Neuropsychological Outcomes of Older Breast Cancer Survivors: Cognitive Features Ten or More Years After Chemotherapy
Previous research has shown that chemotherapy may have deleterious effects on neurocognitive functioning. 2 , 3 A recent meta-analysis by Anderson-Hanley and colleagues 4 examined the effects of systemic treatments of cancer on neuropsychological function across 30 studies and included 838 patients. This meta-analysis revealed consistent impairments in measures of executive function, verbal memory, and motor function (Cohen’s d range =−0.48 to −0.93), 5 suggesting medium to large effect sizes. Furthermore, to control for severity of cancer (e.g., involving CNS disease) and treatment method (e.g., total brain irradiation), nine studies including over 300 “less severe” cancer patients who had received chemotherapy were also analyzed, and the aforementioned findings were retained. Thus, there appears to be cognitive consequences secondary to the receipt of chemotherapy.
Research on the neuropsychological effects of chemotherapy with breast cancer patients specifically has demonstrated decrement in several cognitive domains. One study 6 compared 39 breast cancer patients who had received adjuvant chemotherapy with age-matched breast cancer patients who had not. It was found that the patients who had received chemotherapy demonstrated significantly poorer performance in the domains of attention, mental flexibility, verbal and nonverbal memory, and motor function. Another study 7 compared three groups of women, breast cancer patients receiving chemotherapy, breast cancer patients who had completed adjuvant chemotherapy an average of 2 years previously, and a healthy comparison group, with a 30-minute cognitive screening instrument and a self-report of affective state. It was found that breast cancer patients, regardless of chemotherapy status, were significantly more likely to demonstrate cognitive impairment. Furthermore, breast cancer patients who were currently undergoing chemotherapy had significantly poorer performance in the domains of memory and language in relation to the healthy comparison group, and the breast cancer patients who had received chemotherapy previously had significantly poorer performance in the domains of language and visual-motor skills in relation to the healthy comparison group. Finally, there were no group differences with regard to affective state.
Although the aforementioned research is invaluable, there remains a need to understand how breast cancer survivors over the age of 65—and thus more than a decade past the initial cancer event—fare in later life. One study 8 specifically examined women who have longer-term survival and recruited a sample of cancer survivors who were 5 to 10 years past initial treatment, representing a longer duration than many previous studies. The study involved survivors of either breast cancer or lymphoma and compared those who had received chemotherapy to those who had not received chemotherapy. Among the breast cancer survivors with and without chemotherapy exposure, the mean age was 59.0 and 60.6 years, respectively. Among the lymphoma survivors with and without chemotherapy exposure, the mean age was 55.9 and 48.7 years, respectively. The study observed that the chemotherapy patients scored lower in the domains of verbal memory and psychomotor functioning. Additionally, the chemotherapy patients were more likely to endorse problems with working memory on a self-report memory questionnaire. The authors concluded that these data support the hypothesis that chemotherapy treatments may have long-term deleterious consequences on cognitive functioning. In all such work, however, despite meticulous attempts at matching for cancer diagnosis, there is often the limitation that those who go on to receive chemotherapy have a more severe disease than those who do not, such that the severity of the underlying cancer and the independent effects of cancer itself are quite difficult to delineate entirely.
To our knowledge, no studies to date have examined the role of cognitive functioning in breast cancer patients who are more than a decade postchemotherapy. We recruited women over the age of 65, who were at least 50 years old at the time of treatment, and conducted comprehensive neuropsychological testing on these long-term survivors. We hypothesized that a history of chemotherapy would be associated with cognitive deficits in excess of those observed in demographically matched healthy comparison women. Preliminary findings from our first 30 long-term survivors are presented in this article in relation to 30 healthy community-dwelling older adults with no history of cancer who were matched in terms of age, years of education, and IQ.
METHODS
Breast cancer survivor participants were recruited in collaboration with the Iowa Cancer Registry, a statewide registry of cancer patients begun in 1973. 1 Enrollment criteria specified that the participants were women over the age of 65, at least 50 years old at the time of cancer diagnosis and treatment, and at least 10 years postcancer treatment. Participants for this preliminary report were diagnosed and treated for early malignant breast cancer Stage I through Stage IIIA without evidence of metastasis. All participants received a standard multiagent chemotherapy regimen involving cyclophosphamide, methotrexate and 5-fluorouracil (CMF) or an anthracycline (doxorubicin). Participants were excluded if there had been a recurrence of any kind of cancer in the 10–15 year period since initial diagnosis, excluding basal cell or relatively benign skin lesions. Participants were also excluded if they possessed a CNS disorder, such as multiple sclerosis, Parkinson’s disease, closed head trauma with an extended loss of consciousness, or other CNS lesion. All breast cancer survivor participants were free of currently active and unstable metabolic, psychiatric, and cardiovascular diseases, including cerebrovascular events and substance abuse.
Each breast cancer survivor participant was demographically matched to a noncancer comparison participant from an existing database. These comparison subjects were previously recruited via flyers and advertisements posted in the Iowa City community for an ongoing study examining the effects of aging on decision-making behavior. As such, only healthy community-dwelling adults were included. Participants met inclusionary criteria if they were free of neurological and psychiatric illness, as indicated above. All participants signed a written informed consent document approved by the University of Iowa Institutional Review Board.
Procedures
Each participant completed a 3-hour standardized neuropsychological battery designed to evaluate a broad range of cognitive abilities, involving attention, premorbid and current intellect, memory, language, visuospatial skills, and executive functioning.
Intelligence and General Cognitive Functioning
Intelligence was measured using the four-subtest Wechsler Abbreviated Scale of Intelligence 9 (WASI) to obtain a Full Scale IQ score. The WASI Vocabulary subtest asks participants to describe the meaning of words. In the WASI Block Design subtest, participants replicate two-dimensional patterns with red- and white-colored blocks. The WASI similarities and matrix reasoning subtests tap verbal and nonverbal reasoning, respectively. Premorbid intellect was measured using the Wide Range Achievement Test—III, reading subtest 10 (WRAT-III), a single-word reading task. The Folstein Mini-Mental State Examination 11 (MMSE) was used to assess brief global cognitive functioning.
Attention
Both simple and divided attention (also referred to as working memory) was measured using the digit span, letter-number sequencing, and arithmetic subtests from the WAIS—3rd edition 12 (WAIS-III). In the digit span task, participants are asked to repeat strings of numbers both forward and backward. For the letter-number sequencing subtest, participants must recite back a string of numbers and letters after putting them in numerical and alphabetical order. Participants mentally solve arithmetic problems for the arithmetic subtest. Trail Making test, part A, 13 is both an attentional and psychomotor task, and participants connect dots in numerical order as quickly as they can, without making errors.
Language
The Controlled Oral Word Association Test 14 (COWAT) measures verbal fluency; participants are given 1 minute to say as many words as they can that begin with a specific letter. Participants are shown a series of line drawings of simple objects and asked to name them for the Boston Naming Test. 15
Visuospatial
The Rey-Osterrieth Complex Figure Test Copy Condition 16 is a detailed drawing that must be copied while participants look at the stimuli. On the Facial Recognition Test, 17 participants must identify and discriminate photographs of faces.
Memory
The Rey Auditory-Verbal Learning Test 18 (RAVLT) is a verbal learning and memory task in which participants are given five trials to learn a list of words and are then asked to recite these words again following an incidental 30-minute delay period. Visual memory was assessed with the Rey-Osterrieth Complex Figure Test-Delay Condition, 16 in which participants must reproduce the figure they were asked to draw 30 minutes previously. (Both the RAVLT and the Rey-Osterrieth Complex Figure are incidental memory tasks; therefore patients are not given forewarning that a delayed memory test will be administered.) For the Benton Visual Retention Test—Revised, 19 participants look at simple geometric figures for 10 seconds, and are asked to draw from immediate memory what they saw.
Executive Functioning
The Intradimensional/Extradimensional Shift 20 task is a computerized instrument in which compound stimuli are presented in two stages (intra- and extradimensional shift) as shapes overlaid with lines on a computer screen. Participants must learn rules through feedback to shift between intra- and extradimensional stages. In the Trail Making test, part B, 13 participants connect dots consecutively in an alternating order between numbers and letters. In the Wisconsin Card Sorting Test 21 (WCST), participants must correctly categorize cards based on verbal feedback.
Mood
The Beck Depression Inventory—II, 22 a 21-item self-report measure, was used to measure depressive symptomatology.
Statistical Analyses
A one-way multivariate analysis of variance (MANOVA) was computed using cognitive measures as dependent variables and participant groups (i.e., breast cancer survivors and healthy noncancer comparison subjects) as independent variables. This mode of analysis was chosen to avoid alpha inflation based on the number of variables and comparisons and to take into consideration the intercorrelations of the different cognitive measures. Average performance scores for each measure and group were computed to input missing data.
RESULTS
The breast cancer survivor group had a mean age of 72.8 years old (SD=5.1), 14.4 years of education (SD=2.7), a WASI Full-Scale IQ of 113.1 (SD=12.6), and a WRAT-III reading raw score of 47.9 (SD=4.44). The noncancer comparison group did not significantly differ from the breast cancer survivor group in terms of demographics and overall premorbid and current intelligence, and they had a mean age of 72.6 years old (SD=5.5), 14.3 years of education (SD=2.2), a WASI Full-Scale IQ of 112.5 (SD=9.8), and a WRAT-III reading raw score of 50.2 (SD=5.04). Among the cancer survivors, the mean duration since the initial diagnosis of cancer was 16.8 years (SD=2.8, range=13.8–22.5 years).
Table 1 displays the results of between-subjects effects from MANOVA analysis. We observed significant differences on the MMSE (F=18.95, df=1, 58, p=0.00), a brief screen of global cognitive functioning.
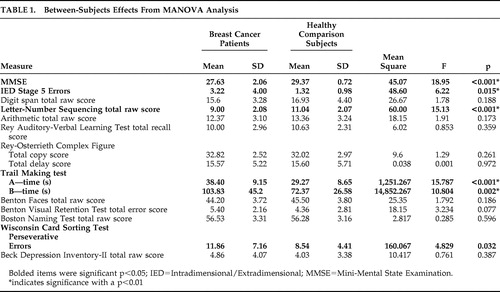 |
Group differences were also observed in aspects of attention. Specifically, there were group differences in the Letter-Number Sequencing subtest (F=15.13, df=1, 58, p=0.00), and the noncancer comparison group outperformed the breast cancer survivor group on Trail Making test, part A (F=15.787, df=1, 58, p=0.00), a measure of attention and psychomotor speed.
In the domain of executive functioning, group differences were observed on the Trail Making test, part B (F=10.804, df=1, 58, p=0.002). Similarly, the breast cancer survivor group committed more reversal errors on the Intradimensional/Extradimensional Shift task (F=6.22, df=1, 58, p=0.015) and demonstrated more perseverative errors on the WCST (F=4.829, df=1, 58, p=0.032).
By contrast, group differences did not emerge in the cognitive domains of language, visuospatial, and memory functioning. There were no group differences in self-reported mood (F=0.761, df=1, 58, p=0.387).
DISCUSSION
The aim of this study was to evaluate the effects of chemotherapy on cognition in long-term survivors of breast cancer. We hypothesized that a history of chemotherapy would be associated with greater cognitive deficits when compared to an age-, education-, and IQ-matched sample of healthy, community-dwelling older adults. A MANOVA demonstrated significant differences between breast cancer survivor and noncancer comparison groups on a brief measure of global cognition. More detailed neuropsychological assessment revealed significant differences in the cognitive domains of attention, working memory, psychomotor speed, and aspects of executive functioning. By contrast, there were no differences in the domains of language, visuospatial, and memory functioning. Mood also did not differ between the groups.
In the domains of attention, psychomotor speed, and executive functioning, the breast cancer survivor group performance ranged from 0.75 to 2.0 standard deviations below their well-matched noncancer comparison peers, suggesting that the group differences have both statistical and practical importance. The discrepancy between these two groups is likely of a magnitude to be noticed in everyday life. 23 Specifically, tasks completed with ease prior to breast cancer diagnosis and treatment may be significantly more effortful. However, in terms of strict clinical definition, the breast cancer survivor group performance would not be classified as bona fide impairment (i.e., less than 2 standard deviations below expectations) but rather as a cognitive weakness.
Furthermore, like other breast cancer samples that rely on volunteer participation (e.g., in Brezden et al. 7 ), our participants were comprised of women with above average levels of education and intellect. However, these demographics make our findings all the more compelling when one considers that cognitive deficits may be compounded in women with less education and intellect. Such women may have less “cognitive reserve” to withstand the potentially negative influence of chemotherapy upon brain function. 24
Our study concurred with prior studies in terms of deficiencies in the domains of attention, working memory, psychomotor speed, and aspects of executive functioning. 4 , 6 – 8 However, our findings differed in terms of memory performance; specifically, we did not find group differences in verbal and nonverbal memory. This could be the result of several features of our sample. First, our participants have survived breast cancer for more than 10 years, which exceeds the mean survivorship of other studies and thus makes comparisons difficult. Second, our participants are substantially older than participants in prior breast cancer studies. Given the preponderance of age-associated memory impairment, younger patients may detect impairment more readily as they are less likely to attribute problems to age-related changes; also, such problems are less likely to be obscured by preexisting age-related deficits. Third, we cannot negate the possibility that our sample with higher intellect was affected by cancer and its associated treatment in a different manner than those with a relatively lower intellect (and thus lower cognitive reserve). All of these features may serve to change the neuropsychological profile of these older long-term survivors.
The lower performance among breast cancer survivors on attentional and executive functioning tasks may reflect potential dysfunction in frontal regions and their subcortical connections. In addition, psychomotor slowing, as measured by poorer performance on the Trail Making test, part A, observed in the breast cancer survivor group could suggest basal ganglia and subcortical white matter involvement. Taken together, the profile that is emerging among long-term breast cancer survivors has some overlap with Alzheimer’s disease insofar as the frontal-subcortical involvement but importantly departs from Alzheimer’s disease, and even the prodromal syndrome of Alzheimer’s disease, as we did not observe involvement of mesial temporal brain regions (per strong performance on tests of anterograde verbal and visual memory). The psychomotor slowing also departs from that commonly seen in Alzheimer’s disease patients. Thus, neuropsychological testing may serve to differentiate the outcomes seen in long-term breast cancer survivors from the cognitive changes that one might expect from an individual with Alzheimer’s disease. It is important to heed this assertion with caution. Specifically, current evidence has suggested that attention, especially divided attention that may affect executive function, could be the first cognitive domain affected by early stages of Alzheimer’s disease. 25
There are some limitations to this study. First, as a preliminary analysis of a larger study, this sample size is relatively small. Second, though a healthy comparison group was used and matched to the best of our ability, an additional important comparison group could be demographically matched breast cancer patients who did not receive chemotherapy. Clearly, such a comparison would allow us to more clearly isolate the effects of chemotherapy alone, although the independent effects of cancer and underlying differences in cancer severity will always represent a potential confounding factor. Furthermore, the nature of combination chemotherapy treatment does not allow us to identify the individual effects of each specific chemotherapy regimen (e.g., cyclophosphamide versus anthracycline). Third, group differences were not observed for mood in our sample; however, other factors, such as stress, could contribute to neurocognitive deficits. As such, efforts to parse out the deleterious consequences of psychosocial variables should necessitate consideration. Lastly, it is possible that there were undetected differences in medical comorbidities between the two groups that could have subtly affected cognition regardless of exclusion criteria meant to screen any unstable medical or neurological conditions that could affect neuropsychological performance.
These preliminary analyses of the effects of chemotherapy on long-term survivorship in breast cancer can provide a foundation for future studies. Specifically, these data may serve as a baseline as our study progresses to include serial neuropsychological evaluations, neuroimaging techniques, and examination of biomarkers. As the number of long-term survivors increases, efforts to better understand the implications of systemic interventions of cancer are necessary.
1. Ries LAG, Melbert D, Krapcho M, et al (eds): SEER Cancer Statistics Review, 1975–2004. Bethesda, Md, National Cancer Institute, 2007Google Scholar
2. Ahles TA, Saykin AS: Cognitive effects of standard-dose chemotherapy in patients with cancer. Cancer Invest 2001; 19:812–820Google Scholar
3. Olin JJ: Cognitive function after systemic therapy for breast cancer. Oncology 2001; 15:613–624Google Scholar
4. Anderson-Hanley C, Sherman ML, Riggs R, et al: Neuropsychological effects of treatments for adults with cancer: a meta-analysis and review of the literature. JINS 2003; 9:967–982Google Scholar
5. Cohen J: Statistical Power Analysis for the Behavioral Sciences, 2nd ed. Hillsdale, NJ, Lawrence Erlbaum Associates, 1988Google Scholar
6. Schagen SB, van Dam Frits SAM, Muller MJ, et al: Cognitive deficits after postoperative adjuvant chemotherapy for breast carcinoma. Cancer 1999; 85:640–650Google Scholar
7. Brezden CB, Phillips K-A, Abdolell M, et al: Cognitive function in breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol 2000; 18:2695–2701Google Scholar
8. Ahles TA, Saykin AJ, Fustenberg CT, et al: Neuropsychological impact of standard-dose systemic chemotherapy in long-term survivors of breast cancer and lymphoma. J Clin Oncol 2002; 20:485–493Google Scholar
9. Wechsler D: The WASI: Wechsler Abbreviated Scale of Intelligence. San Antonio, Tex, Psychological Corp, 1999Google Scholar
10. Wilkinson GS: WRAT-3: Wide Range Achievement Test Administration Manual, 3rd ed. Wilmington, Del, Wide Range, 1993Google Scholar
11. Folstein MF, Folstein SE, McHugh PR: “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198Google Scholar
12. Wechsler D: Wechsler Adult Intelligence Scale–III. San Antonio, Tex, Psychological Corp, 1997Google Scholar
13. Spreen O, Strauss E: A Compendium of Neuropsychological Tests, 2nd ed. New York, Oxford University Press, 1998Google Scholar
14. Benton AL, Hamsher K: Multilingual Aphasia Examination. Iowa City, Iowa, AJA Associates, 1989Google Scholar
15. Kaplan EF, Goodglass H, Weintraub S: Boston Naming Test, 2nd ed. Philadelphia, Lea & Febiger, 1983Google Scholar
16. Rey A: L’examen psychologique dans les cas d’encephalopathie traumatique. Archives de Psychologie 1941; 28:286–340Google Scholar
17. Benton AL, Sivan AB, Hamsher K, et al: Contributions to Neuropsychological Assessment: A Clinical Manual, 2nd ed. New York, Oxford University Press, 1994Google Scholar
18. Rey A: L’examen Clinique en Psychologie. Paris, Presses Universitaires de France, 1964Google Scholar
19. Sivan AB: Benton Visual Retention Test, 5th ed. San Antonio, Tex, Psychological Corp, 1992Google Scholar
20. Sahakian BJ, Owen AM: Computerized assessment in neuropsychiatry using CANTAB: discussion paper. J R Soc Med 1992; 85:399–402Google Scholar
21. Heaton RK, Chelune GJ, Talley JL, et al: Wisconsin Card Sorting Test: Manual Revised and Expanded. Odessa, Fla, Psychological Assessment Resources, 1993Google Scholar
22. Beck AT, Steer RA, Brown G: Beck Depression Inventory–II: Manual. San Antonio, Tex, Psychological Corp, 1996Google Scholar
23. Kerns KA, Mateer CA: Walking and chewing gum: the impact of attentional capacity on everyday activities, in Ecological Validity of Neuropsychological Testing. Edited by Sbordone RJ, Long CJ. Delray Beach, Fla, GR Press/St. Lucie Press, 1996, pp 147–169Google Scholar
24. Stern Y (ed): Cognitive Reserve: Theory and Application. New York, Taylor & Francis, 2007Google Scholar
25. Perry RJ, Hodges JR: Attention and executive deficits in Alzheimer’s disease: a critical review. Brain 1999; 122:383–404Google Scholar



