Herpes Encephalitis in the Immunocompetent Adult: Advances in Neuroimaging
 |
Entry into the nervous system can occur following peripheral infection. 20 Transneuronal spread has been demonstrated for some herpesviruses, as well as infection of multiple cell types within the brain. Symptoms depend upon the location of the infection. Viral infection of the meninges results in aseptic meningitis (meningeal inflammation), characterized by headache, fever and neck stiffness. 20 – 22 Neurological abnormalities (e.g., seizures, cranial nerve palsies) may also be present. The clinical course is much more benign than for bacterial meningitis, and treatment is primarily supportive. Although most cases of aseptic meningitis (>90%) are due to enteroviruses, some cases have been linked to herpesviruses, particularly HSV2. 20 , 21 , 23 Myelitis (inflammation of the spinal cord) may affect both sensory and motor tracts as well as neurons within the spinal cord. 20 , 22 Infection of the gray matter results in acute flaccid paralysis, often without sensory or autonomic symptoms. Infection of the white matter may cause a transverse myelitis, with disturbances in motor, sensory, and autonomic functions. Herpesviruses are more likely to cause myelitis, and prompt antiviral treatment is recommended.
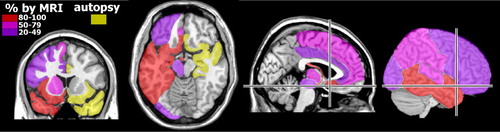
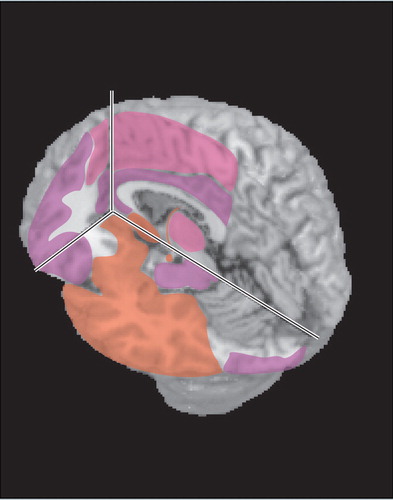
Encephalitis (inflammation of the brain parenchyma) results when brain cells are infected. 20 Common early symptoms include change in consciousness (confusion, then stupor, then coma), fever, headache, and seizures. 20 Behavioral changes are frequently present, and hallucinations have been reported. The predilection of herpesviruses for limbic-related areas (e.g., hippocampal complex, medial temporal cortex, insula, cingulate cortex) ( Figure 1 ) can help to distinguish herpes encephalitis from other etiologies (e.g., Japanese encephalitis). 5 However, cases without medial temporal lobe involvement at presentation have been reported. 24 , 25 Although HSV1 is the most commonly identified agent, multiple reports indicate that the other herpesviruses can cause encephalitis even in immunocompetent adults. 12 , 13 , 20 , 23 , 26 – 29
Clinical history and laboratory investigations, particularly polymerase chain reaction testing of CSF, are the usual basis for diagnosis. 18 , 20 , 21 However, polymerase chain reaction may be negative in the acute stage. 7 , 21 , 30 , 31 This is most commonly a necrotizing encephalitis. Delayed initiation of antiviral treatment when a herpesvirus is the causative agent in a patient presenting with acute symptoms, particularly decreased level of consciousness, is associated with higher mortality and morbidity. 14 , 32
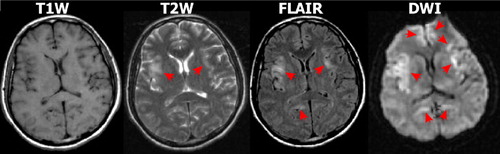
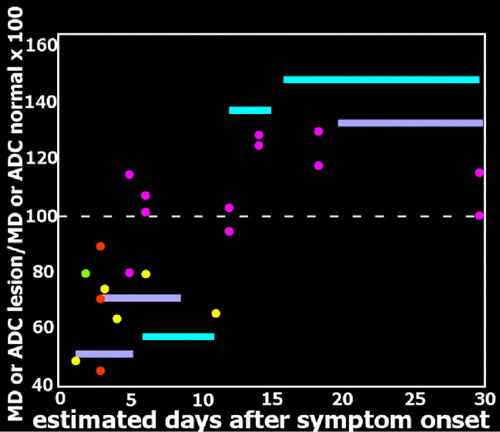
Multiple case reports and series have suggested that diffusion weighted imaging (DWI) MRI may be more sensitive than other imaging modalities in the early acute stage and thus has potential for supporting prompt diagnosis ( Figure 2 ). 5 – 10 , 30 , 33 – 35 In DWI, strong gradient pulses are used to de-phase and rephase the water molecule spins, sensitizing the image to their movement (diffusion) within tissue. Both intracellular and extracellular water movement contribute to imaging appearance. The DWI displays diffusion qualitatively and is also influenced by the T2 of the tissue. Lesions that are bright on T2 weighted images may also be bright on DWI due to “T2 shine through.” It is also possible to calculate the apparent diffusion coefficient (ADC) map, which provides a purer measure. Areas of slow (restricted) diffusion will have high signal on DWI and low signal on the ADC map. Conversely, areas of fast (unrestricted) diffusion will be high signal on the ADC map. The key observation is that the ADC is reduced in lesions in the first few days after symptom onset ( Figure 3 ), resulting in decreased signal intensity on ADC maps and increased signal intensity on DWI, even in the absence of clear changes on T2 weighted (including FLAIR) MRI. 5 , 9 , 30 , 34 At later time points lesions become bright on both T2 weighted MRI and ADC maps ( Figure 3 and Figure 4 ). 5 , 9 , 10 Thus in the acute stage diffusion is below normal (restricted), consistent with presence of cytotoxic edema. 36 , 37 This progresses to above normal (unrestricted) diffusion at later times, consistent with the development of inflammatory vasogenic edema and necrotic processes. 10 , 36 , 37
It has been proposed that the initially reduced diffusion may indicate cellular swelling as a result of viral intrusion/replication. 10 A neuropathological study of patients who died within a week of symptom onset found very high levels of herpes simplex virus antigen (immunoperoxidase technique) in limbic-related areas with relatively little inflammatory reaction. 1 In most cases, structures in one hemisphere were more severely reactive. Antigen levels were slightly lower in patients who died between 8 and 14 days of symptom onset, and levels were more similar between hemispheres. Areas in which necrosis and edema were evident had reduced antigen. Antigen levels were even lower in patients who died between 15 and 19 days of symptom onset, and more similar between hemispheres, while both necrosis and inflammation were more severe and widespread. No antigen was found in patients who died at later times (22 days–3 years) after symptom onset. 1 A similar progression was reported in animal models of herpes encephalitis, with brain viral load increasing to a peak value at 7 days, then gradually decreasing. 38 , 39 It has been reported that lesions within an individual can differ radically in ADC, with some lesions exhibiting slowed diffusion and other lesions exhibiting enhanced diffusion compared to contralateral normal tissue. 8 , 36 An intriguing possibility is that these differences reflect lesions at different pathophysiological stages. Sequential infection of areas as a result of viral propagation may underlie this observed difference, as has been suggested based on postmortem immunohistological mapping of viral antigen. 1
“The impression gained is of a rapidly spreading wave of viral infection within the limbic structures, probably starting on one side of the brain and spreading within it and to the other side, lasting about 3 weeks and leaving in its wake a trail of devastatingly severe necrosis and inflammation of infected parts of the brain.” 1
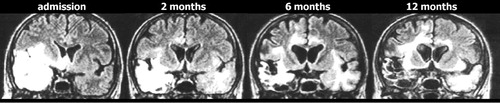
Progressive expansion of lesions on T2 weighted imaging is a common finding in the first few weeks after symptom onset, and is consistent with the expected development of edema, focal hemorrhage and necrosis. Only a few studies have evaluated imaging changes at later times. A few cases consistent with viral reactivation have been reported in both children and adults, with new-onset symptoms and positive polymerase chain reaction. 40 , 41 Clinical relapse in the absence of positive polymerase chain reaction has also been reported. 42 As noted by the authors of that study, the possibility of local viral replication in the absence of viral leakage into the CSF must be considered, but the overall picture is more consistent with immunologically mediated pathogenicity. Although herpes simplex virus antigen was not found in brains of long-term survivors (as noted above), herpes simplex virus DNA has been identified in multiple brain areas in long-term survivors including the midbrain and the medulla. 2 The authors of this study noted that this distribution matched the very widespread distribution of inflammatory infiltrate better than the much more localized destructive lesions (temporal, frontal and insular areas). Persistent widespread inflammation has been reported in an animal model of herpes encephalitis. 39 Although the expected concentration of lesions in limbic-associated areas is the most common MRI finding in patients, the presence of abnormalities in many other areas may reflect inflammatory rather than necrotic processes. 4 , 5
A particularly puzzling pattern has been reported in which clinical symptoms improve but MRI abnormalities continue to progress, with involvement of additional areas including previously uninvolved areas of white matter ( Figure 4 ). 11 , 43 – 45 In some cases this has proven to be a self-limiting process, with eventual full resolution. 44 Although viral reactivation is one possible mechanism, the lack of associated clinical symptoms and negative polymerase chain reaction make this less likely. 45 An alternative hypothesis is that these imaging changes are secondary to an immune-mediated process. 43 A similar phenomenon has been documented in an animal model of herpes encephalitis, with a late progressive increase in MRI findings even with acyclovir treatment. 38 Animals treated with methylprednisolone in addition to acyclovir had greatly reduced MRI findings in the chronic stage, suggesting that adjuvant corticosteroid treatment might be clinically useful. A nonrandomized retrospective study found that addition of corticosteroid treatment was associated with better outcome. 46 This is controversial, however, because of the risk that corticosteroid treatment will increase viral spread. 47 , 48 A multicenter randomized trial is under way that addresses this important issue. 49
The high rate of disability in survivors of herpetic encephalitis supports the need for improved treatment. Although most patients exhibit considerable recovery in cognitive functioning between the acute and chronic stages, long-term impairments are frequently present. 13 – 16 , 32 , 49 , 50 Commonly reported sequelae include epilepsy, memory impairment, personality change, and behavioral abnormalities. 13 , 14 Multiple neuropsychological deficits have been found following HSVE, including anterograde and retrograde amnesia, anomie (naming difficulty), executive deficits, and cognitive decline. 4 , 13 , 16 A recent study suggests that depressive symptoms may be present more frequently than previously thought. 15
As is true in other acquired brain injury populations, therapeutic interventions directed toward remediation and rehabilitation are essential for improving patients’ quality of life in the chronic phase. Recent case reports have described useful approaches to particular issues. Examples include adaptation of cognitive behavior therapy to address changes in identity, use of errorless learning paradigms in memory rehabilitation, written versus pictorial diaries to enhance autobiographical memory, and use of automatic reminder systems to improved independence in tasks of everyday living. 51 – 55 There are indications in the literature that encephalitis due to the various herpesviruses may differ in presentation and prognosis. As screening for a wider range of herpesviruses becomes more common, our understanding of these differences will improve. Future longitudinal neuroimaging studies will be crucial to illuminating pathophysiological progression.
1. Esiri MM: Herpes simplex encephalitis: an immunohistological study of the distribution of viral antigen within the brain. J Neurol Sci 1982; 54:209–226Google Scholar
2. Nicoll JAR, Love S, Kinrade E: Distribution of herpes simplex virus DNA in the brains of human long-term survivors of encephalitis. Neurosci Lett 1993; 157:215–218Google Scholar
3. Corbett D, Harper C: The diversity of presentation of herpes simplex encephalitis. J Clin Neurosci 1994; 1:131–133Google Scholar
4. Kapur N, Barker S, Burrows EH, et al: Herpes simplex encephalitis: long term magnetic resonance imaging and neuropsychological profile. J Neurol Neurosurg Psychiatry 1994; 57:1334–1342Google Scholar
5. Sawlani V: Diffusion-weighted imaging and apparent diffusion coefficient evaluation of herpes simplex encephalitis and Japanese encephalitis. J Neurol Sci 2009; 287:221–226Google Scholar
6. Hatipoglu HG, Sakman B, Yuksel E: Magnetic resonance and diffusion-weighted imaging findings of herpes simplex encephalitis. Herpes 2008; 15:13–17Google Scholar
7. McCabe K, Tyler K, Tanabe J: Diffusion-weighted MRI abnormalities as a clue to the diagnosis of herpes simplex encephalitis. Neurology 2003; 61:1015–1016Google Scholar
8. Heiner L, Demaerel P: Diffusion-weighted MR imaging findings in a patient with herpes simplex encephalitis. Eur J Radiol 2003; 45:195–198Google Scholar
9. Kiroglu Y, Calli C, Yunten N, et al: Diffusion-weighted MR imaging of viral encephalitis. Neuroradiology 2006; 48:875–880Google Scholar
10. Herweh C, Jayachandra MR, Hartmann M, et al: Quantitative diffusion tensor imaging in herpes simplex virus encephalitis. J Neurovirol 2007; 13:426–432Google Scholar
11. Gaviani P, Leone M, Mula M, et al: Progression of MRI abnormalities in herpes simplex encephalitis despite clinical improvement: natural history or disease progression? Neurol Sci 2004; 25:104–107Google Scholar
12. Granerod J, Crowcroft NS: The epidemiology of acute encephalitis. Neuropsychol Rehabil 2007; 17:406–428Google Scholar
13. Hokkanen L, Launes J: Neuropsychological sequelae of acute-onset sporadic viral encephalitis. Neuropsychol Rehabil 2007; 17:450–477Google Scholar
14. McGrath N, Anderson NE, Croxson MC, et al: Herpes simplex encephalitis treated with acyclovir: diagnosis and long term outcome. J Neurol Neurosurg Psychiatry 1997; 63:321–326Google Scholar
15. Fazekas C, Enzinger C, Wallner M, et al: Depressive symptoms following herpes simplex encephalitis: an underestimated phenomenon? Gen Hosp Psychiatry 2006; 28:403–407Google Scholar
16. Pewter SM, Williams WH, Haslam C, et al: Neuropsychological and psychiatric profiles in acute encephalitis in adults. Neuropsychol Rehabil 2007; 17:478–505Google Scholar
17. Kleinschmidt-DeMasters BK, Gilden DH: The expanding spectrum of herpesvirus infections of the nervous system. Brain Pathol 2001; 11:440–451Google Scholar
18. Baringer JR: Herpes simplex infections of the nervous system. Neurol Clin 2008; 26:657–674Google Scholar
19. Chen T, Hudnall SD: Anatomical mapping of human herpesvirus reservoirs of infection. Mod Pathol 2006; 19:726–737Google Scholar
20. Wright EJ, Brew BJ, Wesselingh SL: Pathogenesis and diagnosis of viral infections of the nervous system. Neurol Clin 2008; 26:617–633Google Scholar
21. Tyler KL: Herpes simplex virus infections of the central nervous system: encephalitis and meningitis including Mollaret’s. Herpes 2004; 11:57A–64AGoogle Scholar
22. Irani DN: Aseptic meningitis and viral myelitis. Neurol Clin 2008; 26:635–655Google Scholar
23. Omland LH, Vestergaard BF, Wandall JH: Herpes simplex virus type 2 infections of the central nervous system: a retrospective study of 49 patients. Scand J Infect Dis 2008; 40:59–62Google Scholar
24. Jereb M, Lainscak M, Marin J, et al: Herpes simplex virus infection limited to the brainstem. Wien Klin Wochenschr 2005; 117:495–499Google Scholar
25. Taylor SW, Lee DH, Jackson AC: Herpes simplex encephalitis presenting with exclusively frontal lobe involvement. J Neurovirol 2007; 13:477–481Google Scholar
26. Glaser CA, Honarmand S, Anderson LJ, et al: Beyond viruses: Clinical profiles and etiologies associated with encephalitis. Clin Infect Dis 2006; 43:1565–1577Google Scholar
27. Chadaide Z, Voros E, Horvath S: Epstein-Barr virus encephalitis mimicking clinical and electroencephalographic characteristics of herpes simplex encephalitis. J Med Virol 2008; 80:1930–1932Google Scholar
28. Abul-Kasim K, Palm L, Maly P, et al: The neuroanatomic localization of Epstein-Barr virus encephalitis may be a predictive factor for its clinical outcome: a case report and review of 100 cases in 28 reports. J Child Neurol 2009; 24:720–726Google Scholar
29. Yao K, Honarmand S, Espinosa A, et al: Detection of human herpesvirus-6 in cerebrospinal fluid of patients with encephalitis. Ann Neurol 2009; 65:257–267Google Scholar
30. Akyldz BN, Gumus H, Kumandas S, et al: Diffusion-weighted magnetic resonance is better than polymerase chain reaction for early diagnosis of herpes simplex encephalitis: a case report. Pediatr Emerg Care 2008; 24:377–379Google Scholar
31. Mook-Kanamori B, van de Beek D, Wijdicks EF: Herpes simplex encephalitis with normal initial cerebrospinal fluid examination. J Am Geriatr Soc 2009; 57:1514–1515Google Scholar
32. Raschilas F, Wolff M, Delatour F, et al: Outcome of and prognostic factors for herpes simplex encephalitis in adult patients: results of a multicenter study. Clin Infect Dis 2002; 35:254–260Google Scholar
33. Tsuchiya K, Katase S, Yoshino A, et al: Diffusion-weighted MR imaging of encephalitis. AJR Am J Roentgenol 1999; 173:1097–1099Google Scholar
34. Kuker W, Nagele T, Schmidt F, et al: Diffusion-weighted MRI in herpes simplex encephalitis: a report of three cases. Neuroradiology 2004; 46:122–125Google Scholar
35. Chung SP, You JS, Lee HS: Use of the diffusion-weighted magnetic resonance imaging for early diagnosis of herpes simplex encephalitis in the ED: a case report. Am J Emerg Med 2007; 25:986.e5–986.e6Google Scholar
36. Sener RN: Herpes simplex encephalitis: diffusion MR imaging findings. Comput Med Imaging Graph 2001; 25:391–397Google Scholar
37. Sener RN: Diffusion MRI in Rasmussen’s encephalitis, herpes simplex encephalitis, and bacterial meningoencephalitis. Comput Med Imaging Graph 2002; 26:327–332Google Scholar
38. Meyding-Lamade UK, Oberlinner C, Rau PR, et al: Experimental herpes simplex virus encephalitis: a combination therapy of acyclovir and glucocorticoids reduces long-term magnetic resonance imaging abnormalities. J Neurovirol 2003; 9:118–125Google Scholar
39. Armien AG, Hu S, Little MR, et al: Chronic cortical and subcortical pathology with associated neurological deficits ensuing experimental herpes encephalitis. Brain Pathol 2009 (epub ahead of print)Google Scholar
40. Landau Z, Miller EB, Roif M: Recurrent herpes simplex encephalitis. Eur J Int Med 2005; 16:513–514Google Scholar
41. Spiegel R, Miron D, Yodko H, et al: Late relapse of herpes simplex virus encephalitis in a child due to reactivation of latent virus: clinicopathological report and review. J Child Neurol 2008; 23:344–348Google Scholar
42. Skoldenberg B, Aurelius E, Hjalmarsson A, et al: Incidence and pathogenesis of clinical relapse after herpes simplex encephalitis in adults. J Neurol 2006; 253:163–170Google Scholar
43. Meyding-Lamade UK, Lamade WR, Wildemann BT, et al: Herpes simplex virus encephalitis: chronic progressive cerebral magnetic resonance imaging abnormalities in patients despite good clinical recovery. Clin Infect Dis 1999; 28:148–149Google Scholar
44. Mitsufuji N, Ikuta H: Asymptomatic self-limiting white matter lesions in the chronic phase of herpes simplex encephalitis. Brain Dev 2002; 24:300–303Google Scholar
45. Markoula S, Giannopoulos S, Pelidou S-H, et al: MRI deterioration in herpes simplex encephalitis despite clinical recovery. Neurologist 2009; 15:223–226Google Scholar
46. Kamei S, Sekizawa T, Shiota H, et al: Evaluation of combination therapy using aciclovir and corticosteroid in adult patients with herpes simplex virus encephalitis. J Neurol Neurosurg Psychiatry 2005; 76:1544–1549Google Scholar
47. Openshaw H, Cantin EM: Editorial commentary: corticosteroids in herpes simplex virus encephalitis. J Neurol Neurosurg Psychiatry 2005; 76:1469–1469Google Scholar
48. Steiner I, Budka H, Chaudhuri A, et al: Viral encephalitis: a review of diagnostic methods and guidelines for management. Eur J Neurol 2005; 12:331–343Google Scholar
49. Martinez-Torres F, Menon S, Pritsch M, et al: Protocol for German trial of acyclovir and corticosteroids in herpes-simplex-virus-encephalitis (GACHE): a multicenter, multinational, randomized, double-blind, placebo-controlled German, Austrian and Dutch trial [ISRCTN45122933]. BMC Neurol 2008; 8:40Google Scholar
50. Hokkanen L, Launes J: Cognitive recovery instead of decline after acute encephalitis: a prospective follow up study. J Neurol Neurosurg Psychiatry 1997; 63:222–227Google Scholar
51. Berry E, Kapur N, Williams L, et al: The use of a wearable camera, SenseCam, as a pictoral diary to improve autobiographical memory in a patient with limbic encephalitis: a preliminary report. Neuropsychol Rehabil 2007; 17:582–601Google Scholar
52. Dewar B-K, Gracey F: “Am not was”: cognitive-behavioral therapy for adjustment and identity change following herpes simplex encephalitis. Neuropsychol Rehabil 2007; 17:602–620Google Scholar
53. Emslie H, Wilson BA, Quirk K, et al: Using a paging system in the rehabilitation of encepahalitic patients. Neuropsychol Rehabil 2007; 17:567–581Google Scholar
54. Miotto EC: Cognitive rehabilitation of amnesia after virus encephalitis: a case report. Neuropsychol Rehabil 2007; 17:551–566Google Scholar
55. Dewar B-K, Patterson K, Wilson BA, et al: Re-acquistion of person knowledge in semantic memory disorders. Neuropsychol Rehabil 2009; 19:383–421Google Scholar
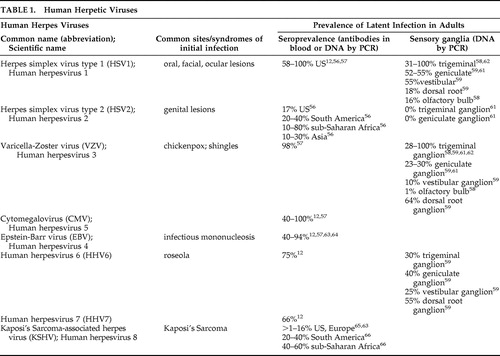
56. Xu F, Sternberg MR, Kottiri BJ, et al: Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA 2006; 296:964–973Google Scholar
57. Wrensch M, Weinberg A, Wiencke J, et al: History of Chickenpox and shingles and prevalence of antibodies to varicella-zoster virus and three other herpesviruses among adults with glioma and controls. Am J Epidemiol 2005; 161:929–938Google Scholar
58. Liedtke W, Opalka B, Zimmermann CW, et al: Age distribution of latent herpes simplex virus 1 and varicella-zoster virus genome in human nervous tissue. J Neurol Sci 1993; 116:6–11Google Scholar
59. Hufner S, Arbusow V, Himmelein S, et al: The prevalence of human herpesvirus 6 in human sensory ganglia and its co-occurrence with alpha-herpesviruses. J Neurovirol 2007; 13:462–467Google Scholar
60. Hill JM, Ball MJ, Neumann DM, et al: The high prevalence of herpes simplex virus type 1: DNA in human trigeminal ganglia is not a function of age or gender. J Virology 2008; 82:8230–8234Google Scholar
61. Richter ER, Dias JK, Gilbert JEI, et al: Distribution of herpes simplex virus type 1 and varicella zoster virus in ganglia of the human head and neck. J Infect Dis 2009; 200:1901–1906Google Scholar
62. Inoue H, Motani-Saitoh H, Sakurada K, et al: Detection of varicella-zoster virus DNA in 414 human trigeminal ganglia from cadavers by the polymerase chain reaction: A comparison of the detection rates of varicella-zoster virus and herpes simplex virus type 1. J Med Virol 2010; 82:345–349Google Scholar
63. Nishiwaki M, Fujimuor M, Teishikata Y, et al: Epidemiology of Epstein-Barr virus, cytomegalovirus, and Kaposi’s Sarcoma-associated herpesvirus infections in peripheral blood leukocytes revealed by a multiplex PCR assay. J Med Virol 2006; 78:1635–1642Google Scholar
64. Higgins CD, Swerdlow AJ, Macsween KF, et al: A study of risk factors for acquisition of Epstein-Barr virus and its subtypes. J Infect Dis 2007; 195:474–482Google Scholar
65. Tedeschi R, Bidoli E, Agren A, et al: Epidemiology of Kaposi’s Sarcoma herpesvirus (HHV8) in Vasterbotten County, Sweden. J Med Virol 2006; 78:372–378Google Scholar
66. Viejo-Bobolla A, Schulz TF: Kaposi’s Sarcoma-associated herpesvirus (KSHV/HHV8): Key aspects of epidemiology and pathogenesis. AIDS Rev 2003; 5:222–229Google Scholar



