Semantic Clustering Inefficiency in HIV-Associated Dementia
A mild “subcortical” memory profile characterized by impaired learning, with broadly intact delayed retention and recognition, is also common in HIV-associated dementia. 5 For example, White et al. 5 reported a deficit in verbal episodic memory (i.e., overall list learning and recall) in HIV-associated dementia, with relative sparing of verbal semantic memory. However, no studies have examined the cognitive underpinnings of the general episodic learning and memory deficit observed in HIV-associated dementia. Such investigations are important because they may facilitate a clearer understanding of the cognitive mechanisms of impairment, as well as inform compensatory interventional strategies designed to minimize the impact of HIV-associated dementia-associated memory deficits on the performance of instrumental activities of daily living (e.g., medication adherence).
To this end, it is generally acknowledged that greater organization of learned material is associated with improved recall. This is especially important considering the limited capacity of short-term information storage. 7 Therefore, the use of “metacognitive” strategies is desirable to maximize recall performance. In the context of word-list learning, semantic clustering, which takes advantage of semantic relationships between items to facilitate efficient encoding and retrieval, represents one such strategy. Decreased use of semantic clustering has been found in patients with frontal lobe lesions. 8 In addition, the relationship between semantic clustering and recall performance has been demonstrated in numerous populations, including healthy elderly adults, 9 Huntington’s disease, 10 phenylketonuria, 11 methamphetamine dependence, 12 and HIV infection. 13 In contrast, increased use of serial clustering, a less active strategy where recalled words are simply recited back in the same order as the learning word-list, may be less reliably associated with frontal systems pathology. 14 Indeed, prior studies of HIV infection have not observed deficits in serial clustering, 13 even in persons with minor cognitive-motor disorder. 15
However, no studies have examined semantic and serial clustering in HIV-associated dementia. It is generally held that cognitive functioning, including episodic verbal memory, declines with advancing HIV disease. 16 Accordingly, the current study focuses on examining the use of active organizational strategies on a verbal memory task in HIV-associated dementia relative to individuals with less severe HIV-associated cognitive disorders. We hypothesized a stepwise decline in the use of semantic clustering across these groups, with the worst performance expected in individuals with HIV-associated dementia. Further, in light of the above-described prior literature on HIV infection, we hypothesized that there would be no between-group differences in serial clustering.
METHOD
Participants
Participants in this study were 24 healthy comparison volunteers and 59 individuals with HIV infection, as indicated by enzyme-linked immunosorbent assays and a Western Blot confirmatory test. Potential participants were excluded if they met any of the following criteria: 1) a history of head injury with loss of consciousness greater than 30 minutes; 2) a history of neurological or psychiatric illness that would adversely affect cognitive functioning (e.g., seizure disorder, CNS opportunistic infections, cerebrovascular accidents, mental retardation, schizophrenia spectrum disorders, or CNS neoplasms); or 3) active illicit substance use confirmed by urine toxicology screening at the time of evaluation.
Consensus diagnoses of HIV-associated neurocognitive disorders were assigned for each participant on the basis of comprehensive neuropsychological, neuromedical, and psychiatric evaluations, and according to modified American Academy of Neurology 2 and Grant and Atkinson 6 criteria. Within this classification system, the term “HIV-associated dementia” is used for individuals with more severe and often more generalized cognitive decline, while “minor cognitive-motor disorder” is used for individuals with definite cognitive impairment but milder instrumental activities of daily living effects than HIV-associated dementia. Subsyndromal neuropsychological impairment is used to describe individuals whose neuropsychological deficits are “subclinical” and do not affect instrumental activities of daily living in a noticeable way. Recent data support the interrater reliability 17 and construct validity 18 of this nosology. Classified in this manner, the HIV-infected participants consisted of 20 individuals with neuropsychological impairment, 24 individuals with minor cognitive-motor disorder, and 15 individuals with HIV-associated dementia.
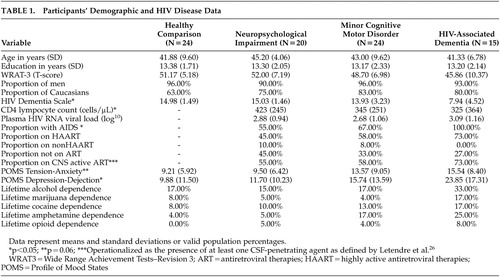
Participants’ demographic and disease characteristics are displayed in Table 1 . Consistent with study group definitions, significant omnibus differences were observed on the HIV Dementia Scale 19 (chi-square=18.60, p<0.0005), with lowest scores evident in the HIV-associated dementia group (Z-score=−4.18, p<0.05). As might be expected, the HIV seropositive study cells differed on CD4 lymphocyte counts (F=73.90, p<0.0001) and the proportion of subjects with AIDS (chi-square= 41.86, p<0.0001). However, no significant group differences were evident in plasma HIV RNA viral load or antiretroviral regimens (p>0.10). The four participant groups were comparable for age, years of education, sex and ethnic composition, and estimated premorbid verbal intellectual functioning based on the oral word reading subtest from the Wide Range Achievement Test–Revision 3 20 (p>0.10). Study groups were also comparable with respect to the prevalence of participants with lifetime histories of alcohol, marijuana, cocaine, amphetamine and opioid dependence per structured interviews using DSM-IV 21 criteria (p<0.05). Omnibus group differences emerged on the Profile of Mood States (POMS) 22 Depression-Dejection (p<0.05) and Tension-Anxiety (p=0.06) scales, with the HIV-associated dementia group endorsing more depressive and anxious symptoms for the week prior to evaluation than the healthy comparison and neuropsychological impairment samples (p<0.05).
 |
Procedure
All participants were administered the Hopkins Verbal Learning Test–Revised (HVLT–R). 23 The HVLT-R involves learning a list of 12 words belonging to three semantic categories (animals, precious stones, and human dwellings) over three successive free-recall trials. The order of items in the word list was randomized such that no items from the same category were in adjacent positions. In each trial, participants were read the word list in the same order and immediately asked to recall the items. Recalled items from the three learning trials were used in the current analyses.
Participants also received standardized clinical ratings on measures of executive functioning, working memory, and speed of information processing that were performed by the study neuropsychologists. The clinical ratings were derived from demographically adjusted T scores from published and well-validated clinical measures. Primary tests within the executive function domain included the Trail Making Test, Part B, 24 and Wisconsin Card Sorting Test–64 Card Version. 25 Tests of working memory included the Paced Auditory Serial Addition Test 27 and Wechsler Adult Intelligence Scale–III (WAIS–III) 28 Letter-Number Sequencing subtest. Finally, speed of information processing was assessed with the WAIS-III Processing Speed Index and Trail Making Test, Part A. Clinical ratings were based on a scale ranging from 1 (above average, T score ≥55) to 0 (severely impaired, T score <20), where scores of five and higher (T score <40) represent definite cognitive impairment (see Woods et al. 17 for technical details regarding this methodology, as well as its interrater reliability and construct validity).
Data Analysis
Three variables of interest were derived from each HVLT-R recall trial: number of items correctly recalled, semantic clusters, and serial clusters. A semantic cluster was defined as two successive correctly recalled items from the same semantic category, whereas a serial cluster was operationalized as an adjacent pair of correctly recalled items that were also adjacent in the presented word list, regardless of order. For each recall trial, indices of category clustering and serial clustering were computed by subtracting the number of clusters expected by chance from the number of observed clusters. 29 Negative clustering index values were rounded up to zero. Clustering index values across the three free recall trials were then averaged to reflect semantic and serial organization.
Based on existing literature, the current study sample size afforded adequate power to detect a large effect size (power >0.80). The unbiased Cohen’s d statistic was used as an estimate of effect size. Both clustering variables of interest were tested for normality of distribution and homogeneity of variance within each HIV-related cognitive status group. Subsequently, a monotonic transformation (natural logarithm) was performed on the semantic clustering index to eliminate inhomogeneity of variance. Additionally, nonparametric statistics were used for group comparisons of both variables due to non-normal distributions. To examine the hypothesized stepwise decline in the use of semantic clustering across HIV-related cognitive status groups, the Jonckheere-Terpstra nonparametric test for ordered alternatives 30 , 31 was conducted. This between-group trend test assesses the null hypothesis that the distribution of the dependent variable does not differ among the groups. Essentially, to reject the null hypothesis, the median level of semantic clustering must decrease in an orderly fashion with disease progression. We conducted follow-up pairwise comparisons using Mann-Whitney tests with Bonferroni adjustment for six possible comparisons (critical α=0.0083). Additionally, we used a Kruskal-Wallis test to examine the between-group difference in serial clustering.
RESULTS
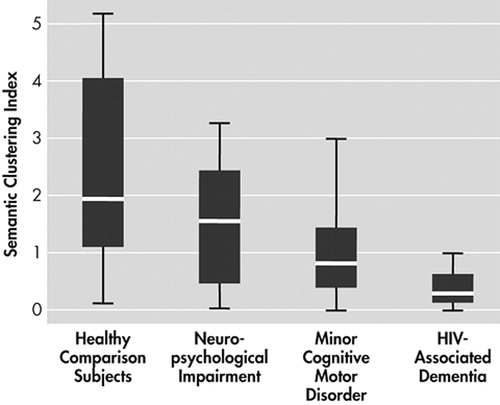
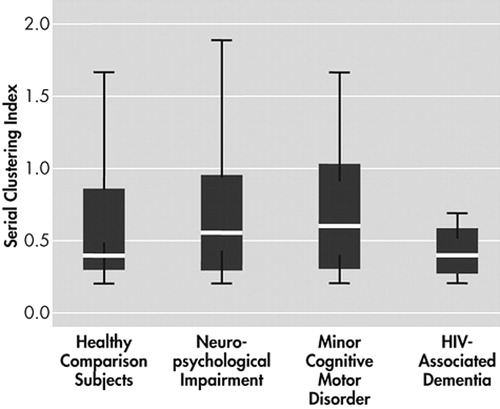
Figure 1 shows the degree of semantic clustering exhibited by participants in each group. A Jonckheere-Terpstra test for ordered alternatives showed an orderly decrease in exhibited degree of semantic clustering from healthy comparison, neuropsychological impairment, minor cognitive-motor disorder, and HIV-associated dementia participants, respectively ( J =5.31, p<0.0005). Follow-up pairwise comparisons with Mann-Whitney tests showed significant group differences between healthy comparison and minor cognitive-motor disorder (chi-square=10.75, p<0.0083, d=1.10), healthy comparison and HIV-associated dementia (chi-square=19.02, p<0.0083, d=1.63), and neuropsychological impairment and HIV-associated dementia (chi-square=11.57, p<0.0083, d=1.39); while minor cognitive-motor disorder and HIV-associated dementia groups exhibited a borderline significant between-group difference after Bonferroni adjustment (chi-square =6.69, p=0.01, d=0.93). Figure 2 shows the degree of serial clustering exhibited by the four participant groups. A Kruskal-Wallis test failed to reject the null hypothesis that the groups differ in their exhibited degree of serial clustering (chi-square=1.85, p>0.05).
As the study groups differed in general cognitive abilities and affective distress, a post hoc analysis was undertaken to more carefully evaluate the hypothesis that deficient executive functions were the driving force behind the semantic clustering findings in HIV-associated dementia. A linear regression was performed using HIV Dementia Scale total, the POMS Depression-Dejection and Tension-Anxiety scales, and the cognitive domain ratings of executive functions, working memory, and information processing speed as predictors of semantic clustering in the entire study sample. Though the overall model was significant (adjusted R 2 =0.15, F=3.12, p<0.01), the executive functions variable emerged as the only significant independent predictor of semantic clustering (F=4.64, p<0.05).
DISCUSSION
To examine the effect of HIV-associated dementia on the use of active organizational strategy during a verbal memory task, we compared the use of semantic and serial clustering between healthy comparison and three groups of HIV-infected individuals with increasing severity of HIV-associated cognitive disorder. Consistent with our hypothesis, there was a statistically significant stepwise decline in the use of semantic clustering, such that the healthy comparison group exhibited the highest clustering, followed by the neuropsychological impairment, minor cognitive-motor disorder and HIV-associated dementia groups, respectively. These findings were associated with large effect sizes and cannot be readily attributed to demographic factors, psychiatric variables, or antiretroviral treatment differences between groups. Moreover, post hoc analysis revealed that semantic clustering was specifically related to executive functioning, and not to performance on measures of working memory, processing speed, or a screening measure of general cognitive abilities. Also consistent with our hypothesis, participant groups were comparable with regard to their use of the serial clustering strategy. It is therefore evident that individuals with HIV-associated dementia experienced the most difficulty utilizing semantic structure as a memory strategy. Consistent with existing literature on HIV-1 infection, 13 , 32 semantic clustering impairments were also evident in individuals with neuropsychological impairment and minor cognitive-motor disorder. This finding also parallels the previously demonstrated declines in other cognitive domains across HIV disease status, including executive functions. 5
The current study demonstrates an HIV-associated impairment in the utilization of a memory organization strategy often linked to frontal systems functioning. These data support previous cognitive studies suggesting a “subcortical” pattern of neuropsychological deficits in HIV-associated dementia. Prior research has linked HIV-associated dementia to deficits in retrieval from remote memory, 33 verbal episodic memory, 5 and semantic memory. 34 For example, a recent finding from our group suggested that semantic memory retrieval impairments in HIV-associated dementia reflect a disruption in the frontally mediated process of a rule-guided lexical-semantic search, rather than depleted semantic memory stores. 34 The current finding of selective impairment in semantic (versus serial) clustering utilization thus suggests that the observed HIV-associated dementia-associated episodic memory impairment may be attributable to executive dyscontrol of encoding and retrieval, which are mechanisms that have consistently been implicated in HIV-associated cognitive disorders. 15 , 31 , 33
Although HIV-associated frontal systems pathology is hypothesized to underlie the deficient semantic clustering performance in HIV-associated dementia, interpretation of the neuropathogenesis of our data is restricted by the observational and inferential nature of the study design. For example, an alternate interpretation of our findings might be that the HIV-associated dementia-associated semantic clustering impairment reflects generalized cognitive decline and diffuse pathology, rather than the specific effects of frontal systems damage. 35
Yet several factors argue against this competing hypothesis. First, executive functions emerged as the only independent predictors of semantic clustering in a statistical model that also included tests of working memory, speed of information processing, and global cognition (i.e., the HDS). Though executive functions are not always synonymous with frontal systems, the specificity of the association between semantic clustering and executive dysfunction demonstrated in this study and the prominent fronto-basal ganglia neuropathophysiology of HIV-associated dementia lend some credence to our interpretation.
Second, functional neuroimaging data in healthy comparison subjects support the primary involvement of the prefrontal cortex in semantic clustering strategy use during free recall. 36 Consistent with this notion, prior research also indicates that semantic clustering deficits are evident in individuals with various frontal systems neuropathologies (e.g., frontal lobe lesions, 8 Huntington’s disease, 10 and methamphetamine dependence). 12 Nevertheless, future studies using neuroimaging and neuropathological techniques are needed to more directly evaluate the neuropathogenesis of semantic clustering deficits in HIV-associated dementia.
Though this study utilized a classification system for HIV-related cognitive disorders with well-demonstrated reliability and validity, the precise trajectory of the observed decline in memory organization is difficult to delineate from a cross-sectional design. A longitudinal study with a similar neuropsychological methodology is therefore desirable to examine the robustness of the current findings. Second, the sample sizes were too small to explore the effects of systemic disease on semantic clustering (e.g., immunosuppression) and data were not available on the qualitative aspects of participants’ histories of substance use (e.g., injection drug use, lifetime quantity, duration of use, and abstinence). Larger study sample sizes will allow for a more thorough investigation of the relationship between semantic clustering and systemic HIV disease (e.g., immunosuppression, CSF viral load, and treatment factors), as well as the potentially additive effects of commonly encountered comorbidities (e.g., substance abuse, hepatitis C coinfection, and aging). Finally, future studies with increased numbers of HIV-infected women and ethnic minorities, especially African Americans and Hispanics, are needed to examine the generalizability of the current findings.
Despite these limitations, the potential clinical implications of deficient semantic clustering in HIV-associated dementia warrant consideration. The benefit of explicit category cuing has been demonstrated in patients with frontal lobe lesions 37 and Parkinson's disease, 38 where previous knowledge of the category structure of a word-list facilitated the use of semantic clustering strategy. This suggests that a cognitive rehabilitation process utilizing mnemonic strategies, such as explicit cuing, may serve to mitigate the functional impact of memory difficulties in patients with HIV-associated dementia. This is especially important in light of the observed relationship between memory deficit and deterioration in instrumental activities of daily living, including management of complex HIV medication regimens. 39
1. McArthur JC, Selnes OA, Glass JD, et al: HIV dementia: incidence and risk factors. Res Publ Assoc Res Nerv Ment Dis 1994; 72:251–272Google Scholar
2. American Academy of Neurology AIDS Task Force: Nomenclature and research case definitions for neurologic manifestations of human immunodeficiency virus-type 1 (HIV-1) infection. Neurology 1991; 41:778–785Google Scholar
3. Aylward EH, Henderer JD, McArthur JC, et al: Reduced basal ganglia volume in HIV-1-associated dementia: results from quantitative neuroimaging. Neurology 1993; 43:2099–2104Google Scholar
4. Glass JD, Wesselingh SL, Selnes OA, et al: Clinical-neuropathologic correlation in HIV-associated dementia. Neurology 1993; 43:2230–2237Google Scholar
5. White DA, Taylor MJ, Butters N, et al: Memory for verbal information in individuals with HIV-associated dementia complex: the HNRC group. J Clin Exp Neuropsychol 1997; 19:357–366Google Scholar
6. Grant I, Atkinson JH: Psychiatric aspects of acquired immune deficiency syndrome, in Comprehensive Textbook of Psychiatry, VI. Edited by Kaplan HI, Sadock BJ. Baltimore, Md, Williams & Wilkins, 1999, pp 1644–1669Google Scholar
7. Miller GA: The magical number seven plus or minus two: some limits on our capacity for processing information. Psychol Rev 1956; 63:81–97Google Scholar
8. Baldo JV, Delis D, Kramer J, et al: Memory performance on the California Verbal Learning Test-II: findings from patients with focal frontal lesions. J Int Neuropsychol Soc 2002; 8:539–546Google Scholar
9. Hazlett EA, Buchsbaum MS, Mohs RC, et al: Age-related shift in brain region activity during successful memory performance. Neurobiol Aging 1998; 19:437–445Google Scholar
10. Massman PJ, Delis DC, Butters N, et al: Are all subcortical dementias alike? verbal learning and memory in Parkinson’s and Huntington’s disease patients. J Clin Exp Neuropsychol 1990; 12:729–744Google Scholar
11. White DA, Nortz MJ, Mandernach T, et al: Deficits in memory strategy use related to prefrontal dysfunction during early development: evidence from children with phenylketonuria. Neuropsychology 2001; 15:221–229Google Scholar
12. Woods SP, Rippeth JD, Conover E, et al: Deficient strategic control of verbal encoding and retrieval in individuals with methamphetamine dependence. Neuropsychology 2005; 19:35–43Google Scholar
13. Peavy G, Jacobs D, Salmon DP, et al: Verbal memory performance of patients with human immunodeficiency virus infection: evidence of subcortical dysfunction: the HNRC group. J Clin Exp Neuropsychol 1994; 16:508–523Google Scholar
14. Delis DC, Kramer JH, Kaplan E, et al: California Verbal Learning Test, 2nd ed. San Antonio, Tex, Psychological Corporation, 2000Google Scholar
15. Delis DC, Peavy G, Heaton R, et al: Do patients with HIV-associated minor cognitive/motor disorder exhibit a “subcortical” memory profile? evidence using the California Verbal Learning Test. Assessment 1995; 2:151–165Google Scholar
16. Reger M, Welsh R, Razani J, et al: A meta-analysis of the neuropsychological sequelae of HIV infection. J Int Neuropsychol Soc 2002; 8:410–424Google Scholar
17. Woods SP, Rippeth JD, Frol AB, et al: Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV. J Clin Exp Neuropsychol 2004; 26:759–778Google Scholar
18. Cherner M, Masliah E, Ellis RJ, et al: Neurocognitive dysfunction predicts postmortem findings of HIV encephalitis. Neurology 2002; 59:1563–1567Google Scholar
19. Power C, Selnes OA, Grim JA, et al: HIV dementia scale: a rapid screening test. J Acquir Immune Defic Syndr Hum Retrovirol 1995; 8:273–278Google Scholar
20. Wilkinson GS: Wide Range Achievement Test: Administration Manual, 3rd ed. Wilmington, Del, Wide Range, 1993Google Scholar
21. American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC, American Psychiatric Association, 1994Google Scholar
22. McNair PM, Lorr M, Droppelman LF: Profile of Mood States Manual, 2nd ed. San Diego, Calif, Educational and Industrial Testing Service, 1981Google Scholar
23. Benedict RHB, Schretlen D, Groninger L, et al: Hopkins Verbal Learning Test–Revised: normative data and analysis of inter-form and test-retest reliability. Clin Neuropsychologist 1998; 12:43–55Google Scholar
24. Heaton RK, Grant I, Matthews CG: Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographic Corrections, Research Findings, and Clinical Applications. Odessa, Fla, Psychological Assessment Resources, 1991Google Scholar
25. Kongs SK, Thompson LL, Iverson GL, et al: Wisconsin Card Sorting Test–64 Card Computerized Version. Odessa, Fla, Psychological Assessment Resources, 2000Google Scholar
26. Letendre SL, McCutchan JA, Childers ME, et al: Enhancing antiretroviral therapy for human immunodeficiency virus cognitive disorders. Ann Neurology 2004; 56:416–423Google Scholar
27. Diehr MC, Heaton RK, Miller W, et al: The paced auditory serial addition task (PASAT): norms for age, education, and ethnicity. Assessment 1998; 5:375–387Google Scholar
28. Wechsler D: Wechsler Adult Intelligence Scale, 3rd ed. San Antonio, Tex, Psychological Corporation, 1997Google Scholar
29. Stricker JL, Brown GG, Wixted J, et al: New semantic and serial clustering indices for the California Verbal Learning Test–2nd ed: background, rationale, and formulae. J Int Neuropsychol Soc 2002; 8:425–435Google Scholar
30. Jonckheere AR: A distribution-free k-sample test against ordered alternatives. Biometricka 1954; 41:133–145Google Scholar
31. Terpstra TJ: The asymptotic normality and consistency of Kendall’s test against trend, when ties are present in one ranking. Indigationes Mathematicae 1952; 14:327–333Google Scholar
32. Woods SP, Scott JC, Dawson MS, et al: Construct validity of Hopkins verbal learning test-revised component process measures in an HIV-1 sample. Arch Clin Neuropsychol 2005; 20:1061–1071Google Scholar
33. Sadek JR, Johnson SA, White DA, et al: Retrograde amnesia in dementia: comparison of HIV-associated dementia, Alzheimer’s disease, and Huntington’s disease. Neuropsychology 2004; 18:692–699Google Scholar
34. Woods SP, Conover E, Rippeth JD, et al: Qualitative aspects of verbal fluency in HIV-associated dementia: a deficit in rule-guided lexical-semantic search processes? Neuropsychologia 2004; 42:801–809Google Scholar
35. Glosser G, Gallo JL, Clark CM, et al: Memory encoding and retrieval in frontotemporal dementia and Alzheimer’s disease. Neuropsychology 2002; 16:190–196Google Scholar
36. Savage CR, Deckersbach T, Heckers S, et al: Prefrontal regions supporting spontaneous and directed application of verbal learning strategies: evidence from PET. Brain 2001; 124(pt 1):219–231Google Scholar
37. Gershberg FB, Shimamura AP: Impaired use of organizational strategies in free recall following frontal lobe damage. Neuropsychologia 1995; 33:1305–1333Google Scholar
38. van Spaendonck KP, Berger HJ, Horstink MW, et al: Memory performance under varying cueing conditions in patients with Parkinson’s disease. Neuropsychologia 1996; 34:1159–1164Google Scholar
39. Hinkin CH, Castellon SA, Durvasula RS, et al: Medication adherence among HIV+ adults: effects of cognitive dysfunction and regimen complexity. Neurology 2002; 59:1944–1950Google Scholar



