Consequences of Aggressive Behavior in Patients With Dementia
METHODS
Participants
Participants were nonaggressive and newly diagnosed with dementia, identified through the 2001–2004 Veterans Administration (VA) outpatient data files and patient treatment files; primary care and geriatrics clinics of the Michael E. DeBakey VA Medical Center in Houston, Texas; and flyers and radio and print ads. Recruitment occurred from September 5, 2003, to June 10, 2005. Details of the recruitment process have been previously described. 19
Inclusion/Exclusion Criteria and Screening of Patients
Patients had to be at least 60 years old, with an initial outpatient diagnosis of one of these forms of dementia in the previous 12 months: 290.xx (dementias, including vascular dementia), 291.2 (alcohol-induced, persisting dementia), 292.82 (substance-induced persistent dementia), 294.1 (dementia due to head trauma), 294.8 (dementia not otherwise specified) or 331.0 (Alzheimer’s disease), according to the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM). This allowed us to identify a cohort likely to have many patients who had not yet developed aggression. Patients were excluded for presenting aggression at baseline, for residing in a nursing home, or for having a caregiver fewer than 8 hours per week. We screened for aggression during the past year during a telephone interview with caregivers, using three probes from the Ryden Aggression Scale. 20 Caregivers of patients identified as nonaggressive were assessed during a face-to-face evaluation with the full Ryden scale to confirm the absence of aggression during the preceding year.
Degree of cognitive impairment was assessed using the Dementia Rating Scale (DRS), 21 shown to have adequate internal consistency, test-retest reliability, and convergent and predictive validity. 21
The Houston VA Research and Development Committee and the institutional review board of Baylor College of Medicine approved this study. All participants, both patients and caregivers, signed informed consent forms.
Schedule of Assessments
Dyads were assessed monthly for 24 months, during home visits at baseline, and then every 4 months (months 5, 9, 13, 17, 21 and 25) and during telephone assessments at months 2–4, 6–8, 10–12, 14–16, 18–20, and 22–24. Admission of a patient to a nursing home terminated study follow-up. Patient-caregiver dyads received $15.00 for each of the first five completed home visits and $45.00 at the last visit.
Instruments for Assessing Aggression (Dependent Variable)
We evaluated aggression using the Cohen-Mansfield Agitation Inventory (CMAI), 22 with its seven-point Likert scale that rates frequency of behaviors and five-point Likert scale that rates disruptiveness. It yielded both categorical and continuous scores: total scores summed all 29 frequency and distress ratings. Aggression was defined as scores higher than zero on both frequency and disruptiveness on any of the 13 questions representing spitting, cursing/verbal aggression, hitting, kicking, grabbing people/things inappropriately, pushing, throwing things, biting, scratching, hurting self/others, tearing things/destroying property, making inappropriate verbal sexual advances, or making inappropriate physical sexual advances or exposing genitals.
Instruments for Assessing Outcomes
Nursing-Home Placement
Nursing-home placement was defined as moving from home to a long-term care facility, including a personal-care home, an assisted-living facility, or a skilled/nonskilled nursing home. This was assessed by trained research assistants who queried caregivers. We created a dichotomous variable to indicate nursing-home placement.
Injury to Caregiver, Self or Others
During assessments, trained research assistants asked caregivers the following questions 23 : “Over the past month, has the physical (hitting, pushing, throwing things) or verbal aggression of [patient’s name] caused physical injury to himself/herself? To you? To others? Did these injuries require medical attention? Was medical attention sought?” If there was report of a physical injury, we asked, “Please describe the physical injury.” We created a dichotomous variable to indicate the occurrence of injury to the patient or caregiver.
Use of Physical Restraints
Use of physical restraint was determined by asking the caregiver, “Has the physical or verbal aggression of [patient’s name] necessitated the use of a physical restraining mechanism? In the past month have you used the following to decrease the chance of injury: Vest restraint? Bed rails? Tie restraint? Geriatric chair with lap bar? Other physical restraint?” 24 Using this information, we created a dichotomous variable to indicate the use of physical restraints.
Anxiolytic or Antipsychotic Use
Use of an anxiolytic was defined as use of a benzodiazepine (e.g., diazepam, alprazolam, lorazepam), hypnotic (e.g., zolpidem, chloral hydrate), or antipsychotic (e.g., haloperidol, risperidone). Use was assessed by caregiver report. We created a dichotomous variable to examine this outcome.
Health-Service Use
Health-service use was assessed through electronic medical records. We calculated the use of health services for neurology, mental health and medical or surgical visits for each patient, as recorded in the VA administrative database. Inpatient information was gathered from the bed-section variable; outpatient information was gathered from the clinic-stop variable. The number of inpatient and outpatient visits was determined for each patient during the study.
Analyses
We examined differences between aggressive and nonaggressive patients for several outcomes and made two comparisons. First, we compared outcomes of nonaggressive patients with those of patients who became aggressive, comparing them during the preaggressive period (before the aggressives developed aggression). Second, we conducted a longitudinal comparison of outcomes for aggressive patients during their preaggressive period with outcomes during their postaggressive period to examine whether onset of aggression was related to change in outcome measures. To determine the rate of each outcome, we established periods of risk for both groups. For aggressive patients, two periods of risk were calculated: the total number of person-years during their preaggression period, and the number of person-years during their postaggression period. For nonaggressive patients, the period of risk was calculated as the total number of person-years in the study (years of observation time per person in each category).
The main analyses examined the relationship between aggression and nursing-home placement, use of anxiolytic/antipsychotic medication, injuries, use of restraints, outpatient-clinic visits, and inpatient admissions. We treated nursing-home placement differently because it terminated follow-up in the study; no other outcomes were study-exit criteria. For nursing-home placement, we conducted a time-to-event comparison between aggressive and nonaggressive patients.
To calculate the rate for medication use, injuries, and restraint use, the total number of individuals with observed events was divided by the total number of person-years at risk. Thus, for these measures, our rates represent the rate of individuals per person-year observed with at least one incident of the relevant measure. For aggressive individuals, rates were calculated separately for their pre- and postaggression periods. The event rate for inpatient admissions and outpatient visits was calculated by dividing the mean number of admissions or visits by the total number of person-years at risk.
Because of the high frequency of zero-cells in our data, we added 0.0001 to all cells for our Poisson and negative binomial-regression models. We used a discrete time-hazard model to test for differences in nursing-home placement between aggressive and nonaggressive patients.
These analyses were conducted using the Statistical Analysis Systems Software (SASS, Carey, NC), version 9.1.
Covariates
We evaluated our models to examine the effect of adjusting for gender, race, baseline age, and dementia severity. Because of the low frequency of most outcomes, we included covariates in the final model for each outcome only if they demonstrated a significant (p≤0.05) univariate relationship with the outcome.
RESULTS
Description of Cohort
Recruitment yielded 615 potential participants for prescreening. Most were successfully contacted (91%, n=562). Of those, 71% (n=400) consented to participate (5% refused screening, 22% opted out or declined, and 2% of caregivers refused consent).
All patients successfully prescreened were screened by phone after verbally consenting. Most (81%, n=325) were not aggressive, but almost 19% of the total caregivers (n=75) acknowledged aggressive behavior. For the 325 with a negative screen for aggression, attempts were made to schedule and complete baseline home visits. Of these, 110 could not be completed for the following reasons: 45 participants (14%) were deemed ineligible, 33 participants (10%) refused assessment, 15 participants (5%) were unreachable, 11 participants (3%) had caregiver-related issues, five participants (1%) could not complete the interview, and one participant (1%) died before the visit. A total of 215 (66%) newly diagnosed dementia patients/caregiver dyads were successfully enrolled.
The mean age of the participants was 76 years (SD=6.2). Because participants were veterans, most (95%) were men (n=205). Approximately 76% were white (n=163), 20% were black (n=43), and 4% were another race (n=9). Twenty-nine were taking antipsychotic medications at baseline. Use of antipsychotics at baseline was not associated with development of aggression (p=0.31).
Of the 215 patients in our cohort, 88 became aggressive during the 24-month study and 127 remained nonaggressive. Among those remaining nonaggressive, the distribution of dementia severity by DRS criteria 21 was 56 as severe (45%), 21 as moderate (17%), and 47 as mild (38%). For those who became aggressive, the rates were 56 as severe (66%), 14 as moderate (17%), and 14 as mild (17%). The mean dementia-severity score at baseline was significantly lower (worse) among patients who became aggressive than among those who did not (p=0.004).
Because participants might have been followed for different lengths of time, we calculated each patient’s period of follow-up. For the 127 nonaggressive patients, follow-up totaled 194.4 person-years (average=1.5 years per patient). For patients who became aggressive, we calculated two follow-up periods, preaggression and postaggression. The total follow-up during the preaggression period was 57.3 person-years (0.7 years per patient) and during the postaggression period was 82.5 person-years, or 0.9 years per patient. Total follow-up was similar between groups (1.5 versus 1.6 years).
Nursing-Home Placement
Because nursing-home admissions were terminal events for study participation, we tested differences in nursing-home admissions across the entire follow-up period for both aggressive and nonaggressive patients. Ten aggressive patients (0.11 patients per patient/year) were admitted to nursing homes, compared with six nonaggressive patients (0.05 patients per patient/year; hazard ratio [95% confidence interval]=2.98 [1.05–8.49], p≤0.004; Figure 1 ). All aggressive patients admitted to nursing homes were admitted after they became aggressive.
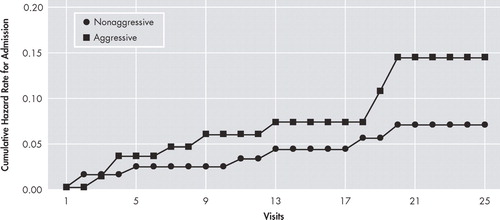
Age was included as a covariate. No other covariates met our criteria for inclusion.
Medication Use
Preaggressive and nonaggressive patients showed comparable rates of use of anxiolytic or antipsychotic medications. However, aggressive patients showed a statistically significant increase from pre- to postaggression in use of psychotropic medications (0.2 persons/year to 0.41 persons/year, p≤0.04; Figure 2 ).
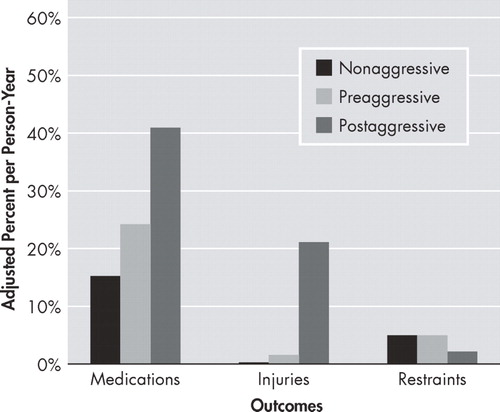
For medication use, dementia severity was included as a covariate in our test of nonaggressive versus preaggressive patients. No covariates met our criteria for inclusion in our pre- versus postaggression test.
For injuries, age was included as a covariate in our test of pre- versus postaggression differences. No covariates met our criteria for inclusion in our nonaggressive versus preaggressive model.
No covariates met our criteria for inclusion in either our nonaggressive versus preaggressive or our pre- versus postaggression tests.
Injuries
Preaggressive and nonaggressive patients did not significantly differ in number of injuries ( Figure 2 ). However, patients who became aggressive had a 10-fold increase in injuries (0.02 persons/year to 0.21 persons/year, p≤0.0001) between their pre- and postaggressive periods.
Restraints
Preaggressive and nonaggressive patients did not significantly differ in use of restraints ( Figure 2 ). There was also no increase in the very low rate of restraint use ( Figure 2 ) between the pre- and postaggression periods in patients who became aggressive.
Use of Health Services
Preaggressive and nonaggressive patients did not differ significantly in use of health services (inpatient admissions or outpatient visits) ( Table 1 ). Patients who became aggressive had no increase in outpatient visits or inpatient admissions between pre- and postaggression periods ( Table 1 ).
 |
DISCUSSION
In this study 88 of 215 nonaggressive patients (40.9%) became aggressive within 24 months, corroborating the findings of previous studies that aggression is common in persons with dementia. The use of antipsychotic medications increased significantly in patients after they became aggressive, and this group also had a 10-fold greater occurrence of injuries. In addition, 10 aggressive patients were admitted to nursing homes, almost twice as many as nonaggressive patients. There were no differences in rates of restraint use and in- and outpatient visits between those who became aggressive and those who did not from the pre- to postaggressive periods.
Aggression has been repeatedly associated with nursing-home placement in individuals with dementia 4 , 5 , 7 , 25 – 28 and Alzheimer’s disease, 5 and our findings confirm this. In the future, it will be important to identify other factors that lead to nursing-home placement, as even nonaggressive patients were placed. However, 90% of patients who became aggressive were not admitted to nursing homes.
Although the literature contains little about injuries in patients with dementia, 29 , 30 some have suggested that self-injury appears to stem from frustration and the inability to communicate needs to others. 30 Results of one study found self-injury occurring in 22% of dementia patients, primarily involving pinching and scratching themselves, pulling out hair and hitting the wall with their fist. 11 This same group found a modest correlation between self-injury and aggression but no relation between self-injury and dementia severity. Another study found that 40% of staff caring for elderly patients had been exposed to violence during the preceding year 31 and that another 33.1% of caretakers had experienced abuse. 32 Gates et al. 33 have suggested that caregivers who are victims of assault are more likely to be aggressive with those for whom they care, establishing the potential for a cycle of caregiver-patient abuse. We found a 10-fold increase in rate of injuries (to both self and others) in patients who became aggressive, compared with their preaggression baseline, but clearly more research is needed.
Little systematic research exists on the prevalence of using physical restraints in a community-dwelling sample of patients newly diagnosed with dementia or on people who are aggressive and have dementia. 17 The term restraints includes any limitations on an individual’s ability to move freely, including tying patients to restraining chairs or bedsides 34 or holding a person’s arms during grooming or other personal-care activities. 17 Although we had hypothesized that onset of aggression would initiate restraint use, we found a low level of use, which did not differ significantly between preaggressive and nonaggressive individuals or between pre- and postaggressive individuals. This is reassuring because some researchers have reported that mechanical restraints can actually provoke aggression. 24 , 35 , 36 We suspect that the use of restraints could be more prevalent in institutions than in the community because staff have heavy demands on their time, responsibility for multiple patients, and familiarity with and access to such devices (although other investigators posit that, even in institutional settings, physical restraints might be used primarily with individuals prone to falls, or those with physical problems, rather than as a way to manage aggression). 37 , 38 It might also be that the nation-wide move toward less use of restraints is having a positive impact.
The relationship between aggression and the use of psychotropic medications in persons with newly diagnosed dementia has not been documented. For persons with established dementia, a study estimating the prevalence of agitation in community-dwelling older adults with Alzheimer’s disease found that 31% of individuals with agitation were prescribed psychotropics, compared with 7% without agitation. 39 Kolanowski et al. 40 found that antipsychotic drugs were prescribed to 27% of community-dwelling persons with dementia and that they, in turn, were significantly associated with delirium, depression, hip fracture and falls. Our study did not find a significant difference in medication use between preaggressive and nonaggressive patients; however, as we had hypothesized, there was a significant increase (p≤0.04) in the pre- to postaggression group. This could be of concern because of reports outlining the limited efficacy and possible increased risk of death associated with the use of antipsychotics 41 ; possible adverse effects, 42 – 44 especially for older persons; and the potential for problems with polypharmacy and adverse outcomes, such as falls, fractures and syncope. 40 More research is needed to establish clear risk-benefit ratios for the use of such drugs, as well as to develop new interventions that could be helpful. That is especially true for patients newly diagnosed with dementia, for whom there is no evidence-based literature from which to draw guidance. This study did not examine the use of anti-dementia drugs such as donepezil, which has been weakly linked to decreased behavioral disturbances.
No research has focused specifically on the impact of aggression on the use of health services by individuals with dementia or newly diagnosed dementia. However, studies have found that the comorbidity of dementia with other psychiatric diagnoses is associated with a 50% greater use of psychiatric outpatient visits 45 and that patients with comorbid depression and Alzheimer’s disease use nearly twice as many psychiatric inpatient services and more medical inpatient services than patients with dementia alone. 46 We did not find that aggression significantly affected use of services, except for the greater likelihood of institutionalization. However, this lack of effect might be attributable to system of care and baseline frequency of care. In addition, non-VA care was not captured. These patients were already making 20 or more visits a year, and it might be hard to increase this level of use.
This study benefits from a longitudinal design and use of a cohort diagnosed with dementia within 12 months before screening that was not previously aggressive. However, it is limited by a predominance of male veterans and the large number of newly diagnosed subjects excluded because they scored positive for aggression, which restrict generalizability. It is also possible that once aggression was noted, nursing-home placement could have been prevented or delayed by providing some type of intervention. In addition, we did not differentiate between types of dementia because primary care physicians usually do not specify type.
Aggression is highly prevalent in individuals with dementia, even in those newly diagnosed; however, its true prevalence in community-residing patients is poorly understood, and its incidence in newly diagnosed patients has previously not been well-studied. Data regarding the consequences of aggression come primarily from cross-sectional studies and institutional samples. Thus, results may not be generalizable, and presumed causal relationships are weak. In contrast, the current study looked at a community sample at baseline and over time, avoiding biases inherent in studying institutionalized or clinic-based (e.g., dementia or psychiatry clinic) patients, using cross-sectional designs, and strengthening causal inferences. Interventional studies aimed at preventing aggression or the consequences of aggression have great potential to reduce patient and caregiver suffering, side effects of medications used to treat aggression and risk of injury to patients and loved ones.
1. Gonzalez-Salvador MT, Arango C, Lyketsos CG, et al: The stress and psychological morbidity of the Alzheimer patient caregiver. Int J Geriatr Psychiatry 1999; 14:701–710Google Scholar
2. Beeri MS, Werner P, Davidson M, et al: The cost of behavioral and psychological symptoms of dementia (BPSD) in community dwelling Alzheimer’s disease patients. Int J Geriatr Psychiatry 2002; 17:403–408Google Scholar
3. O’Brien J: Caring for caregivers. Am Fam Physician 2000; 62:2584–2587Google Scholar
4. Brodaty H, Low L: Aggression in the elderly. J Clin Psychiatry 2003; 64:36–43Google Scholar
5. Gilley DW, Bienias JL, Wilson RS, et al: Influence of behavioral symptoms on rates of institutionalization for persons with Alzheimer’s disease. Psychol Med 2004; 34:1129–1135Google Scholar
6. Kopetz S, Steele CD, Brandt J, et al: Characteristics and outcomes of dementia residents in an assisted living facility. Int J Geriatr Psychiatry 2000; 15:586–593Google Scholar
7. Knopman DS, Berg JD, Thomas R, et al: Nursing home placement is related to dementia progression. Neurology 1999; 52:714Google Scholar
8. Haup M, Romero B, Kurz A: Delusions and hallucination in Alzheimer’s disease: results from a two-year longitudinal study. Int J Geriatr Psychiatry 1996; 11:965–972Google Scholar
9. Lehmann LS, McCormick RA, Kizer KW: A survey of assaultive behavior in veterans health administration facilities. Psychiatr Serv 1999; 50:384–389Google Scholar
10. Malone ML, Thompson L, Goodwin JS: Aggressive behaviors among the institutionalized elderly. J Am Geriatr Soc 1993; 41:853–856Google Scholar
11. de Jonghe-Rouleau AP, Pot AM, de Jonghe FM: Self-injurious behavior in nursing home residents with dementia. Int J Geriatr Psychiatry 2005; 20:651–657Google Scholar
12. Sink KM, Holden KF, Yagge K: Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA 2005; 293:596–608Google Scholar
13. Lee PE, Gill SS, Freedman M, et al: Atypical antipsychotic drugs in the treatment of behavioral and psychological symptoms of dementia: systematic review. BMJ 2004; 329:75Google Scholar
14. Kirkevold Ø, Sanvik L, Engedal K: Use of constraints and their correlates in Norwegian nursing homes. Int J Geriatr Psychiatry 2004; 19:980–988Google Scholar
15. Talerico KA, Evans LK, Strumpf NE: Mental health correlates of aggression in nursing home residents with dementia. Gerontologist 2002; 42:169–177Google Scholar
16. Menon AS, Gruber-Baldini AL, Hebel R, et al: Relationship between aggressive behaviors and depression among nursing home residents with dementia. Int J Geriatr Psychiatry 2001; 16:139–146Google Scholar
17. Pulsford D, Duxbury J: Aggressive behavior by people with dementia in residential care settings: a review. J Psychiatr Ment Health Nurs 2006; 13:611–618Google Scholar
18. Gilley DW, Bienias JL, Wilson RS, et al: Influence of behavioral symptoms on rates of institutionalization for persons with Alzheimer’s disease. Psychol Med 2004; 34:1129–1135Google Scholar
19. Orengo CA, Kunik ME, Snow AL, et al: Aggression in individuals newly diagnosed with dementia. Am J Alzheimers Dis Other Demen 2008; 23:227–232Google Scholar
20. Ryden MB: Aggressive behavior in persons with dementia living in the community. Alzheimer Dis Assoc Disord 1988; 2:342–355Google Scholar
21. Jurica PJ, Leitten CL, Mattis S: DRS-2: Dementia Rating Scale-2. Lutz, Fla, Psychological Assessment Resources, Inc, 2001Google Scholar
22. Cohen-Mansfield J: Agitated behaviors in the elderly, II: preliminary results in the cognitively deteriorated. J Am Geriatr Soc 1986; 34:722–727Google Scholar
23. Lanza ML: The reactions of nursing staff to physical assault by a patient. Hosp Community Psychiatry 1983; 34:44–47Google Scholar
24. Ryden MB, Feldt KS, Ohio HL, et al: Relationships between aggressive behavior in cognitively impaired nursing home residents and use of restraints, psychoactive drugs, and secured units. Arch Psychiatr Nurs 1999; 13:170–178Google Scholar
25. Hamel M, Gold DP, Andres D, et al: Predictors and consequences of aggressive behavior by community-based dementia patients. Gerentologist 1990; 30:206–211Google Scholar
26. Knopman DS, Kitto J, Deinard S, et al: Longitudinal study of death and institutionalization in patients with primary degenerative dementia. J Am Geriatr Soc 1988; 36:108–112Google Scholar
27. Haupt M, Kurz A: Reversibility of dementia in hypothyroidism. J Neurol 1993; 240:333–335Google Scholar
28. O’Donnell DF, Drachman DA, Barnes HJ, et al: Incontinence and troublesome behaviors predict institutionalization in dementia. J Geriatr Psychiatry Neurol 1992; 5:45–52Google Scholar
29. Warnock JK, Burke WJ, Huerter C: Self-injurious behavior in elderly patients with dementia: four case reports. Am J Geriatr Psychiatry 1999; 7:166–170Google Scholar
30. Parks SM, Feldman SM: Self-injurious behavior in the elderly. Consult Pharm 2006; 21:905–910Google Scholar
31. Anström S, Karlsson S, Sandvide Å, et al: Staff’s experience of and the management of violent incidents in elderly care. Scand J Caring Sci 2004; 18:410–416Google Scholar
32. Coyne AC, Reighman WE, Berbig LJ: The relationship between dementia and elder abuse. Am J Psychiatry 1993; 150:643–646Google Scholar
33. Gates DM, Fitzwater E, Succop P: Relationships of stressors, strain, and anger to caregiver assaults. Issues Ment Health Nurs 2003; 24:775–793Google Scholar
34. Hantikainen V: Physical restraint: a descriptive study in Swiss nursing homes. Nurs Ethics 1998; 5:330–346Google Scholar
35. Flannery RB Jr: Restraint procedures and dementia sufferers with psychological trauma. Am J Alzheimers Dis Other Demen 2003; 8:227–230Google Scholar
36. Evans LK, Strumpf NE: Tying down the elderly: a review of the literature on physical restraint. J Gerontol Nurs 1989; 18:21–23Google Scholar
37. Karlsson S, Bucht G, Rasmussen B, et al: Restraint use in elder care: decision making among registered nurses. J Clin Nurs 2000; 9:842–850Google Scholar
38. Retsas AP: Survey findings describing the use of physical restraints in nursing homes in Victoria, Australia. Int J Nurs Stud 1998; 35:184–191Google Scholar
39. Wills P, Claesson CB, Fratiglioni L, et al: Drug use by demented and non-demented elderly people. Age Ageing 1997; 26:383–391Google Scholar
40. Kolanowski A, Fick D, Waller JL, et al: Outcomes of antipsychotic drug use in community-dwelling elders with dementia. Arch Psychiatr Nurs 2006; 20:217–225Google Scholar
41. Schneider LS, Dagerman KS, Insel P: Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA 2005; 294:1934–1943Google Scholar
42. van Reekum R, Clarke D, Conn D, et al: A randomized, placebo-controlled trial of the discontinuation of long-term antipsychotics in dementia. Int Psychogeriatr 2002; 14:197–210Google Scholar
43. Suto B, Rummans TA, Smith GE: Assessment and management of behavioral disturbances in nursing home patients with dementia. Mayo Clin Proc 2001; 76:540–550Google Scholar
44. US Food and Drug Administration Center for Drug Evaluation and Research: FDA Public Health Advisory: Deaths with antipsychotics in elderly patients with behavioral disturbances, April 11, 2005. Available at www.fda.gov/cder/drug/advisory/antipsychotics.htmGoogle Scholar
45. Kunik ME, Snow AL, Molinari VA, et al: Health care utilization in dementia patients with psychiatric comorbidity. Gerontologist 2003; 43:86–91Google Scholar
46. Kales HC, Chen P, Blow FC, et al: Rates of clinical depression, diagnosis, functional impairment, and nursing home placement in coexisting dementia and depression. Am J Geriatr Psychiatry 2005; 13:441–449Google Scholar



