Low-Dose Trazodone-Related Drug Eruption
To the Editor: Trazodone is a triazole pyridine derivative antidepressant that was first marketed in the United States in March 1982. Structurally, it is unrelated to tricyclic or tetracyclic antidepressants or to monoamine oxidase inhibitors. Because of its favorable anticholinergic and cardiac side effects, it has always been a popular antidepressant with standard dosages ranging from 250 mg to 600 mg daily. It is also a first-line agent for the treatment of insomnia, with typical initial dosage ranges from 25 mg to 100 mg daily. 1 We report a patient with a rare dermatologic reaction to low-dose trazodone.
Case Report
A 26-year-old man developed depressed mood, insomnia, poor appetite with weight loss, psychomotor retardation, and suicidal ideation after enlistment in late September 2008. He had no previous personal or family history of psychiatric disorders. His medical history and physical examination were also unremarkable. He had his first depressive episode in May 2007, and then this episode remitted spontaneously 5 months later.
The patient was admitted to a psychiatric inpatient ward on October 10, 2008, with an initial diagnosis of recurrent major depressive disorder. On the second day in the hospital, the patient was given the following agents nightly: sertraline, 50 mg; zolpidem, 10 mg; and alprazolam, 0.5 mg. After 10 days in the hospital, his mood improved rapidly. However, his insomnia persisted, and he responded poorly to nightly doses of clonazepam, 4 mg; flurazepam, 30 mg; and low-dose quetiapine, 25 mg. A low dose of trazodone (50 mg at night time) was started on hospital day 19, and then insomnia improved. However, on hospital day 20 he reported an itchy red rash over his anterior chest. On hospital day 21, the rash had become full blown and covered his entire trunk, presternum and back with morbilliform erythematous macules and papules ( Figure 1 ). After a dermatologic consultation, the trazodone was discontinued and an antihistamine was begun. On hospital day 22, no new lesions were noted. By day 24, the maculopapular rash had resolved.
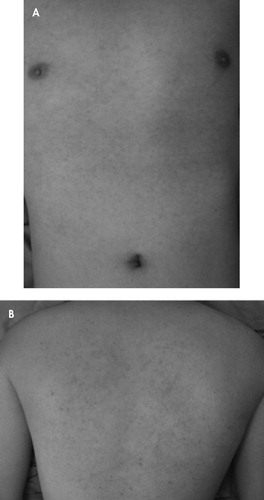
On the third day of taking trazodone, 50 mg daily, the patient developed typical morbilliform erythematous macules and papules over the presternal region (A) and on his back (B).
Discussion
A review of the literature for the period between 1984 and 1986 revealed a total of four cases of dermatologic reactions following use of trazodone. Two cases involved dermatologic eruptions when trazodone was given in daily dosages of 200 mg and 25–50 mg, respectively. 2 , 3 One case of erythema multiforme 4 occurred at a daily dosage of 400 mg, and one case of leukocytoclastic vasculitis 5 was reported at a daily dosage of 350 mg. These dermatologic reactions secondary to trazodone occurred mainly within the regular dosage range given for depression. In our case, the dermatologic reactions occurred after the patient received a much lower dose of trazodone, for alleviating sleep disturbances, and the lesions localized on the presternal region and back. Because of safety issues, we did not use a rechallenge, but the clinical picture and the fact that the lesions resolved after trazodone was discontinued suggest the reaction was a low-dose trazodone-induced eruption.
In addition, there have been no more reports about trazodone-related drug eruptions during the past 20 years. Our experience was a reminder that the common use of trazodone to treat insomnia, even at a low dosage, may lead to drug reactions.
1. Sadock BJ, Kaplan HI, Sadock VA: Kaplan & Sadock’s Synopsis of Psychiatry: Behavioral Sciences/Clinical Psychiatry. Philadelphia, Wolter Kluwer/Lippincott Williams & Wilkins, 2007Google Scholar
2. Cohen LE: Drug eruption secondary to trazodone: a recently introduced antidepressant. J Am Acad Dermatol 1984; 10:303–305Google Scholar
3. Rongioletti F, Rebora A: Drug eruption from trazodone. J Am Acad Dermatol 1986; 14:274–275Google Scholar
4. Ford HE, Jenike MA: Erythema multiforme associated with trazodone therapy: case report. J Clin Psychiatry 1985; 46:294–295Google Scholar
5. Mann SC, Walker MM, Messenger GG, et al: Leukocytoclastic vasculitis secondary to trazodone treatment. J Am Acad Dermatol 1984; 10:669–670Google Scholar