APOE ε4 and Cognitive Dysfunction in Multiple Sclerosis: A Review
Genetics offers the possibility of bridging the gap between cerebral MR indices on one hand and neuropsychological variables on the other. In the process, potential clues pertaining to the etiological underpinnings of cognitive dysfunction in multiple sclerosis may be elucidated. Apolipoprotein E ( APOE ) is one of the most studied genes in relation to human cognition. 23 Three common allelic variants, APOE ε2, ε3, and ε4, give rise to three protein isoforms that differ in the combination of amino acids at two residues of the 299 amino acids polypeptide. The consequent variations in tertiary and quaternary structure have significant repercussions for the risk of Alzheimer’s disease. 24 In Alzheimer’s disease, one copy of the ε4 allele raises the risk of acquiring the late onset form of the disease three to four times; two copies raise it 10 to 12 times. 25 – 28 Brain imaging of Alzheimer’s disease patients demonstrates the presence of neuropathology that distinguishes ε4 carriers from noncarriers, 29 , 30 changes that may in turn be linked to the nature of cognitive deficits in ε4+ Alzheimer’s disease patients. 31 In the general population, APOE allelic frequencies range from 5% to 10% for ε2, 65%–70% for ε3, and 15%–20% for ε4. 23 Cognitive decline 32 – 34 and neuroimaging changes in healthy aging 35 – 38 have also been linked to APOE genotype, though not without controversy. 39 , 40
Discovery of the major genetic risk factor for sporadic Alzheimer’s disease in the 1990s ushered in considerable research on the relationship between APOE and neurocognition. Experimental studies continue to delineate putative mechanisms of ε4-mediated neuropathology, 41APOE -targeted therapeutics are in development, 42 , 43 and possible correlations of ε4 with other complex neuropsychiatric diseases are being investigated. 44 – 51
In the case of multiple sclerosis, more than a dozen studies sought an association between ε4 carrier status and disease severity, with mixed results. In a quantitative review of this literature, Burwick et al. 52 recently concluded that APOE is not involved in the severity of multiple sclerosis. A second comprehensive review came to the same conclusion. 53 However, in both reviews, a significant limitation of the reports examined was their reliance on the EDSS as a primary measure of disease severity. Potentially more rewarding avenues of inquiry may be the relationship between the ε4 allele and cognition and brain imaging.
The purpose of this review is twofold. First, given the important role cognition plays in the quality of life of multiple sclerosis patients and the potential relevance of the APOE ε4 allele as a determinant of cognitive decline in Alzheimer’s disease, we undertook a multiple sclerosis-focused literature review exploring the association, or lack thereof, between these two variables. Second, since magnetic resonance imaging (MRI) is a sensitive predictor of cognitive dysfunction and disease activity in multiple sclerosis, we reviewed the literature comparing MR-demonstrable differences in brain pathology between ε4+ and ε4− multiple sclerosis patients.
Cognitive Studies
Five studies have specifically examined cognition in the context of APOE ε4 status. Table 1 shows sample demographics, disease variables, cognitive domains, and neuropsychological tests for each report.
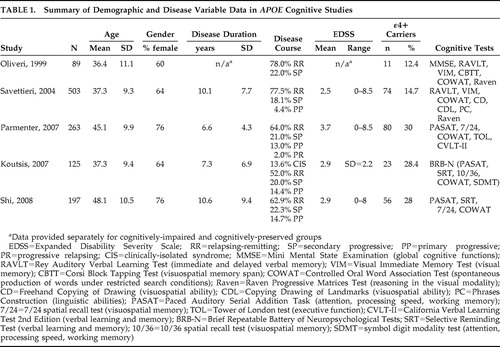 |
Oliveri et al. 54 studied 89 multiple sclerosis subjects, 12 of whom were ε4+. Demographic and disease variables were provided for the entire sample as shown in Table 1 , but how these factors compared between ε4+ and ε4− groups was not specified. Based on a brief battery of tests, some of which are seldom used in multiple sclerosis research, the neuropsychological scores of multiple sclerosis patients were compared to age-, gender-, and education-matched healthy control subjects in previous studies. Patients were classified as cognitively impaired if their results on at least one cognitive test were deemed to be abnormal. The criteria for this designation were again unspecified. No difference was found in the frequency of the three alleles (ε2, ε3, ε4) and four genotypes (ε2/ε3, ε2/ε4, ε3/ε3, ε3/ε4) between cognitively impaired and cognitively preserved multiple sclerosis subjects. Acknowledging the small size of the ε4+ group, the authors concluded that their data did not support an adverse effect of the ε4 allele on cognition in multiple sclerosis. A subsequent revision of these analyses produced a different result, however. 55 With multiple sclerosis subjects divided into one of two groups based on ε4 carrier status, a significantly higher proportion of ε4+ patients were present in the impaired group.
Savettieri et al. 56 studied 503 multiple sclerosis patients also using a brief cognitive battery ( Table 1 ). As in the previous study, no data were provided on whether ε4+ and ε4− subjects were matched on demographic and disease variables. Cutoff scores for cognitive impairment were derived from a previous study of normative data. Failure on one to four tests signified mild impairment, while those failing five or more tests were characterized as having severe impairment. In the primary analysis, ε4+ subjects were represented in equal proportions in the cognitively impaired (mild + severe) and preserved groups. However, the cognitively impaired group had significantly more males, longer disease duration, more secondary progressive disease, lower education, and more physical disability. Taking these potential influences into account, the authors performed a number of subgroup analyses that ultimately led them to conclude that high EDSS, low education, and ε4 carriage were significant risk factors for severe cognitive impairment in males. In females, no variables were identified as risk factors for cognitive impairment.
Here, it should be noted that greater cognitive impairment in male versus female multiple sclerosis patients finds limited support in the literature. 1 , 3 , 4 , 57 , 58 The data in this study are further challenged by the lack of association in female subjects between cognitive indices and variables known to influence cognition such as age and education.
In the third study, 55 ε4 carriers were matched to ε4 noncarriers on age, gender, education, duration of multiple sclerosis, age at onset of multiple sclerosis, EDSS, depression, anxiety, and fatigue ( Table 1 ). The often-cited, validated Neuropsychological Screening Battery for multiple sclerosis (NSBMS) 1 was modified by substituting the Selective Reminding Test with the California Verbal Learning Test—II (CVLT-II). Patients’ raw scores on each cognitive test were converted to z scores based on published means from normative samples. The z scores from all tests were then averaged and transformed to a single standard cognitive composite score, which did not differ between ε4+ and ε4− groups. Two categorical analyses were then undertaken. The first dichotomized the sample into cognitively intact and cognitively impaired, with the latter defined as having a standard cognitive composite score at least one standard deviation below the mean (n=95, 40% of the sample). No significant differences were found with respect to the frequency of ε4 carriage in these groups. In the second analysis, a stricter criterion for cognitive impairment was used. To be classified as impaired, a subject had to score at least one standard deviation below the mean on the Paced Auditory Serial Addition Task (PASAT), on two or more measures derived from the CVLT-II, and on two or more of the remaining tests in their battery. This substantively reduced the size of the cognitively impaired sample (n=25, 11% of the sample). However, with the stricter criterion, 23% (13/56) of ε4 carriers were deemed to be cognitively impaired, compared to only 8% (12/150) of non-ε4 carriers, a statistically significant difference.
In the fourth study, Koutsis et al. 59 used the Brief Repeatable Battery of Neuropsychological Tests (BRB-N), which consists of the four tests in the NSBMS plus the Symbol Digit Modalities Test 60 ( Table 1 ). The cutoff for failure on an individual cognitive test was designated as below the fifth percentile of scores obtained by age, gender, and education matched controls (n=43), a threshold deemed by previous studies to be clinically meaningful. 1 , 2 Overall cognitive impairment was defined as failure on at least three tests. ε4 carriers did not differ from non-ε4 carriers on age, gender, education, age at onset, disease duration, disease course, EDSS, disease-modifying drugs, or depression. Potential differences in the frequencies of overall and domain-specific cognitive impairment were analyzed by logistic regression with ε4 status as a predictor variable. This showed that presence of the ε4 allele did not affect the risk of overall cognitive impairment. However, of the individual cognitive domains defined, ε4 carriage did emerge as a significant predictor of verbal learning impairment with a sixfold increase in relative risk of failure compared to noncarriers. Failure of verbal learning was defined as failure on both long-term storage and consistent long-term retrieval components of the Selective Reminding Test (SRT). The key finding of this study was the link between ε4 and a specific cognitive domain, namely verbal memory, a finding obscured by composite cognitive score.
The possibility of domain-specific cognitive impairment in ε4+ multiple sclerosis patients was also addressed in the next study. Shi et al. 61 used the NSBMS to study ε4+ subjects who were slightly older (mean=50.9, SD=9.3 years) than ε4− subjects (mean=47, SD=10.8 years, p=0.017). The two groups were otherwise similar with respect to other demographic and disease parameters. Raw scores for each cognitive test were converted to standardized t scores corrected for age, gender, and education, and the cutoff for failure was taken as below the fifth percentile of the t score. The odds ratio of failing one index of the verbal memory test (Selective Reminding Test), namely, consistent long-term retrieval, in ε4+ versus ε4− patients was 2.1 (p=0.035). There were no other significant cognitive differences. In a secondary analysis, the authors compared a subgroup of the youngest ε4 carriers to age-matched noncarriers (mean=36.4, SD=2.4 years, range=31 to 40 years) and found that a significantly higher proportion of ε4 carriers had verbal memory impairment. When the overall sample was stratified by age (decades), the young cohort of ε4+ patients (ages 31–40) was found to be especially susceptible to verbal learning deficits. These secondary results are difficult to interpret, however, since subgroup sample sizes were not provided.
Comment
Overall, results of the five studies suggest a possible link between ε4 carriage and greater cognitive dysfunction in multiple sclerosis. A cautionary note is that in some of these studies, the association only became apparent on secondary, post hoc analyses where the original definition of cognitive impairment had been adjusted. 55 , 56 Similarly, the single negative study 54 was subsequently reanalyzed by a different research group, with a positive result emerging. 55 The broad agreement between studies should therefore be viewed within the context of a number of potential limitations including cognitive batteries yet to be validated in an multiple sclerosis population, variable thresholds for test failure, and different definitions of cognitive impairment.
Magnetic Resonance Studies of APOE in Multiple Sclerosis
Brain pathology as elucidated by MRI is a robust predictor of cognitive impairment. Significant correlations have been found between decreased cognition, T2 and T1 lesion volume, and various indices of brain atrophy. 62 – 76 To date, only one study has collected APOE genotypes, cognitive variables, and quantitative MRI data in the same sample. 77 This was an open-label evaluation of IFN beta-1b in 46 relapsing-remitting patients, of whom seven were ε4+. Although the authors mentioned that APOE polymorphisms were not associated with cognitive decline or MRI parameters, the omission of important information such as whether ε4+ and ε4− patients were matched on demographic and disease variables renders this report difficult to evaluate, a challenge compounded further by the absence of cognitive and MRI data parsed by ε4 carrier status.
A small literature of seven studies has examined the possible relationship between ε4 carrier status and MR-elicited brain abnormalities in multiple sclerosis. Table 2 illustrates sample demographics, disease parameters, MR modality and field strength, and dependent variable(s) for each report.
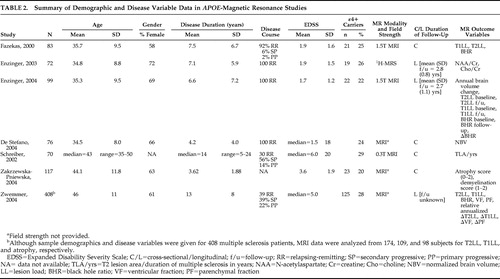 |
Fazekas et al. 78 studied ε4+ and ε4− multiple sclerosis patients matched on age, gender, disease duration, disease course, number of relapses, and EDSS ( Table 2 ). While T2 lesion load (LL) did not differ significantly between the groups, T1LL was significantly higher in ε4 carriers. “Black hole ratio” was also reported, a term defined by: (T1LL/T2LL) × 100. The percentages of black holes in ε4+ and ε4− multiple sclerosis patients were 13.3% and 8.3%, respectively, a statistically significant difference. Given that T1 lesions reflect more destructive tissue pathology, the implication here is that ε4 may be a potential marker of disease severity according to MRI parameters. This is supported by postmortem data linking black holes with axonal swelling and loss, reactive astrocytes, and gliosis. 79 – 82
The same group followed up their initial study with a longitudinal proton magnetic resonance spectroscopy ( 1 H-MRS) investigation 83 ( Table 2 ). They reported biochemical measures, N -acetylaspartate (NAA)/creatine and choline/creatine, from a single large volume of interest that comprised parts of the periventricular white matter, the deep white matter, and the basal ganglia. Age, disease duration, EDSS, and treatment with immunomodulatory drugs did not differ between ε4 carriers and noncarriers. At baseline, ε4 carriers showed significantly lower NAA/creatine ratios, a nonspecific marker of neuronal integrity. Two-year follow-up data were available in 44 patients which included 9 ε4+ subjects. The drop in NAA/creatine ratio was significantly greater in ε4 carriers over 2.8 years, while choline/creatine levels, indicative of membrane turnover and/or cellular infiltration, were stable. In a regression model, APOE ε4 carriage emerged as a significant independent predictor of a low NAA/creatine ratio at follow-up when age at onset, gender, disease duration, and interval between MRS studies were accounted for.
The MRS findings 83 validated and extended the structural MRI data 78 and suggested that ε4 carriage may be independently associated with greater neuropathology, whether it was measured by T1LL or NAA/creatine. Additionally, the drop in NAA/creatine over time in the ε4 carriers suggested that this allele may mediate a faster rate of tissue destruction.
APOE and the dynamics of tissue damage in multiple sclerosis were investigated by the same group in a third study. 84 Rates of brain atrophy, T1LL, and black hole ratios that evolved over a mean follow-up period of 2.7 years were evaluated in ε4+ and ε4− multiple sclerosis patients matched across a host of demographic and disease modifying variables. ε4 carriers had a significantly higher rate of annual brain volume loss and an increase in black hole ratio over the follow-up period. Contrary to their initial study, 78 however, T1LL and black hole ratio—like T2LL—did not significantly differ between the groups either at baseline or at follow-up; it was only the change in black hole ratio over 2.7 years that achieved statistical significance. Regression modeling that incorporated all demographic and clinical variables plus changes in T1 and T2 lesion load and black hole ratio identified the presence of the ε4 allele as the only significant predictor of brain volume changes. Also undertaken was a secondary analysis in which the sample was dichotomized by disease duration above or below 5 years. The findings of the primary analysis—higher annualized brain volume loss and increase in black hole ratio in ε4 carriers—persisted in the cohort with shorter disease duration (n=16) but lost statistical significance in those with disease duration greater than 5 years (n=7), suggesting that atrophy rates in ε4 carriers may be dependent on disease duration.
Smaller brain volumes in ε4+ multiple sclerosis patients were confirmed by De Stefano et al. 85 in ε4+ and ε4− cohorts matched on demographic and disease variables ( Table 2 ). A subgroup analysis looking at patients with short disease duration (<3 years) and relatively low physical disability (EDSS<2) found that the lower normalized brain volumes in ε4+ patients (n=8) compared to ε4− patients (n=22) were evident early in the disease process.
In the studies reviewed thus far, T2LL did not differ between the ε4+ and ε4− patients with multiple sclerosis. 78 , 84 , 85 Schreiber et al., 86 using a less sensitive MRI technique ( Table 2 ), were also unable to find a correlation between possession of the ε4 allele and an index of T2 lesions that controlled for disease duration. This report did not examine any other imaging parameters, however.
Zwemmer et al. 87 undertook a retrospective study of 408 multiple sclerosis subjects, of whom 115 were ε4 carriers ( Table 2 ). T2 lesion data, T1 lesion data, and serial atrophy measurements were present for 174, 109, and 98 subjects, respectively. The numbers of ε4 carriers were not given for any subgroups, demographic and disease characteristics were not presented for ε4 carriers and noncarriers, time between scans was not mentioned, and, with the exception of p values, no raw data were shown. Nonetheless, in this largest imaging/ APOE study in multiple sclerosis to date, no MRI differences were reported between ε4 carriers and noncarriers. These findings were unchanged when corrected for age, gender, onset type of multiple sclerosis, duration of disease, and IFN treatment.
The failure to find an association between the ε4 allele and MRI-elicited brain pathology was replicated in a study that had significant methodological problems. 88 MRI scans were evaluated using an “arbitrarily proposed semiqualitative scores system” in which atrophy was scored on a scale of 0 to 2 and demyelination was scored from 1 to 2. The use of nonquantitative, subjective, ordinal scales for MR measures with uncertain floor and ceiling effects is problematic and thwarts critical review of the data.
Comment
Of the seven imaging studies, four support a correlation between ε4 and brain pathology, particularly T1LL, 78 , 84 black hole ratio, 78 , 84 brain atrophy, 84 , 85 and NAA/creatine. 83 These studies had the benefit of sound methodologies that matched ε4+ and ε4− multiple sclerosis patients on demographic and disease variables. Two studies had the additional advantage of a longitudinal study design. However, three of the four studies were conducted by the same research team. 78 , 83 , 84 While this does not detract from the results, the fact that the subjects were drawn from the same population tempers the generalizability of the findings. Therefore, conclusions regarding an association of the ε4 allele and MR-derived indices of brain pathology cannot be reliably drawn until the above data are replicated in different multiple sclerosis populations.
An interesting addendum to these issues is the potential interaction of ε4-affiliated brain pathology with shorter disease duration. 84 , 85 Results here are equivocal. In a longitudinal study, the presence of atrophy was most marked in ε4+ patients with disease duration of less than 5 years. 84 No attempt to control for age was made, however. This finding was not replicated in a second study using a cross-sectional design. 85 Of the two remaining studies that did not find a gene-imaging association, one examined only T2LL 86 and the other had significant methodological problems discussed above. 88
DISCUSSION
The impetus for this review was twofold. First, given the high prevalence of cognitive dysfunction in multiple sclerosis and the associated deleterious effects on quality of life, we reviewed the putative influence of the ε4 allele on neuropsychological function in multiple sclerosis. This review was given added salience by the firm link established between the ε4 allele and Alzheimer’s disease. 26 – 28 Second, the strong association between cognitive decline and MRI abnormalities 62 – 76 led us to review the data pertaining to the possible influence of the ε4 allele on brain imaging parameters. The studies reviewed here 54 – 56 , 59 , 61 , 78 , 83 – 88 are not without their limitations, and definitive answers await additional research. The most parsimonious conclusion is that an association between the presence of the APOE ε4 allele and cognitive dysfunction remains possible pending further, well-controlled studies.
The evidence suggests that multiple sclerosis patients with the ε4 allele have poorer cognitive function 55 , 56 , 59 , 61 and possibly more brain pathology 78 , 83 – 85 than their ε4-negative counterparts. Cognitive impairment, when present in ε4 carriers, may manifest as greater deficits in verbal memory in particular. 59 , 61 More extensive neuropathology in ε4+ multiple sclerosis patients may be reflected by increased T1LL, 78 , 83 black hole ratio, 78 , 83 and brain atrophy on conventional MRI 84 , 85 ; lower NAA/creatine on MRS 84 ; and accelerated development of atrophy, T1LL, and black hole ratio over time. 83 , 84 Whether these findings are influenced by age and disease duration cannot be reliably ascertained from the data as it currently exists.
Cognitive studies in Alzheimer’s disease generally suggest that ε4 influences disease risk by exerting a relatively selective effect on episodic memory, 31 a finding consistent with that observed in two of five of the multiple sclerosis studies of APOE . 59 , 61 Given the risk that ε4 carriage confers upon developing Alzheimer’s disease, it is possible that a proportion of the ε4+ subjects with verbal memory deficits were ultimately destined to develop Alzheimer’s disease. Clarifying this issue would require further study of multiple sclerosis populations stratified by age. Additional deficits in ε4+ multiple sclerosis patients including measures of global impairment have emerged from studies employing different cognitive measures and arbitrary cutoff points for impairment. 54 – 56 This multiplicity of cognitive measures makes it difficult to arrive at a consensus with certainty, for it highlights one of the problems that has bedeviled the multiple sclerosis cognitive literature to date. Future research can offset this by confining methodologies to cognitive batteries with proven sensitivity and specificity in multiple sclerosis population. One example is the Minimal Assessment of Cognitive Function in Multiple Sclerosis (MACFIMS), a broad-ranging, 90-minute assessment developed by expert consensus. 90 , 91
The seven studies that examined the possible relationship between ε4 carrier status and MR-elicited brain abnormalities in multiple sclerosis 78 , 83 – 88 pale in comparison to at least 145 brain imaging studies of ε4 correlates in Alzheimer’s disease where robust associations have been demonstrated. 29 These may be summarized as increases in general cerebral atrophy and decreases in cerebral blood flow with disproportionate atrophic changes in the right temporal lobe and more perfusion/metabolic deficits in the left hemisphere. These changes are further accentuated in ε4 homozygotes. 30 Studies in cognitively healthy ε4 carriers have suggested a continuum of changes beginning with focal thinning of the entorhinal cortex or other hippocampal subregions, findings that have been observed in ε4+ pediatric and adolescent populations 92 as well as cognitively healthy ε4+ elderly subjects. 38 , 93 – 96 With age and/or Alzheimer’s disease, these changes become more diffuse and pronounced across the medial temporal lobe, and degeneration may then spread sequentially to the temporoparietal association cortices, the frontal regions, and finally the primary sensory, motor, and occipital areas.
Viewed alongside the Alzheimer’s disease literature, neuroimaging studies of APOE in multiple sclerosis are less varied. Techniques with the potential to yield important information beyond that afforded by conventional MRI are, in all but one spectroscopy study, lacking. In addition, studies have not reported regional lesion or atrophy measures, nor indices of pathology in normal-appearing brain tissue. From a mentation standpoint, the most notable imaging finding to emerge thus far is that two of three studies that looked at generalized cerebral atrophy reported an association with the ε4 allele. The clinical importance of this lies in the robust association between atrophy and cognitive dysfunction reported previously. 97 – 102
In summary, the literature investigating a possible relationship between the ε4 allele and cognitive dysfunction in multiple sclerosis patients is small and on balance suggests a link. The same cannot as yet be said for any gene-MRI association. These conclusions should be viewed as tentative in light of the limitations mentioned above. What is notable about the literature as it currently stands is an omission. With one flawed exception, 77 no study has yet combined detailed neuropsychological inquiry, brain imaging, and genetic determination, an approach that has the potential to pull together various pieces of the puzzle. Answering the question is not only of considerable theoretical importance; it is clinically relevant too. Cognitive dysfunction looms large in the lives of many multiple sclerosis patients. Access to neuropsychological testing and cognitive rehabilitation services is often limited. Predicting who is most at risk may therefore help focus resources where they are most needed.
1. Rao SM, Leo GJ, Bernardin L, et al: Cognitive dysfunction in multiple sclerosis, I: frequency, patterns, and prediction. Neurology 1991; 41:685–691Google Scholar
2. Rao SM, Leo GJ, Ellington L, et al: Cognitive dysfunction in multiple sclerosis, II: impact on employment and social functioning. Neurology 1991; 41:692–696Google Scholar
3. McIntosh-Michaelis SA, Roberts MH, Wilkinson SM, et al: The prevalence of cognitive impairment in a community survey of multiple sclerosis. Br J Clin Psychol 1991; 30(part 4):333–348Google Scholar
4. Chiaravalloti ND, DeLuca J: Cognitive impairment in multiple sclerosis. Lancet Neurol 2008; 7:1139–1151Google Scholar
5. Patti F: Cognitive impairment in multiple sclerosis. Mult Scler 2009; 15:2–8Google Scholar
6. Amato MP, Zipoli V, Portaccio E: Multiple sclerosis-related cognitive changes: a review of cross-sectional and longitudinal studies. J Neurol Sci 2006; 245:41–46Google Scholar
7. Foong J, Rozewicz L, Chong WK, et al: A comparison of neuropsychological deficits in primary and secondary progressive multiple sclerosis. J Neurol 2000; 247:97–101Google Scholar
8. Amato MP, Zipoli V, Goretti B, et al: Benign multiple sclerosis: cognitive, psychological and social aspects in a clinical cohort. J Neurol 2006; 253:1054–1059Google Scholar
9. Marsh GG: Disability and intellectual function in multiple sclerosis patients. J Nerv Ment Dis 1980; 168:758–762Google Scholar
10. Rao SM, Glatt S, Hammeke TA, et al: Chronic progressive multiple sclerosis relationship between cerebral ventricular size and neuropsychological impairment. Arch Neurol 1985; 42:678–682Google Scholar
11. Wishart HA, Flashman L, Saykin AJ: The neuropsychology of multiple sclerosis: contributions of neuroimaging research. Curr Psychiatry Rep 2001; 3:373–378Google Scholar
12. Tartaglia MC, Arnold DL: The role of MRS and fMRI in multiple sclerosis. Adv Neurol 2006; 98:185–202Google Scholar
13. Rovaris M, Comi G, Filippi M: MRI markers of destructive pathology in multiple sclerosis-related cognitive dysfunction. J Neurol Sci 2006; 245:111–116Google Scholar
14. Mainero C, Pantano P, Caramia F, et al: Brain reorganization during attention and memory tasks in multiple sclerosis: insights from functional MRI studies. J Neurol Sci 2006; 245:93–98Google Scholar
15. Dineen RA, Vilisaar J, Hlinka J, et al: Disconnection as a mechanism for cognitive dysfunction in multiple sclerosis. Brain 2009; 132:239–249Google Scholar
16. Bonzano L, Pardini M, Mancardi GL, et al: Structural connectivity influences brain activation during PVSAT in multiple sclerosis. Neuroimage 2009; 44:9–15Google Scholar
17. Mesaros S, Rovaris M, Pagani E, et al: A magnetic resonance imaging voxel-based morphometry study of regional gray matter atrophy in patients with benign multiple sclerosis. Arch Neurol 2008; 65:1223–1230Google Scholar
18. Sicotte NL, Kern KC, Giesser BS, et al: Regional hippocampal atrophy in multiple sclerosis. Brain 2008; 131:1134–1141Google Scholar
19. Warlop NP, Achten E, Debruyne J, Vingerhoets G: Diffusion weighted callosal integrity reflects interhemispheric communication efficiency in multiple sclerosis. Neuropsychologia 2008; 46:2258–2264Google Scholar
20. Houtchens MK, Benedict RH, Killiany R, et al: Thalamic atrophy and cognition in multiple sclerosis. Neurology 2007; 69:1213–1223Google Scholar
21. Amato MP, Portaccio E, Goretti B, et al: Association of neocortical volume changes with cognitive deterioration in relapsing-remitting multiple sclerosis. Arch Neurol 2007; 64:1157–1161Google Scholar
22. Tekok-Kilic A, Benedict RH, Weinstock-Guttman B, et al: Independent contributions of cortical gray matter atrophy and ventricle enlargement for predicting neuropsychological impairment in multiple sclerosis. Neuroimage 2007; 36:1294–1300Google Scholar
23. Mahley RW, Rall SC Jr: Apolipoprotein E: far more than a lipid transport protein. Annu Rev Genomics Hum Genet 2000; 1:507–537Google Scholar
24. Zhong N, Weisgraber KH: Understanding the association of apolipoprotein E4 with Alzheimer’s disease: clues from its structure. J Biol Chem 2009; 284:6027–6031Google Scholar
25. Corder EH, Saunders AM, Strittmatter WJ, et al: Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 1993; 261:921–923Google Scholar
26. Weisgraber KH, Mahley RW: Human apolipoprotein E: the Alzheimer’s disease connection. FASEB J 1996; 10:1485–1494Google Scholar
27. Ashford JW, Mortimer JA: Non-familial Alzheimer’s disease is mainly due to genetic factors. J Alzheimers Dis 2002; 4:169–177Google Scholar
28. Raber J, Huang Y, Ashford JW: APOE genotype accounts for the vast majority of AD risk and AD pathology. Neurobiol Aging 2004; 25:641–650Google Scholar
29. Cherbuin N, Leach LS, Christensen H, et al: Neuroimaging and APOE genotype: a systematic qualitative review. Dement Geriatr Cogn Disord 2007; 24:348–362Google Scholar
30. Filippini N, Rao A, Wetten S, et al: Anatomically-distinct genetic associations of APOE epsilon4 allele load with regional cortical atrophy in Alzheimer’s disease. Neuroimage 2009; 44:724–728Google Scholar
31. Smith GE, Bohac DL, Waring SC, et al: Apolipoprotein E genotype influences cognitive phenotype in patients with Alzheimer’s disease but not in healthy control subjects. Neurology 1998; 50:355–362Google Scholar
32. Deary IJ, Whiteman MC, Pattie A, et al: Cognitive change and the APOE epsilon 4 allele. Nature 2002; 418:932Google Scholar
33. Small BJ, Rosnick CB, Fratiglioni L, et al: Apolipoprotein E and cognitive performance: a meta-analysis. Psychol Aging 2004; 19:592–600Google Scholar
34. Flory JD, Manuck SB, Ferrell RE, et al: Memory performance and the apolipoprotein E polymorphism in a community sample of middle-aged adults. Am J Med Genet 2000; 96:707–711Google Scholar
35. Bartzokis G, Lu PH, Geschwind DH, et al: Apolipoprotein E genotype and age-related myelin breakdown in healthy individuals: implications for cognitive decline and dementia. Arch Gen Psychiatry 2006; 63:63–72Google Scholar
36. Wishart HA, Saykin AJ, McAllister TW, et al: Regional brain atrophy in cognitively intact adults with a single APOE epsilon4 allele. Neurology 2006; 67:1221–1224Google Scholar
37. Chen K, Reiman EM, Alexander GE, et al: Correlations between apolipoprotein E epsilon4 gene dose and whole brain atrophy rates. Am J Psychiatry 2007; 164:916–921Google Scholar
38. Burggren AC, Zeineh MM, Ekstrom AD, et al: Reduced cortical thickness in hippocampal subregions among cognitively normal apolipoprotein E e4 carriers. Neuroimage 2008; 41:1177–1183Google Scholar
39. Nilsson LG, Nyberg L, Backman L: Genetic variation in memory functioning. Neurosci Biobehav Rev 2002; 26:841–848Google Scholar
40. Savitz J, Solms M, Ramesar R: Apolipoprotein E variants and cognition in healthy individuals: a critical opinion. Brain Res Rev 2006; 51:125–135Google Scholar
41. Jiang Q, Lee CY, Mandrekar S, et al: APOE promotes the proteolytic degradation of Abeta. Neuron 2008; 58:681–693Google Scholar
42. Poirier J: Apolipoprotein E represents a potent gene-based therapeutic target for the treatment of sporadic Alzheimer’s disease. Alzheimers Dement 2008; 4:S91–97Google Scholar
43. Tukhovskaya EA, Yukin AY, Khokhlova ON, et al: COG1410, a novel apolipoprotein-E mimetic, improves functional and morphological recovery in a rat model of focal brain ischemia. J Neurosci Res 2009; 87:677–682Google Scholar
44. Tzourio C, Arima H, Harrap S, et al: APOE genotype, ethnicity, and the risk of cerebral hemorrhage. Neurology 2008; 70:1322–1328Google Scholar
45. Hopkins RO, Weaver LK, Valentine KJ, et al: Apolipoprotein E genotype and response of carbon monoxide poisoning to hyperbaric oxygen treatment. Am J Respir Crit Care Med 2007; 176:1001–1006Google Scholar
46. Rao R, Tah V, Casas JP, et al: Ischemic stroke subtypes and their genetic basis: a comprehensive meta-analysis of small and large vessel stroke. Eur Neurol 2009; 61:76–86Google Scholar
47. Lane R, He Y, Morris C, et al: BuChE-K and APOE epsilon4 allele frequencies in Lewy body dementias, and influence of genotype and hyperhomocysteinemia on cognitive decline. Mov Disord 2009; 24:392–400Google Scholar
48. Ezquerra M, Campdelacreu J, Gaig C, et al: Lack of association of APOE and tau polymorphisms with dementia in Parkinson’s disease. Neurosci Lett 2008; 448:20–23Google Scholar
49. Zetterberg H, Jacobsson J, Rosengren L, et al: Association of APOE with age at onset of sporadic amyotrophic lateral sclerosis. J Neurol Sci 2008; 273:67–69Google Scholar
50. Allen NC, Bagade S, McQueen MB, et al: Systematic meta-analyses and field synopsis of genetic association studies in schizophrenia: the SzGene database. Nat Genet 2008; 40:827–834Google Scholar
51. Zhou W, Xu D, Peng X, et al: Meta-analysis of APOE4 allele and outcome after traumatic brain injury. J Neurotrauma 2008; 25:279–290Google Scholar
52. Burwick RM, Ramsay PP, Haines JL, et al: APOE epsilon variation in multiple sclerosis susceptibility and disease severity: some answers. Neurology 2006; 66:1373–1383Google Scholar
53. Pinholt M, Frederiksen JL, Christiansen M: The association between apolipoprotein E and multiple sclerosis. Eur J Neurol 2006; 13:573–580Google Scholar
54. Oliveri RL, Cittadella R, Sibilia G, et al: APOE and risk of cognitive impairment in multiple sclerosis. Acta Neurol Scand 1999; 100:290–295Google Scholar
55. Parmenter BA, Denney DR, Lynch SG, et al: Cognitive impairment in patients with multiple sclerosis: association with the APOE gene and promoter polymorphisms. Mult Scler 2007; 13:25–32Google Scholar
56. Savettieri G, Messina D, Andreoli V, et al: Gender-related effect of clinical and genetic variables on the cognitive impairment in multiple sclerosis. J Neurol 2004; 251:1208–1214Google Scholar
57. Benedict RH, Cookfair D, Gavett R, et al: Validity of the minimal assessment of cognitive function in multiple sclerosis (MACFIMS). J Int Neuropsychol Soc 2006; 12:549–558Google Scholar
58. Feinstein A: Cognitive impairment in multiple sclerosis, in The Clinical Neuropsychiatry of Multiple Sclerosis, 2nd ed. Feinstein A. New York, Cambridge University Press, 2007, pp 115–144Google Scholar
59. Koutsis G, Panas M, Giogkaraki E, et al: APOE epsilon4 is associated with impaired verbal learning in patients with MS. Neurology 2007; 68:546–549Google Scholar
60. Rao SM, Cognitive Function Study Group of the National Multiple Sclerosis Society: A Manual for the Brief Repeatable Battery of Neuropsychological Tests in Multiple Sclerosis. Milwaukee, Wi, Medical College of Wisconsin, 1990Google Scholar
61. Shi J, Zhao CB, Vollmer TL, et al: APOE epsilon 4 allele is associated with cognitive impairment in patients with multiple sclerosis. Neurology 2008; 70:185–190Google Scholar
62. Summers M, Fisniku L, Anderson V, et al: Cognitive impairment in relapsing-remitting multiple sclerosis can be predicted by imaging performed several years earlier. Mult Scler 2008; 14:197–204Google Scholar
63. Archibald CJ, Wei X, Scott JN, et al: Posterior fossa lesion volume and slowed information processing in multiple sclerosis. Brain 2004; 127:1526–1534Google Scholar
64. Amato MP, Portaccio E, Goretti B, et al: Association of neocortical volume changes with cognitive deterioration in relapsing-remitting multiple sclerosis. Arch Neurol 2007; 64:1157–1161Google Scholar
65. Houtchens MK, Benedict RH, Killiany R, et al: Thalamic atrophy and cognition in multiple sclerosis. Neurology 2007; 69:1213–1223Google Scholar
66. Brass SD, Benedict RH, Weinstock-Guttman B, et al: Cognitive impairment is associated with subcortical magnetic resonance imaging grey matter T2 hypointensity in multiple sclerosis. Mult Scler 2006; 12:437–444Google Scholar
67. Hildebrandt H, Hahn HK, Kraus JA, et al: Memory performance in multiple sclerosis patients correlates with central brain atrophy. Mult Scler 2006; 12:428–436Google Scholar
68. Sanfilipo MP, Benedict RH, Weinstock-Guttman B, et al: Gray and white matter brain atrophy and neuropsychological impairment in multiple sclerosis. Neurology 2006; 66:685–692Google Scholar
69. Benedict RH, Ramasamy D, Munschauer F, et al: Memory impairment in multiple sclerosis: correlation with deep grey matter and mesial temporal atrophy. J Neurol Neurosurg Psychiatry 2009; 80:201–206Google Scholar
70. Mineev KK, Prakhova LN, Il’ves AG, et al: Characteristics of neurological and cognitive status in patients with multiple sclerosis in relation to the location and volumes of demyelination foci and the severity of brain atrophy. Neurosci Behav Physiol 2009; 39:35–38Google Scholar
71. Lin X, Tench CR, Morgan PS, Constantinescu CS: Use of combined conventional and quantitative MRI to quantify pathology related to cognitive impairment in multiple sclerosis. J Neurol Neurosurg Psychiatry 2008; 79:437–441Google Scholar
72. Rashid W, Miller DH: Recent advances in neuroimaging of multiple sclerosis. Semin Neurol 2008; 28:46–55Google Scholar
73. Tekok-Kilic A, Benedict RH, Weinstock-Guttman B, et al: Independent contributions of cortical gray matter atrophy and ventricle enlargement for predicting neuropsychological impairment in multiple sclerosis. Neuroimage 2007; 36:1294–1300Google Scholar
74. Bermel RA, Bakshi R: The measurement and clinical relevance of brain atrophy in multiple sclerosis. Lancet Neurol 2006; 5:158–170Google Scholar
75. Ranjeva JP, Audoin B, Au Duong MV, et al: Structural and functional surrogates of cognitive impairment at the very early stage of multiple sclerosis. J Neurol Sci 2006; 245:161–167Google Scholar
76. Rovaris M, Comi G, Filippi M: MRI markers of destructive pathology in multiple sclerosis-related cognitive dysfunction. J Neurol Sci 2006; 245:111–116Google Scholar
77. Lanzillo R, Prinster A, Scarano V, et al: Neuropsychological assessment, quantitative MRI and APOE gene polymorphisms in a series of MS patients treated with IFN beta-1b. J Neurol Sci 2006; 245:141–145Google Scholar
78. Fazekas F, Strasser-Fuchs S, Schmidt H, et al: Apolipoprotein E genotype related differences in brain lesions of multiple sclerosis. J Neurol Neurosurg Psychiatry 2000; 69:25–28Google Scholar
79. van Walderveen MA, Kamphorst W, Scheltens P, et al: Histopathologic correlate of hypointense lesions on T1-weighted spin-echo MRI in multiple sclerosis. Neurology 1998; 50:1282–1288Google Scholar
80. van Walderveen MA, Truyen L, van Oosten BW, et al: Development of hypointense lesions on T1-weighted spin-echo magnetic resonance images in multiple sclerosis: relation to inflammatory activity. Arch Neurol 1999; 56:345–351Google Scholar
81. van Walderveen MA, Barkhof F, Pouwels PJ, et al: Neuronal damage in T1-hypointense multiple sclerosis lesions demonstrated in vivo using proton magnetic resonance spectroscopy. Ann Neurol 1999; 46:79–87Google Scholar
82. Fisher E, Chang A, Fox RJ, et al: Imaging correlates of axonal swelling in chronic multiple sclerosis brains. Ann Neurol 2007; 62:219–228Google Scholar
83. Enzinger C, Ropele S, Strasser-Fuchs S, et al: Lower levels of N-acetylaspartate in multiple sclerosis patients with the apolipoprotein E epsilon4 allele. Arch Neurol 2003; 60:65–70Google Scholar
84. Enzinger C, Ropele S, Smith S, et al: Accelerated evolution of brain atrophy and “black holes” in MS patients with APOE-epsilon 4. Ann Neurol 2004; 55:563–569Google Scholar
85. De Stefano N, Bartolozzi ML, Nacmias B, et al: Influence of apolipoprotein E epsilon4 genotype on brain tissue integrity in relapsing-remitting multiple sclerosis. Arch Neurol 2004; 61:536–540Google Scholar
86. Schreiber K, Otura AB, Ryder LP, et al: Disease severity in Danish multiple sclerosis patients evaluated by MRI and three genetic markers (HLA-DRB1*1501, CCR5 deletion mutation, apolipoprotein E). Mult Scler 2002; 8:295–298Google Scholar
87. Zwemmer JN, van Veen T, van Winsen L, et al: No major association of APOE genotype with disease characteristics and MRI findings in multiple sclerosis. Mult Scler 2004; 10:272–277Google Scholar
88. Zakrzewska-Pniewska B, Styczynska M, Podlecka A, et al: Association of apolipoprotein E and myeloperoxidase genotypes to clinical course of familial and sporadic multiple sclerosis. Mult Scler 2004; 10:266–271Google Scholar
89. Parmenter BA, Weinstock-Guttman B, Garg N, et al: Screening for cognitive impairment in multiple sclerosis using the Symbol Digit Modalities Test. Mult Scler 2007; 13:52–57Google Scholar
90. Benedict RH, Cookfair D, Gavett R, et al: Validity of the minimal assessment of cognitive function in multiple sclerosis (MACFIMS). J Int Neuropsychol Soc 2006; 12:549–558Google Scholar
91. Benedict RH, Fischer JS, Archibald CJ, et al: Minimal neuropsychological assessment of MS patients: a consensus approach. Clin Neuropsychol 2002; 16:381–397Google Scholar
92. Shaw P, Lerch JP, Pruessner JC, et al: Cortical morphology in children and adolescents with different apolipoprotein E gene polymorphisms: an observational study. Lancet Neurol 2007; 6:494–500Google Scholar
93. Reiman EM, Uecker A, Caselli RJ, et al: Hippocampal volumes in cognitively normal persons at genetic risk for Alzheimer’s disease. Ann Neurol 1998; 44:288–291Google Scholar
94. Tohgi H, Takahashi S, Kato E, et al: Reduced size of right hippocampus in 39- to 80-year-old normal subjects carrying the apolipoprotein E epsilon4 allele. Neurosci Lett 1997; 236:21–24Google Scholar
95. Plassman BL, Welsh-Bohmer KA, Bigler ED, et al: Apolipoprotein E epsilon 4 allele and hippocampal volume in twins with normal cognition. Neurology 1997; 48:985–989Google Scholar
96. Schmidt H, Schmidt R, Fazekas F, et al: Apolipoprotein E e4 allele in the normal elderly: neuropsychologic and brain MRI correlates. Clin Genet 1996; 50:293–299Google Scholar
97. Hildebrandt H, Hahn HK, Kraus JA, et al: Memory performance in multiple sclerosis patients correlates with central brain atrophy. Mult Scler 2006; 12:428–436Google Scholar
98. Benedict RH, Weinstock-Guttman B, Fishman I, et al: Prediction of neuropsychological impairment in multiple sclerosis: comparison of conventional magnetic resonance imaging measures of atrophy and lesion burden. Arch Neurol 2004; 61:226–230Google Scholar
99. Sanchez MP, Nieto A, Barroso J, et al: Brain atrophy as a marker of cognitive impairment in mildly disabling relapsing-remitting multiple sclerosis. Eur J Neurol 2008; 15:1091–1099Google Scholar
100. Benedict RH, Bruce JM, Dwyer MG, et al: Neocortical atrophy, third ventricular width, and cognitive dysfunction in multiple sclerosis. Arch Neurol 2006; 63:1301–1306Google Scholar
101. Sanfilipo MP, Benedict RH, Weinstock-Guttman B, et al: Gray and white matter brain atrophy and neuropsychological impairment in multiple sclerosis. Neurology 2006; 66:685–692Google Scholar
102. Bermel RA, Bakshi R: The measurement and clinical relevance of brain atrophy in multiple sclerosis. Lancet Neurol 2006; 5:158–170Google Scholar



