Neuroanatomical Correlates of Depression in Post Traumatic Brain Injury: Preliminary Results of a Pilot Study
Given this high incidence of post-TBI depression, investigators have sought neuropathologic correlates. Levin et al. 6 reported that in 129 mild TBI patients, an abnormal brain computerized tomogram (CT) was associated with a sevenfold increased risk of incident depression within 3 months of TBI. Jorge et al. 7 noted that left dorsolateral frontal and left basal ganglia lesions were associated with major depression in the first year after injury in a cohort of 66 TBI patients. Similarly, in 99 TBI patients, an association of depression with reduced left prefrontal gray matter volumes—especially in the ventrolateral and dorsolateral regions—as well as with deficits in executive function was reported. 8
These studies used structural brain imaging to assess lesion localization and/or regional brain volumes as correlates of presumed neuronal damage. An alternative noninvasive technique which can probe brain biochemistry in vivo is proton magnetic resonance spectroscopy imaging (MRSI) which can measure brain levels of selected neurochemicals that may reflect neuronal viability and integrity and number, including N -acetylaspartate, creatine and phosphocreatine, and choline-containing compounds. 9 Proton MRSI can identify metabolic abnormalities in brain regions with normal appearance on conventional MRI. 9 We have previously shown that a cohort of post-TBI depressed subjects had significantly reduced N -acetylaspartate/choline and N -acetylaspartate/creatine ratios compared to non-TBI, nondepressed healthy comparison subjects in frontal cortex, basal ganglia, and thalamus. 10
We present preliminary results of a pilot study that sought to examine cognitive and neuroanatomical correlates of post-TBI depression using MRSI and volumetric MRI, comparing TBI patients with and without depression.
METHODS
Participants were recruited by advertisements in local newspapers. Subjects 18 years of age or older, with closed head injury, date of TBI between 3 and 60 months prior to the evaluations, no history of diagnosable mood disorder prior to TBI, Mini-Mental State Examination (MMSE) score >18, stable medical history prior to injury, and sufficient cognitive capacity to provide consent were included. The Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-1) was administered to all participants. TBI-depressed patients (cases) were required to meet DSM-IV criteria for a major depressive episode after TBI (296.83; major depressive-like episode) and to have never met the criteria prior to TBI. Nondepressed TBI subjects (comparison subjects) were required to have never met criteria for major depressive episode (296.83).
Neuropsychological Tests
The following neuropsychological tests were administered to all study participants: Verbal fluency (letters S&P) and category (animals & supermarket items), Trail Making Test, Stroop Color and Word Test, Wisconsin Card Sorting Test, Brief Test of Attention, MMSE, National Adult Reading Test, Brief Visuospatial Memory Test—Revised, and the Hopkins Verbal Learning Test—Revised.
MRI Methodology
Two scan acquisitions were used to perform the volumetric analysis: a T1-weighted coronal Fast Field Echo (FFE) acquisition (256×256×124 matrix, 0.9375 mm in plane, 1.5 mm slice thickness, TR/TE=35/6 msec, flip angle of 45°, FOV=24 cm) and an axial Fluid Attenuated Inversion Recovery (FLAIR) acquisition (256×256×50 matrix, 0.9 mm in plane, 3.0 mm slice thickness, TR/TE/TI=11000/140/2725 msec, FOV=23 cm). Brain volumes were extracted using a semiautomatic procedure based on in-house software developed as a plug-in for the Medical Image Processing, Analysis, and Visualization (MIPAV) package. 11 Gray matter, white matter, and CSF were then segmented using an automated clustering analysis. FLAIR images were then registered to the T1-weighted FFE using a rigid-body normalized mutual information cost function. Lesions, defined as regions of enhancement on the FLAIR images, were manually segmented using the MIPAV software package. Talairach lobar definitions were then overlaid on the images using the approach also described by Studholme et al. 12
MRSI Methodology
Proton MRSI was performed using a multislice spin-echo (SE) sequence with outer volume suppression. 13 Three oblique axial slices were acquired with a 15 mm thickness and a gap of 2.5 mm (TR/TE=2,000/280 msec, acquisition matrix 28×28×256, FOV=24 cm). The echo signal was digitized with 256 data points, and the spectral width was 1000 Hz. Water suppression was accomplished with a single chemical shift selective pulse with a bandwidth of 110 Hz. T1-weighted spin echo MR images (TR/TE=400/20 msec, 15 mm slice thickness) were recorded at the same slice locations as the MRSI data set for anatomical correlation. The in-house software “csx” was used to process the MRSI data sets.
Statistical Analysis
The nondepressed and depressed subjects were compared on categorical variables using chi-squared statistics and on continuous variables using Student’s t test. The criterion for statistical significance was set at p<0.05, and for statistical trend at p<0.10. Given the small sample size, our hypothesis testing must be considered preliminary rather than definitive. For this reason we did not correct for multiple comparisons.
RESULTS
Clinical Characteristics
A total of 17 subjects were included in the study. Of these, 10 (59%) were depressed (cases) and seven (41%) were not depressed (comparison subjects). 100% of comparison subjects, but only 40% of cases, had moderate or severe TBI as assessed by the Glasgow Coma Scale. Cases were older than comparison subjects (52.4 versus 27.3 years old, p<0.001), but the two groups did not differ on gender or duration of time since injury.
Cognitive Tests ( Table 1 )
Cases performed significantly worse on part B of the Trail Making test (104.1 seconds versus 60 seconds, p=0.04) and the Brief Visuospatial Memory Test, total recall (i.e., trials 1–3; 15.9 versus 25.7, p=0.03). Cases showed a trend toward worse performance on tests of delayed recall, including HVLT-R (verbal episodic recall; 5.8 versus 9.1, p=0.09) and BVMT-R (visuospatial episodic recall; 7 versus 9.9, p=0.09), but a trend toward better performance on the Wisconsin Card Sorting Test (WCST) categories (3.9 versus 2.1, p=0.07).
 |
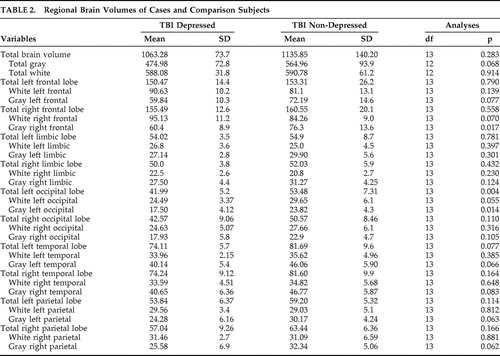
Regional Brain Volumes Measured by MRI ( Table 2 )
Cases had significantly reduced gray matter volume in right frontal (p=0.017) and total left occipital lobes (p=0.004), left occipital lobe white matter (p=0.055), and left occipital lobe gray matter (p=0.014). In addition, cases also had trends toward lower gray matter volume of total brain, left frontal cortex, bilateral temporal cortex, and right parietal cortex.
 |
Magnetic Resonance Spectroscopy Imaging (MRSI)
The only significant difference was noted in the right basal ganglia. Choline/creatine ratio was found to be reduced in cases (mean=1.6, SD=2.0) relative to comparison subjects (mean=2.0, SD=0.43) (p=0.02). N -acetylaspartate/creatine ratio was also reduced in cases (mean=1.7, SD=0.36) relative to comparison subjects (mean=2.2, SD=0.68) (p=0.059).
DISCUSSION
These results suggest a possible role for frontal cortex, temporal lobe, and basal ganglia pathology in post-TBI depression, as well as reduced left occipital volume. This may be due to the known association of frontal and occipital lesions post-TBI, likely due to the contrecoup lesions common in motor vehicle accidents. 14
Post-TBI depression subjects had reduced N -acetylaspartate/creatine ratio (statistical trend) and reduced choline/creatine ratio (statistically significant) in the right basal ganglia. N -acetylaspartate is generally believed to be an indicator of neuronal and axonal integrity. 9 It is reduced following TBI, reflecting diffuse axonal injury or metabolic dysfunction. 9 Choline-containing compounds are involved in cell membrane metabolism; increased choline levels suggest cell membrane breakdown or cell proliferation. 9 Reduced choline in post-TBI patients may suggest absence of cell membrane inflammation, cell membrane repair, or glial cell proliferation. The creatine peak contains both creatine and phosphocreatine, which are involved in cellular energy metabolism. Increased creatine might suggest increased neuronal cell repair or neuronal cell proliferation. It is decreased in conditions associated with cell death. 9 Thus, our observations of reduced N -acetylaspartate/creatine and choline/creatine are suggestive of decreased neuronal health and viability in the basal ganglia.
The cognitive testing is consistent with frontotemporal pathology. Depressed subjects (cases) had slowed psychomotor processing speed and poorer executive functioning (Trail Making test, part B) than comparison subjects, both domains thought to be subsumed by frontal cortex. This is more likely due to brain pathology than major depression itself, since the effect of major depression on set-shifting paradigms (including Trail Making test, part B) is inconsistent at best. 15 Also some studies have noted poor performance on tests of mental flexibility in dysphoric nondepressed patients suggesting that mental inflexibility may be related to the symptom of depressed mood rather than the syndrome of major depression. 16 , 17 TBI is associated with neuronal loss and reduced white matter integrity which can result in dysfunction of neuronal networks and poor performance in cognitive functioning for a prolonged period. 18 , 19 Another explanation, however, could be the increased age in the depressed group. Older age is also associated with increased neuronal loss and reduced white matter integrity, which can reduce cognitive reserve. 20 Immediate recall on the first three trials of the Brief Visuospatial Memory Test can be conceptualized as a test of visual learning likely mediated by the frontal lobes, while delayed recall assesses visual memory mediated primarily by the temporal lobes (although frontal lobe functioning is considered important for retrieval of information that has been adequately encoded and consolidated by mesial temporal lobe structures). Depressed subjects performed worse than comparison subjects on the delayed recall tests of both verbal and visual memory and had reduced right and left gray matter temporal lobe volumes (statistical trend). This is consistent with other studies 21 , 22 that have implicated temporal lobe abnormalities in the pathophysiology of major depression.
An interesting finding, however, was the better performance by the depressed group on the WCST categories. This is probably secondary to the increased severity of brain injury in the comparison group.
In summary, preliminary findings from this pilot study suggest that post-TBI depression is associated with temporal lobe pathology (with concurrent cognitive and MRI findings) but not as clearly with frontal lobe pathology. The finding of basal ganglia pathology on MRSI suggests that MRSI might be complementary to MRI and display different patterns of sensitivity to brain pathology.
This is the first report of proton MRSI findings in TBI subjects with and without new-onset major depression following TBI. Also, this is the first study to replicate the lesion location findings in TBI depression published by Jorge et al. 7 , 8 Limitations include a small sample size, discordance in age and severity of TBI for cases and comparison subjects, limited coverage of temporal lobes, and lack of control for use of psychotropic medications.
CONCLUSION
The preliminary results of our pilot study suggest a possible role of frontotemporal lobes and basal ganglia pathology in depression after TBI.
These results also highlight the usefulness of volumetric MRI and MRSI in determining patterns of brain injury associated with post-TBI depression. Future research should focus on the use of these techniques for both disease detection and response to treatment.
1. National Institute of Neurological Disorders and Stroke: Traumatic brain injury: hope through research. Bethesda, Md, National Institutes of Health, NIH Publication No. 02–158, 2002Google Scholar
2. Deb S, Lyons I, Koutzoukis C, et al: Rate of psychiatric illness 1 year after traumatic brain injury. Am J Psychiatry 1999; 156:374–378Google Scholar
3. Hibbard MR, Uysal S, Kepler K, et al: Axis I psychopathology in individuals with traumatic brain injury. J Head Trauma Rehabil 1998; 13:24–39Google Scholar
4. Marsh NV, Kersel DA, Havill JA, et al: Caregiver burden during the year following severe traumatic brain injury. J Clin Exp Neuropsychol 2002; 24:434–447Google Scholar
5. Inzaghi MG, De Tanti A, Sozzi M: The effects of traumatic brain injury on patients and their families: a follow-up study. Eura Medicophys 2005; 41:265–273Google Scholar
6. Levin HS, McCauley SR, Josic CP, et al: Predicting depression following mild traumatic brain injury. Arch Gen Psychiatry 2005; 62:523–528Google Scholar
7. Jorge R, Robinson R, Arndt S, et al: Comparison between acute and delayed onset depression following traumatic brain injury. J Neuropsychiatry Clin Neurosci 1993; 5:43–49Google Scholar
8. Jorge RE, Robinson RG, Moser D, et al: Major depression following traumatic brain injury. Arch Gen Psychiatry 2004; 61:42–50Google Scholar
9. Brooks WM, Friedman S, Gasparovic C: Magnetic resonance spectroscopy in TBI. J Head Trauma Rehabil 2001; 16:149–164Google Scholar
10. Rao V, Spiro J, Degoankar M, et al: Lesion location in depression post traumatic brain injury using magnetic resonance spectroscopy: preliminary results from a pilot study. Eur J Psychiatry 2006; 20:65–73Google Scholar
11. Bazin PL, Cuzzocreo JL, Yassa MA, et al: Volumetric neuroimage analysis extensions for the MIPAV software package. J Neurosci Methods 2007; 165:111–121Google Scholar
12. Studholme C, Hill DLG, Hawkes DJ: An overlap invariant entropy measure of 3D medical image alignment. Pattern Recognit 1999; 32:71–86Google Scholar
13. Duyn JH, Gillen J, Sobering G, et al: Multisection proton MR spectroscopic imaging of the brain. Radiology 1993; 188:277–282Google Scholar
14. Gerber DJ, Weintraub AH, Cusick CP, et al: MRI of traumatic brain injury: relationship of T2*SE and T2GE to clinical severity and outcome. Brain Inj 2004; 18:1083–1097Google Scholar
15. Debattista C: Executive dysfunction in major depressive disorder. Expert Rev Neurother 2005; 5:79–83Google Scholar
16. Degl’Innocenti A, Agren H, Backman L: Executive deficits in major depression. Acta Psychiatr Scand 1998; 97:182–188Google Scholar
17. Merriam EP, Thase ME, Haas GL, et al: Prefrontal cortical dysfunction in depression determined by Wisconsin Card Sorting Test performance. Am J Psychiatry 1999; 156:780–782Google Scholar
18. Kurca E, Sivák S, Kucera P: Impaired cognitive functions in mild traumatic brain injury patients with normal and pathologic MRI. Neuroradiology 2006; 48:661–669Google Scholar
19. Hoskison MM, Moore AN, Hu B, et al: Persistent working memory dysfunction following traumatic brain injury: evidence for a time-dependent mechanism. Neuroscience 2009; 159:483–491Google Scholar
20. Green RE, Colella B, Christensen B, et al: Examining moderators of cognitive recovery trajectories after moderate to severe traumatic brain injury. Arch Phys Med Rehabil 2008; 89(suppl 12):S16–24Google Scholar
21. Mayberg HS: Frontal lobe dysfunction in secondary depression. J Neuropsychiatry Clin Neurosci 1994; 6:428–442Google Scholar
22. Videbech P: PET measurements of brain glucose metabolism and blood flow in major depressive disorder: a critical review. Acta Psychiatr Scand 2000; 101:11–20Google Scholar



