes, total white and gray matter, and hippocampal volumes. Though the earlier negative study 24 involved younger subjects (mean ages 22.3 and 22.6 years for men and women, respectively) where most subjects had used for less than 5 years, use histories were much longer in the more recent report. 25 The positive volumetric MRI study 26 reported chronic users compared to nonusing comparison subjects. Chronic users had significantly lower gray matter volumes in the right parahippocampal region while the corresponding left structure had higher white matter volumes. Differences were present in a number of other brain regions as well. Cannabis has been reported to have neurotoxic effects on the hippocampus in animal studies of brain pathology, 27 , 28 but the evidence in human subjects is still lacking 29 and would not explain results differing between hemispheres unless cannabis exposure preferentially affected one hemisphere more than another.
In a study 30 of chronic cannabis users stratified by age of first use and gender, a significant correlation between age of first use and decreased total brain volume was seen. Unfortunately, nonusing subjects were not involved for comparison, so it is unclear if the findings represent differences from comparison subjects or whether they relate to other nonspecific factors that predict earlier onset of substance use and being at risk. 17 Later MRI studies 25 , 26 did not find correlations based on age of onset in secondary analyses of imaging data.
In summary, minimal evidence has been found to suggest an association between cannabis use and structural brain abnormalities. Differences in subjects make comparisons between studies difficult, particularly with regards to amount and duration of cannabis used, as it could be hypothesized that toxicological effects may be more prominent only in heavy, long-standing users. Only one report using the highest resolution imaging technologies and analytic techniques available has identified differences, and it has not been replicated in other comparable studies, suggesting at this time that chronic cannabis use does not result in identifiable structural brain changes.
Functional Imaging Studies
Functional imaging may enable the identification of brain changes too subtle to be detected by current structural imaging techniques. The functional imaging studies reviewed here ( Tables 2 and 3 ) include studies of direct cannabis administration to, and variable periods of abstinence in, occasional and chronic (or heavy) users. The majority of studies involve subjects passively scanned, although more recent ones have employed task-based paradigms. This section focuses on anatomic structures that are implicated in the findings of the reviewed studies and subsequent cognitive and behavioral correlations.
 |
 |
I. Cerebral Cortex
Global cortical effects have been found in functional imaging studies of chronic cannabis users with decreased activity during abstinence, and with generally increased activity with the administration of cannabis or THC, particularly in experienced users.
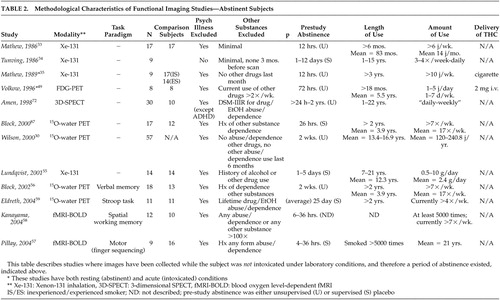
Initial studies utilized 131 Xe-inhalation scintigraphy ( Tables 2 and 3 ), which is less sensitive to identifying subcortical or specific brain region activity changes than more modern functional imaging methods. 31 , 32 The first report by Mathew et al. 33 compared abstinent, previously “heavy” cannabis users to individuals who had never used cannabis. There were no significant differences in cerebral blood flow (CBF) between the groups, although there was a trend toward decreased global CBF in cannabis users. In addition, this study suffers from the same problem that the majority of other functional imaging studies described herein, in that cannabis use (in this case “heavy”) is variably defined based on self-report with widely varying use patterns from subjects of varying backgrounds, making the comparison of study findings difficult. The second 131 Xe-inhalation scintigraphy study 34 examined nine subjects, in various stages of abstinence, who had been hospitalized for cannabis use problems and found significantly lower resting global brain CBF. The sample was not representative of habitual cannabis users, though, as subjects were recruited based on hospitalization without psychiatric comorbidity being excluded. Of interest, the study rescanned four subjects at various intervals 9 to 60 days after the first scan, reporting a 12% increase in CBF with one subject’s scan returning to baseline activity after 9 months of abstinence from cannabis. While rescanned subjects were not selected systematically, it suggests that the hypometabolism may have been a condition normalizing with abstinence. In a later study by Mathew et al., 35 abstinent, “experienced” cannabis smokers exhibited significantly decreased resting global CBF compared to inexperienced cannabis smokers. “Inexperienced” cannabis smokers were required to be abstinent from cannabis for 3 years, but their use histories prior to that were not described.
In a continuation of their structural MRI study comparing early- and late-onset cannabis users, Wilson et al. 30 compared groups during abstinence using positron emission tomography (PET), further subdivided by gender. CBF was significantly greater in the male early-onset group compared to the male late-onset group. No significant differences were found, however, between the two female subgroups or between the early-onset men and women. Although it was postulated that alterations in cerebral volume and blood flow might be linked to cannabis inhibiting sex hormone production, affecting brain development in adolescence, a lack of healthy subjects made the conclusions highly speculative. Debate exists over the effect of age of first use: though some neurophysiological data have suggested that early use is a potential determinant of later brain function, 36 , 37 neuropsychological data do not support this when accounting for verbal IQ. 7 Early-onset users may then simply represent a population at risk for multiple problems rather than cannabis use representing an etiologic basis for brain abnormalities. 17 , 38
The majority of studies have demonstrated global cortical activity increases during administration of smoked cannabis or infused THC. The 1989 study by Mathew et al. 35 examined subjects before and 60 minutes after smoking cannabis. Inexperienced users had a decrease in global cortical CBF while experienced users showed an increase. Anxiety scores were also significantly greater in the inexperienced group and negatively correlated with CBF. When compared with the placebo smoking session, inexperienced users were reported to have a significantly decreased global cortical CBF, but the finding may have just related to anxiety as no differences in CBF were seen in experienced users. The measurements of brain function, however, were taken at 60 minutes, longer than in most other studies discussed here, as well as exceeding likely times for peak neurophysiological activity for cannabis in the brain. 39
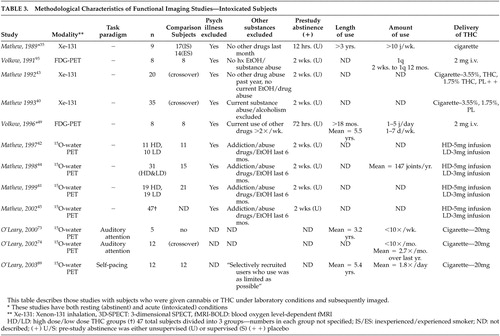
Six studies by Mathew et al. 40 – 45 examined “occasional” users whose use histories were not well-defined. This group likely possessed different demographic, psychosocial, and neurobiological properties when compared with heavy using or better-defined groups, such as cannabis-dependent subjects. Despite this limitation, findings were fairly consistent. In two studies, 40 , 43 increased global cortical activation was reported when comparing smoked (high- and low-dose) cannabis to placebo, where subjective intoxication correlated positively with global cortical CBF. Later studies 41 , 42 , 44 , 45 using 15 O-water PET also found consistent increases in global cortical CBF after infused THC. In one study, 45 dose-dependent activation was found. Although the majority of the above studies have reported bilateral hemispheric activation during intoxication, some studies have reported significantly greater right hemispheric activation. 41 , 43 , 45 It has been theorized that differential right hemisphere activation may relate to cannabis’ purported tendency to promote creativity and increased emotional awareness. 45 – 47
Two studies using 18 F-fluorodeoxyglucose (FDG)-PET to measure cerebral metabolic rate (CMR), however, have failed to demonstrate significant global cortical changes when subjects were administered intravenous THC. One study 48 reported variable changes in eight occasional users, with three subjects showing an increase, three a decrease, and two no change. A later study 49 using cannabis-dependent subjects compared with occasionally-using subjects did not show global cortical changes. It is not clear whether the absence of this finding in these two studies related to potential differences in users’ experience between smoked cannabis and intravenous THC, amounts of THC infused (both studies 48 , 49 used a lower dose infusion of 2 mg versus 3 mg or 5 mg in the studies by Matthew et al. 41 , 42 , 44 , 45 ) or to FDG-PET studying CMR rather than CBF. In general, however, the majority of studies, with the noted exceptions, have reported that smoked and infused cannabis result in increased global cortical activity, particularly in experienced users.
A. Frontal Lobes
Like the global cortical changes reported, differential activity in specific lobes (particularly the frontal and temporal lobes) of the cerebral cortex has been reported, both with abstinence from chronic cannabis use and during the administration of smoked cannabis or infused THC ( Table 4 ). The frontal lobes are thought to play a significant role in substance use disorders 50 by their involvement in cognitive functions, including executive control, working memory, novelty processing, attention and awareness, and integration of multimodal sensory information. 51
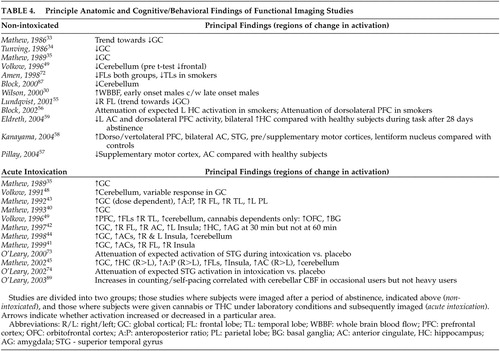 |
Chronic exposure to cannabis in rodents has been reported to result in decreased mesolimbic dopaminergic activity with associated reduced prefrontal cortex activity. 52 – 54 Lundqvist et al. 55 compared 14 abstinent DSM-IV cannabis-dependent subjects with 14 healthy subjects using 131 Xe-inhalation and found significantly reduced blood flow to the right frontal lobe. Their results differed from a similar study 34 which found only decreased global activity. The authors of the former study cited the improved resolution of their imager and shorter average time of abstinence prior to imaging as possible explanations. No correlations were found between flow and duration of use or total cannabis consumption. Volkow et al. 49 also noted decreased resting frontal lobe CMR via FDG-PET in abstinent cannabis-dependent subjects, but following post hoc t tests, this finding was no longer significant.
Mathew et al. have reported consistently increased right frontal lobe 42 – 44 and bilateral frontal lobe 40 , 42 , 45 activation following administration of smoked cannabis or infused THC in occasional cannabis users. While increased bilateral frontal lobe activity has been correlated with reports of subjective intoxication, temporal disintegration (the disrupted ability to subjectively perceive the passage of time) and depersonalization in some studies, 40 , 42 others have reported right frontal activation more strongly correlating with subjective intoxication. 42 , 44 Comparisons of anterior and posterior regional cortical changes in blood flow also support bilateral frontal lobe activation with smoked cannabis and infused THC correlating with reports of subjective intoxication. 43 , 45 Volkow et al. 49 also reported increases in CMR 35 to 50 minutes post-THC infusion in the frontal lobes, including the prefrontal cortex. Both comparison and cannabis-dependent subjects demonstrated the increase, but only cannabis-dependent subjects had increased orbitofrontal cortex (and basal ganglia) activity.
To date there have been four task-based functional neuroimaging studies of recently abstinent regular cannabis users identifying differential frontal lobe activity. 56 – 59 Differences in task paradigms, sample characteristics, and imaging modalities appear to have led to differences in reported findings. Eldreth et al. 59 reported decreased bilateral dorsolateral prefrontal cortex (DLPFC) and right anterior ventromedial prefrontal cortex (VMPFC) activity with a modified Stroop test (a test requiring subjects to ignore distractors during target detection; it has been shown to activate medial and lateral prefrontal cortices and the anterior cingulate in healthy subjects) 60 , 61 in heavy users abstinent for 25 days compared to healthy subjects. No differences were seen in task performance between the two groups, who were matched for IQ and years of education, among other characteristics. The authors postulated that exposure to chronic, heavy cannabis use may result in the activation of alternate neural pathways to process information and thus maintain task performance. Block et al. 56 reported attenuated left DLPFC and left hippocampal activity with a verbal memory task in heavy users abstinent for 2 weeks compared to healthy subjects. Cannabis users had more difficulty with the task, although IQ was not measured in either group, nor was educational background. Their finding parallels neuropsychological testing results in which verbal recall was reported as impaired in heavy users at 7 days, but returned to normal at 28 days. 7 Pillay et al. 57 reported decreased anterior cingulate and supplementary motor cortex (SMC) activity with a finger sequencing task used to examine fine motor function in abstinent heavy users compared to healthy subjects. Although subjects had a brief and somewhat variable (4- to 36-hour) period of abstinence, quantified urinary cannabinoid metabolites did not correlate with task performance, nor was performance correlated with IQ. Subject and comparison groups were not compared for IQ, but there was no difference in years of education. In contrast, one study 58 reported increased bilateral DLPFC and ventrolateral prefrontal cortex (VLPFC) activity with a visuospatial memory task in abstinent cannabis users compared to healthy subjects. The differences found in this last study may have related to a slightly longer period of abstinence prior to testing and potential compensatory changes. No correlation to task performance with urinary cannabinoid metabolites or IQ was seen within the subject group, but the latter variable was not measured in comparison subjects. Years of education were comparable between groups. While the report by Block et al. 56 demonstrated definitive differences in task performance, they were not apparent in the other three, despite differences in regional activation. The task-based studies, then, by finding changes in comparison to healthy subjects in expected areas of activation, suggest that chronic cannabis use may attenuate or at least alter cerebral functions responsible for higher level cognitive processing, which persists at least during initial abstinence.
In summary, functional neuroimaging studies have reported generally consistent frontal lobe differences. Attenuated frontal lobe activity has been reported in recently abstinent heavy cannabis users compared to healthy subjects in resting and task-based studies, while increased frontal lobe activity has been generally reported with cannabis exposure in both heavy and occasional users, correlating with subjective reports of intoxication. The endocannabinoid system is believed to be functionally linked to the extended dopamine reward pathway involving the ventral tegmental area, nucleus accumbens, prefrontal cortex (including the orbitofrontal and DLPFC), and anterior cingulate, 62 , 63 viewed as central to the development of addictive behavior. 64 In rats, administration of THC is reported to increase firing of dopaminergic neurons in the ventral tegmental area. 65 – 68 Differential activity to substance-related cues in the prefrontal cortex has been consistently reported in substance-dependent subjects. 50 , 69 , 70 Changes in the reported frontal lobe activity from neuroimaging studies to date have been consistent with those found with other substance use disorders.
B. Temporal Lobes
The cortical areas of the temporal lobes are related to audition, speech, and integration of sensory information. 71 The only study to report findings during abstinence was by Amen and Waugh, 72 who used three-dimensional SPECT, and reported decreased temporal lobe activity in heavy cannabis users with comorbid attention deficit hyperactivity disorder (ADHD) compared with subjects with ADHD alone, but who were variably bilateral, right-, or left-sided. The psychiatric comorbidity may have confounded the findings, as both diagnoses may have specific interactional effects as opposed to simply additive ones. Subject differences were also marked regarding durations of abstinence (ranging from 24 hours to 2 years) and duration of use.
In two studies, Mathew et al. 40 , 43 reported right temporal lobe activation with smoked cannabis in regular cannabis users which, in one study, 40 correlated with subjective reports of intoxication, depersonalization, and temporal disintegration. Right temporal lobe increases in CMR were also reported in an FDG-PET study 49 in both dependent and occasional users.
Two PET studies by O’Leary et al. 73 , 74 evaluated cannabis users via a dichotic listening task to evaluate auditory attention when administered smoked cannabis. This task has previously been reported to result in activation of the right superior temporal gyrus in healthy, nonexposed subjects. 75 The first was a pilot study of five subjects and lacked a comparison group. Although no differences in task performance were identified between cannabis and placebo administered groups, those who smoked cannabis showed attenuated superior temporal gyrus CBF. Other regions in frontal and parietal areas also showed lower CBF compared with placebo. The decreased activity reported stands in contrast to the pattern of increased global and frontal activity described above. O’Leary et al. concluded that cannabis intoxication may inhibit structures forming an attentional network, but intelligence or education background was not described in either report.
Overall, results of functional neuroimaging studies identifying the temporal lobes are limited and somewhat conflicting. They suggest increased activity with intoxication, correlating to subjective effects in regular cannabis users, but attenuation of activity in tasks requiring attention. Any conclusions would be speculative at this point.
II. Limbic/Paralimbic Regions
A. Anterior Cingulate
The anterior cingulate (AC) is a component of the limbic system, reported to be involved in the diverse realms of memory, cognition, behavior, and motor function. 76 Functional neuroimaging studies to date have reported differential activity in the AC of cannabis users, paralleling that found in the frontal lobes ( Table 4 ).
Mathew et al. 42 reported increased bilateral 41 , 44 , 45 or right AC 42 activity during acute intoxication with infused THC in regular cannabis users compared with healthy subjects. Subjective intoxication 41 , 42 and depersonalization 41 were positively correlated with AC activation, the latter being linked to AC function in other populations. 77 Dose-dependent AC activation has also been reported. 41 , 45
Eldreth et al. 59 used 15 O-water PET to study abstinent chronic cannabis smokers performing a modified Stroop test. Despite no significant differences on task performance, decreased activation was reported in the left AC compared with healthy subjects. Pillay et al. 57 also reported decreased AC activity in abstinent cannabis users compared to healthy subjects during a finger sequencing task. Overall, increased AC activity, correlating with subjective intoxication and depersonalization, has been reported with cannabis administration, while decreased AC activity has been reported during abstinence in regular cannabis users during performance tasks. The differences in AC activity correlate with those seen in other substance use disorders. 78 – 80
B. Insula
The insular cortex has connections to many cortical and subcortical brain regions, including reciprocal pathways to areas with higher densities of cannabinoid receptors, including the hippocampus, amygdala, frontal lobe, and anterior cingulate. 81 Increased right, 41 left, 42 and bilateral 44 , 45 metabolism during administration of THC have been reported ( Table 4 ). Although the insula has a relative dearth of receptors, 3 it forms part of a limbic integration circuit 81 and, therefore, may be activated secondarily after regions with higher cannabinoid receptor density. The insula is involved with sensory integration (including auditory attention) 82 , 83 and autonomic control; 81 both sensory perception and cardiac function are affected during cannabis intoxication. 84
C. Hippocampus
The hippocampus is involved in the encoding of memory and has lateralized functionality; the dominant side is generally responsible for verbal material while the nondominant structure processes nonverbal information. 71 Despite the high concentration of cannabinoid receptors in the hippocampus, 3 there is less data reporting differential activation in this structure with the administration of THC than other brain regions. Right unilateral increased activity was reported by Mathew et al. 42 in the hippocampus and amygdala occurring in a temporal fashion. Hippocampal activity was also described as being inversely correlated with subjective reports of intoxication. The authors of that study postulated that this may relate to the short-term memory dysfunction seen with cannabis intoxication. Increased CBF in the hippocampus was also reported after THC infusion in a follow-up study by Mathew et al. 45 In the high dose condition (5 mg), global cortical increases in activity were reported with increased CBF in the hippocampus and amygdala 30 minutes post-infusion, but at 60 minutes these changes were not significant. No other studies, despite similar methodologies involving THC infusion, have reported hippocampal activity changes during acute intoxication; this is possibly due, in part, to the inability of SPECT or the earlier PET studies to detect changes in this structure.
In a task-based study, Block et al. 56 examined recently abstinent chronic cannabis smokers via a verbal memory task. The group of smokers required significantly more trials to learn a list of words successfully than comparison subjects. 15 O-water PET scans taken during each of the memory tasks failed to show a normally expected increase in left hippocampal CBF (and left DLPFC) in subjects compared with non-using subjects. Subjects were abstinent for only 26 hours, however, so ongoing intoxication or subclinical withdrawal symptoms may have confounded the results. This would be consistent with the findings of a neuropsychological study of heavy cannabis users where verbal memory was impaired at 7 days, but returned to baseline by 28 days. 7
Despite high concentrations of cannabinoid receptors in the hippocampus, increased activation in this region in heavy cannabis smokers has only been found either early with high dose infusions or not at all, with subjective reports of intoxication being inversely correlated with hippocampal activity. Initial abstinence from prior chronic cannabis use appears also to result in problems with memory and an inability to appropriately activate the hippocampus for memory-related tasks. Whether this lack of activation pertains to hippocampus-specific neurotoxicity, as seen in some animal data, or transient effects of cannabis remains to be delineated.
III. Cerebellum
There is mounting evidence for cerebellar involvement in higher cognition, including executive function, memory, language, timing, as well as emotional processing. 85 , 86 Differential cerebellar activity has been reported in several functional neuroimaging studies with chronic cannabis users and acute exposure to cannabis ( Table 4 ).
Earlier studies using SPECT generally would not show changes in cerebellar metabolism as this technique does not visualize subcortical structures well. 32 Volkow et al. 49 compared eight abstinent cannabis-dependent subjects to eight healthy subjects via FDG-PET measuring CMR and reported cerebellar hypometabolism in the cannabis-dependent subjects compared to the healthy subjects. No other regional changes were seen. Greatly reduced cerebellar CBF was also reported in a well-designed volumetric MRI study by Block et al. 87 using 15 O-water PET to compare 17 abstinent regular cannabis users with 12 non-using healthy subjects. Decreased cerebellar metabolism has been demonstrated following chronic in vivo administration of THC to rodents. 88 This effect is thought to be secondary to impaired spontaneous network activity of cerebellar granule cells, suggesting a direct mechanism of action on cerebellar dysfunction, in addition to indirect phenomena, discussed below.
With the administration of smoked cannabis or infused THC, increases in cerebellar activity have been reported in occasional and regular cannabis smokers ( Table 4 ). Mathew et al. 44 reported significant (although variable in individual subjects) increases in cerebellar blood flow in one study and a trend toward increased cerebellar blood flow (not significant) in another. 42 The former study also reported a significant correlation of pontine CBF to cerebellar blood flow, leading the authors to suggest that the findings related to a frontocerebellar network in which the pons is involved. 44 A later study by the same authors 45 found cerebellar activation to be present up to 60 minutes after smoking cannabis, but it was not significant compared with placebo at 90 minutes. Volkow et al. 48 , 49 also reported significant increases in cerebellar activity with infused THC in both occasional cannabis users 48 and cannabis-dependent subjects 49 compared to healthy subjects. Subjective reports of intoxication were positively correlated with cerebellar blood flow, 48 , 49 but inversely correlated with the subjective reporting of temporal disintegration. 44
In a task-based study, O’Leary et al. 89 evaluated neural correlates of self-pacing during acute intoxication with smoked cannabis via 15 O-water PET in occasional and heavy cannabis smokers compared with healthy subjects. No differences were present in IQ or education. Subjects were scanned twice (1 week apart), while counting verbally at a predetermined, practiced rate before and after smoking a cannabis cigarette in one session and a placebo in the other. Counting rate increased in both groups after smoking cannabis but did not change after placebo. Following cannabis, counting rate negatively correlated with left inferior posterior cerebellar metabolism in the occasional cannabis smokers, although this was not present in the heavy cannabis-smoking group (a trend toward negative correlation using a lower statistical threshold was found for the anterior vermis in this group).
In general, cannabis-dependent and regular cannabis-using subjects during abstinence have been found to show reduced cerebellar activity, but with cannabis exposure, both groups show increased cerebellar activity correlating with subjective reports of intoxication. Temporal disintegration, however, has been reported to be inversely correlated with cerebellar activity, while increased metabolism in this region during acute intoxication alters self-pacing, another timing function. These data are consistent with the hypothesis that the cerebellum is highly involved in the subjective experience of time passage as well as self-pacing, and that these functions are disrupted during acute cannabis intoxication with persistence during (early) abstinence.
DISCUSSION
Comparing results between studies is hampered by wide differences in subject selection and study design. Study subjects included a range of cannabis-dependent individuals (based on DSM criteria) as well as “chronic,” “heavy,” or “occasional” cannabis users, all with differing definitions. Healthy comparison subjects also varied from being occasional cannabis users to individuals who had never used in their lifetimes. Differences in inclusion/exclusion criteria (such as other substance or alcohol use and how they are defined) and other demographic features (i.e., gender, education, age) also were present, limiting the “generalizability” of reported findings. It is somewhat remarkable then that the findings of the reported studies to date have been reasonably consistent, so the differences in study design likely further substantiate the reproducibility of the most consistent findings. Quantification of use and duration of abstinence appear to be the most critical factors potentially influencing study outcome.
Only two studies 49 , 55 utilized DSM criteria to define cannabis dependence. Even with the use of specific criteria, however, quantification of previous THC exposure could not necessarily be accounted for by characterizing frequency of use. Not only do smoking styles and quantities vary, but it has also been documented that the THC content of smoked cannabis varies markedly between sources and preparations, with potency reported to have increased substantially over the past 20 years. 84 The comparability then of earlier studies to later studies may not be appropriate. In addition, doses of THC used with exposure paradigms may be very low in comparison to those typically used, again potentially making the results difficult to interpret (e.g., many typical joints have 150 mg of THC where the most delivered in a study here was 20 mg). 84 More recent studies have attempted to quantify more accurately recent use by measuring normalized levels of urinary THC-COOH, a renally excreted carboxylic acid metabolite of tetrahydrocannabinol. 57 , 58
Regarding durations of abstinence, total durations ranged from hours to years. Neuropsychological data suggest residual effects of cannabis are detectable shortly after cessation of use (i.e., within 24 hours), 8 , 13 but likely decrease or resolve significantly thereafter as discussed above, although some data suggest otherwise. 14 It has been reported previously that the onset of cannabis withdrawal occurs within 1 to 2 days postcessation and can last up to 1 to 2 weeks, with residual symptoms sometimes present thereafter. 90 , 91 Therefore, study subjects likely reflect an even more heterogeneous group, as prestudy smoking patterns, as well as the period of abstinence before data collection, could influence results: subjects abstinent for 24 to 48 hours may resemble those who have residual symptoms of intoxication (depending on prestudy amounts and frequencies used); those abstinent for several days to several weeks likely represent varying stages of withdrawal; and periods of abstinence of several months or more would not manifest either of these syndromes.
Variance in study methods, a paucity of studies having data points past 60 minutes, and infrequent (e.g., every 30 minutes) sampling of brain activity limit conclusions relating to the time course of changes in blood flow or metabolism that occur during intoxication. In studies to date, peak activations in brain metabolism post-drug administration are seen at 30 minutes and gradually return toward baseline levels thereafter. 41 , 45 This correlates with studies in which plasma THC concentrations are determined at baseline and every 30 minutes after, 41 , 42 , 44 , 45 but when samples are taken more frequently, 40 , 43 serum levels peak much earlier, like other pharmacokinetic studies of inhaled marijuana and intravenous THC, 92 – 94 where levels peak 3 to 10 minutes postexposure. 94 Serum levels do not necessarily reflect brain levels or receptor occupancy, however, and data in this area are lacking.
Study designs also differed in regards to neuroimaging methods and data analysis. SPECT is less sensitive to subcortical changes 31 and thus is unable to demonstrate later findings more consistently seen with PET and fMRI, such as changes in AC, insula, and cerebellum. Earlier studies examined areas of brain activation as averages of changes in predefined regions of interest (as opposed to later studies using voxel-based morphometry), which would have reduced sensitivity to smaller brain volume changes.
Thus, the current neuroimaging data are yet to help clarify whether residual cognitive deficits either resolve or not with abstinence alone. While changes in brain activity can be surmised from the neuroimaging studies done to date, they have not been consistently reported to correlate with task performance or cognitive testing impairment. It remains unclear as to whether the functional neuroimaging findings relate to subtle residual effects where brain activity has adapted to limit impairment, significantly different neuroimaging findings lacking in clinically significant effects, or functional neuroimaging and neuropsychological testing evaluating different constructs altogether.
CONCLUSIONS
Current data suggest that there are convergent findings regarding the chronic and acute effects of cannabis on brain activity. However, further refinements in study methodology may help answer lingering questions regarding potential differences between those persons who become dependent on cannabis versus those who use cannabis recreationally, potential residual effects of chronic use, consequences of earlier age of exposure to cannabis, acute and chronic effects on task performance, and possible neurobiological similarities between comorbid psychiatric disorders and cannabis use.
Exposure to cannabis, both acutely and chronically, is associated with changes in brain activity, many paralleling those found with other substance use disorders. These findings predominantly involve frontal, limbic and also cerebellar regions, with generally increased activity during exposure and generally decreased activity during abstinence. Findings seen during abstinence may persist even though they are not necessarily correlated with neuropsychological test results. Regions of differential brain activity appear to involve those implicated with other substance use disorders involving the extended dopamine reward pathways, but also a potential frontocerebellar network. Future research employing the use of specific diagnostic criteria, pairing of appropriate neurocognitive testing to functional imaging, adequate exposures to cannabis, and greater lengths of abstinence may help address remaining questions including the basis of any residual cognitive deficits from cannabis use and any potential factors differentiating cannabis-dependent subjects from cannabis users.
1. Tjepkema M: Alcohol and illicit drug dependence. Health Rep 2004; 15:9–19Google Scholar
2. Substance Abuse and Mental Health Services Administration: Results from the 2003 National Survey on Drug Use and Health: National Findings. Rockville, Md, Office of Applied Studies, NSDUH Series H-25, DHHS Publication No. SMA 04–3964, 2004Google Scholar
3. Glass M, Dragunow M, Faull RL: Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience 1997; 77:299–318Google Scholar
4. Ameri A: The effects of cannabinoids on the brain. Prog Neurobiol 1999; 58:315–348Google Scholar
5. Johns A: Psychiatric effects of cannabis. Br J Psychiatry 2001; 178:116–122Google Scholar
6. Fant RV, Heishman SJ, Bunker EB, et al: Acute and residual effects of marijuana in humans. Pharmacol Biochem Behav 1998; 60:777–784Google Scholar
7. Pope HG Jr, Gruber AJ, Hudson JI, et al: Neuropsychological performance in long-term cannabis users. Arch Gen Psychiatry 2001; 58:909–915Google Scholar
8. Solowij N, Stephens RS, Roffman RA, et al: Cognitive functioning of long-term heavy cannabis users seeking treatment. J Am Med Assoc 2002; 287:1123–1131Google Scholar
9. Solowij N: Cannabis and Cognitive Functioning. Cambridge, Cambridge University Press, 1998Google Scholar
10. McDowell D: Marijuana, hallucinogens, and club drugs, in Clinical Textbook of Addictive Disorders. Edited by Richard Frances SM, Avram Mack. New York, Guilford, 2005, pp 157–183Google Scholar
11. Iversen L: Cannabis and the brain. Brain 2003; 126:1252–1270Google Scholar
12. Fried PA, Watkinson B, Gray R: Neurocognitive consequences of marihuana—a comparison with pre-drug performance. Neurotoxicol Teratol 2005; 27:231–239Google Scholar
13. Pope HG Jr, Yurgelun-Todd D: The residual cognitive effects of heavy marijuana use in college students. J Am Med Assoc 1996; 275:521–527Google Scholar
14. Bolla KI, Brown K, Eldreth D, et al: Dose-related neurocognitive effects of marijuana use. Neurology 2002; 59:1337–1343Google Scholar
15. Pope HG, Jr, Gruber AJ, Hudson JI, et al: Early-onset cannabis use and cognitive deficits: what is the nature of the association? Drug Alcohol Depend 2003; 69:303–310Google Scholar
16. Gonzalez R, Carey C, Grant I: Nonacute (residual) neuropsychological effects of cannabis use: a qualitative analysis and systematic review. J Clin Pharmacol 2002; 42:48–57Google Scholar
17. Macleod J, Oakes R, Copello A, et al: Psychological and social sequelae of cannabis and other illicit drug use by young people: a systematic review of longitudinal, general population studies. Lancet 2004; 363:1579–1588Google Scholar
18. Campbell AM, Evans M, Thomson JL, et al: Cerebral atrophy in young cannabis smokers. Lancet 1971; 2:1219–1224Google Scholar
19. Hannerz J, Hindmarsh T: Neurological and neuroradiological examination of chronic cannabis smokers. Ann Neurol 1983; 13:207–210Google Scholar
20. Co BT, Goodwin DW, Gado M, et al: Absence of cerebral atrophy in chronic cannabis users: evaluation by computerized transaxial tomography. J Am Med Assoc 1977; 237:1229–1230Google Scholar
21. Kuehnle J, Mendelson JH, Davis KR, et al: Computed tomographic examination of heavy marijuana smokers. J Am Med Assoc 1977; 237:1231–1232Google Scholar
22. Wiesbeck GA, Taeschner KL: A cerebral computed tomography study of patients with drug-induced psychoses. Eur Arch Psychiatry Clin Neurosci 1991; 241:88–90Google Scholar
23. Aasly J, Storsaeter O, Nilsen G, et al: Minor structural brain changes in young drug abusers: a magnetic resonance study. Acta Neurol Scand 1993; 87:210–214Google Scholar
24. Block RI, O’Leary DS, Ehrhardt JC, et al: Effects of frequent marijuana use on brain tissue volume and composition. Neuroreport 2000; 11:491–496Google Scholar
25. Tzilos GK, Cintron CB, Wood JB, et al: Lack of hippocampal volume change in long-term heavy cannabis users. Am J Addict 2005; 14:64–72Google Scholar
26. Matochik JA, Eldreth DA, Cadet JL, et al: Altered brain tissue composition in heavy marijuana users. Drug Alcohol Depend 2005; 77:23–30Google Scholar
27. Scallet AC, Uemura E, Andrews A, et al: Morphometric studies of the rat hippocampus following chronic delta-9-tetrahydrocannabinol (THC). Brain Res 1987; 436:193–198Google Scholar
28. Landfield PW, Cadwallader LB, Vinsant S: Quantitative changes in hippocampal structure following long-term exposure to delta 9-tetrahydrocannabinol: possible mediation by glucocorticoid systems. Brain Res 1988; 443:47–62Google Scholar
29. Sarne Y, Mechoulam R: Cannabinoids: between neuroprotection and neurotoxicity. Curr Drug Target CNS Neurol Disord 2005; 4:677–684Google Scholar
30. Wilson W, Mathew R, Turkington T, et al: Brain morphological changes and early marijuana use: a magnetic resonance and positron emission tomography study. J Addict Dis 2000; 19:1–22Google Scholar
31. Sadek JR, Hammeke TA: Functional neuroimaging in neurology and psychiatry. CNS Spectr 2002; 7:286–290, 295–289Google Scholar
32. Hoffman JM, Coleman RE: Perfusion quantitation using positron emission tomography. Invest Radiol 1992; 27(suppl 2):S22–26Google Scholar
33. Mathew RJ, Tant S, Burger C: Regional cerebral blood flow in marijuana smokers. Br J Addict 1986; 81:567–571Google Scholar
34. Tunving K, Thulin SO, Risberg J, et al: Regional cerebral blood flow in long-term heavy cannabis use. Psychiatry Res 1986; 17:15–21Google Scholar
35. Mathew RJ, Wilson WH, Tant SR: Acute changes in cerebral blood flow associated with marijuana smoking. Acta Psychiatr Scand 1989; 79:118–128Google Scholar
36. Ehrenreich H, Rinn T, Kunert HJ, et al: Specific attentional dysfunction in adults following early start of cannabis use. Psychopharmacology (Berl) 1999; 142:295–301Google Scholar
37. Huestegge L, Radach R, Kunert HJ, et al: Visual search in long-term cannabis users with early age of onset. Prog Brain Res 2002; 140:377–394Google Scholar
38. Degenhardt L, Hall W, Lynskey M: The relationship between cannabis use, depression and anxiety among Australian adults: findings from the national survey of mental health and well-being. Soc Psychiatry Psychiatr Epidemiol 2001; 36:219–227Google Scholar
39. Grotenhermen F: Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet 2003; 42:327–360Google Scholar
40. Mathew RJ, Wilson WH: Acute changes in cerebral blood flow after smoking marijuana. Life Sci 1993; 52:757–767Google Scholar
41. Mathew RJ, Wilson WH, Chiu NY, et al: Regional cerebral blood flow and depersonalization after tetrahydrocannabinol administration. Acta Psychiatr Scand 1999; 100:67–75Google Scholar
42. Mathew RJ, Wilson WH, Coleman RE, et al: Marijuana intoxication and brain activation in marijuana smokers. Life Sci 1997; 60:2075–2089Google Scholar
43. Mathew RJ, Wilson WH, Humphreys DF, et al: Regional cerebral blood flow after marijuana smoking. J Cereb Blood Flow Metab 1992; 12:750–758Google Scholar
44. Mathew RJ, Wilson WH, Turkington TG, et al: Cerebellar activity and disturbed time sense after THC. Brain Res 1998; 797:183–189Google Scholar
45. Mathew RJ, Wilson WH, Turkington TG, et al: Time course of tetrahydrocannabinol-induced changes in regional cerebral blood flow measured with positron emission tomography. Psychiatry Res 2002; 116:173–185Google Scholar
46. Weinstein S, Graves RE: Are creativity and schizotypy products of a right hemisphere bias? Brain Cogn 2002; 49:138–151Google Scholar
47. Demaree HA, Everhart DE, Youngstrom EA, et al: Brain lateralization of emotional processing: historical roots and a future incorporating “dominance.” Behav Cogn Neurosci Rev 2005; 4:3–20Google Scholar
48. Volkow ND, Gillespie H, Mullani N, et al: Cerebellar metabolic activation by delta-9-tetrahydro-cannabinol in human brain: a study with positron emission tomography and 18F-2-fluoro-2-deoxyglucose. Psychiatry Res 1991; 40:69–78Google Scholar
49. Volkow ND, Gillespie H, Mullani N, et al: Brain glucose metabolism in chronic marijuana users at baseline and during marijuana intoxication. Psychiatry Res 1996; 67:29–38Google Scholar
50. Goldstein RZ, Volkow ND: Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry 2002; 159:1642–1652Google Scholar
51. Knight RT, Stuss DT: Prefrontal Cortex: the present and future, in Principles of Frontal Lobe Function. New York, Oxford University Press, 2002Google Scholar
52. Diana M, Melis M, Muntoni AL, et al: Mesolimbic dopaminergic decline after cannabinoid withdrawal. Proc Natl Acad Sci U S A 1998; 95:10269–10273Google Scholar
53. Verrico CD, Jentsch JD, Roth RH: Persistent and anatomically selective reduction in prefrontal cortical dopamine metabolism after repeated, intermittent cannabinoid administration to rats. Synapse 2003; 49:61–66Google Scholar
54. Verrico CD, Jentsch JD, Roth RH, et al: Repeated, intermittent delta(9)-tetrahydrocannabinol administration to rats impairs acquisition and performance of a test of visuospatial divided attention. Neuropsychopharmacology 2004; 29:522–529Google Scholar
55. Lundqvist T, Jonsson S, Warkentin S: Frontal lobe dysfunction in long-term cannabis users. Neurotoxicol Teratol 2001; 23:437–443Google Scholar
56. Block RI, O’Leary DS, Hichwa RD, et al: Effects of frequent marijuana use on memory-related regional cerebral blood flow. Pharmacol Biochem Behav 2002; 72:237–250Google Scholar
57. Pillay SS, Rogowska J, Kanayama G, et al: Neurophysiology of motor function following cannabis discontinuation in chronic cannabis smokers: an fMRI study. Drug Alcohol Depend 2004; 76:261–271Google Scholar
58. Kanayama G, Rogowska J, Pope HG, et al: Spatial working memory in heavy cannabis users: a functional magnetic resonance imaging study. Psychopharmacology (Berl) 2004; 176:239–247Google Scholar
59. Eldreth DA, Matochik JA, Cadet JL, et al: Abnormal brain activity in prefrontal brain regions in abstinent marijuana users. Neuroimage 2004; 23:914–920Google Scholar
60. Coull JT, Nobre AC: Where and when to pay attention: the neural systems for directing attention to spatial locations and to time intervals as revealed by both PET and fMRI. J Neurosci 1998; 18:7426–7435Google Scholar
61. Coull JT: Neural correlates of attention and arousal: insights from electrophysiology, functional neuroimaging and psychopharmacology. Prog Neurobiol 1998; 55:343–361Google Scholar
62. Loeber R, Yurgelun-Todd D: Human neuroimaging of acute and chronic marijuana use: implications for frontocerebellar dysfunction. Human Psychopharmacology (Berl) 1999; 14:291–304Google Scholar
63. Gardner EL: Endocannabinoid signaling system and brain reward: emphasis on dopamine. Pharmacol Biochem Behav 2005; 81:263–284Google Scholar
64. Kalivas PW, Volkow ND: The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry 2005; 162:1403–1413Google Scholar
65. Chen J, Marmur R, Pulles A, et al: Ventral tegmental microinjection of delta 9-tetrahydrocannabinol enhances ventral tegmental somatodendritic dopamine levels but not forebrain dopamine levels: evidence for local neural action by marijuana’s psychoactive ingredient. Brain Res 1993; 621:65–70Google Scholar
66. Gessa GL, Melis M, Muntoni AL, et al: Cannabinoids activate mesolimbic dopamine neurons by an action on cannabinoid CB1 receptors. Eur J Pharmacol 1998; 341:39–44Google Scholar
67. French ED, Dillon K, Wu X: Cannabinoids excite dopamine neurons in the ventral tegmentum and substantia nigra. Neuroreport 1997; 8:649–652Google Scholar
68. Diana M, Melis M, Gessa GL: Increase in meso-prefrontal dopaminergic activity after stimulation of CB1 receptors by cannabinoids. Eur J Neurosci 1998; 10:2825–2830Google Scholar
69. Wang GJ, Volkow ND, Fowler JS, et al: Regional brain metabolic activation during craving elicited by recall of previous drug experiences. Life Sci 1999; 64:775–784Google Scholar
70. Dom G, Sabbe B, Hulstijn W, et al: Substance use disorders and the orbitofrontal cortex: systematic review of behavioural decision-making and neuroimaging studies. Br J Psychiatry 2005; 187:209–220Google Scholar
71. Clark DL, Boutros NN: The Brain and Behavior: An Introduction to Behavioral Anatomy. Malden, Mass, Blackwell Scientific Science, 1999Google Scholar
72. Amen DG, Waugh M: High resolution brain spect imaging of marijuana smokers with AD/HD. J Psychoactive Drugs 1998; 30:209–214Google Scholar
73. O’Leary DS, Block RI, Flaum M, et al: Acute marijuana effects on rcbf and cognition: a PET study. Neuroreport 2000; 11:3835–3841Google Scholar
74. O’Leary DS, Block RI, Koeppel JA, et al: Effects of smoking marijuana on brain perfusion and cognition. Neuropsychopharmacology 2002; 26:802–816Google Scholar
75. Hugdahl K, Bronnick K, Kyllingsbaek S, et al: Brain activation during dichotic presentations of consonant-vowel and musical instrument stimuli: a 15O-PET study. Neuropsychologia 1999; 37:431–440Google Scholar
76. Yucel M, Wood SJ, Fornito A, et al: Anterior cingulate dysfunction: implications for psychiatric disorders? J Psychiatry Neurosci 2003; 28:350–354Google Scholar
77. Sierra M, Berrios GE: Depersonalization: neurobiological perspectives. Biol Psychiatry 1998; 44:898–908Google Scholar
78. Breiter HC, Gollub RL, Weisskoff RM, et al: Acute effects of cocaine on human brain activity and emotion. Neuron 1997; 19:591–611Google Scholar
79. Volkow ND, Wang GJ, Fowler JS, et al: Association of methylphenidate-induced craving with changes in right striato-orbitofrontal metabolism in cocaine abusers: implications in addiction. Am J Psychiatry 1999; 156:19–26Google Scholar
80. Stein EA, Pankiewicz J, Harsch HH, et al: Nicotine-induced limbic cortical activation in the human brain: a functional MRI study. Am J Psychiatry 1998; 155:1009–1015Google Scholar
81. Augustine JR: Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev 1996; 22:229–244Google Scholar
82. Small DM, Voss J, Mak YE, et al: Experience-dependent neural integration of taste and smell in the human brain. J Neurophysiol 2004; 92:1892–1903Google Scholar
83. Bamiou DE, Musiek FE, Luxon LM: The insula (island of reil) and its role in auditory processing: literature review. Brain Res Brain Res Rev 2003; 42:143–154Google Scholar
84. Ashton CH: Pharmacology and effects of cannabis: a brief review. Br J Psychiatry 2001; 178:101–106Google Scholar
85. Marien P, Engelborghs S, De Deyn PP: Cerebellar neurocognition: a new avenue. Acta Neurol Belg 2001; 101:96–109Google Scholar
86. Schmahmann JD: Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci 2004; 16:367–378Google Scholar
87. Block RI, O’Leary DS, Hichwa RD, et al: Cerebellar hypoactivity in frequent marijuana users. Neuroreport 2000; 11:749–753Google Scholar
88. Ghozland S, Aguado F, Espinosa-Parrilla JF, et al: Spontaneous network activity of cerebellar granule neurons: impairment by in vivo chronic cannabinoid administration. Eur J Neurosci 2002; 16:641–651Google Scholar
89. O’Leary DS, Block RI, Turner BM, et al: Marijuana alters the human cerebellar clock. Neuroreport 2003; 14:1145–1151Google Scholar
90. Kouri EM, Pope HG Jr: Abstinence symptoms during withdrawal from chronic marijuana use. Exp Clin Psychopharmacol 2000; 8:483–492Google Scholar
91. Budney AJ, Moore BA, Vandrey RG, et al: The time course and significance of cannabis withdrawal. J Abnorm Psychol 2003; 112:393–402Google Scholar
92. Chiang CW, Barnett G: Marijuana and delta-9-tetrahydrocannabinol plasma level. Clin Pharmacol Ther 1984; 36:234–238Google Scholar
93. Huestis MA, Henningfield JE, Cone EJ: Blood cannabinoids, I: absorption of THC and formation of 11-OH-THC and THCCOOH during and after smoking marijuana. J Anal Toxicol 1992; 16:276–282Google Scholar
94. Naef M, Russmann S, Petersen-Felix S, et al: Development and pharmacokinetic characterization of pulmonal and intravenous delta-9-tetrahydrocannabinol (THC) in humans. J Pharm Sci 2004; 93:1176–1184Google Scholar



