Substance Use Disorders: Do Males and Females Have Differential Vulnerability?
Substance abuse is defined as a problematic pattern of substance use lasting at least 1 year that leads to impaired functioning in at least one major life domain and/or physical or legal consequences. 12 Substance dependence requires presence of at least three additional symptoms (e.g., physical tolerance, withdrawal, or attempts to reduce use). There is not a uniform pathway from substance use to abuse to dependence. Males generally have a higher rate of alcohol abuse and dependence than females, and to a lesser degree a higher rate of drug abuse and dependence. 9 – 11 Amphetamines tend to be abused only slightly more by males than females. 10 The only drug category that women abuse more than men is prescription medications. Women are approximately twice as likely to misuse pain killers or benzodiazepines as men. 10 Of note, comparison of birth cohorts and surveys of adolescents indicate that these differences are decreasing. 13 – 15
Telescoping, the concept that females tend to exhibit adverse effects from substance use more quickly than males, is supported by several lines of evidence. A faster progression from substance use to dependence is a common finding. 9 – 11 , 16 Although women appear to be less likely to enter substance abuse treatment than men, those who seek treatment generally tend to do so after a shorter duration of substance abuse. 9 , 10 , 16 , 17 This appears to be the case for most substances (e.g., alcohol, opioids, cannabis) with the possible exception of cocaine. 17 Women also tend to experience significant negative effects of their substance use (e.g., social, psychological, and physical difficulties) more quickly than men. 9 – 11 , 16 Some studies indicate differences between men and women in the manner in which they enter substance use treatment, with males more likely to attend at the recommendation of the justice system and females more likely to be referred by mental health providers or social service agencies (e.g., child protective services). 10
The relapse rate following substance abuse treatment is 40%–60%. 6 The evidence for differential treatment outcomes based on gender is quite mixed. 9 , 10 , 18 – 21 Some research indicates that both males and females have better outcomes after receiving substance abuse treatment if intervention strategies are tailored to their particular challenges (e.g., psychosocial issues, provides child care) and/or is single gender. 9 , 10 , 18 , 22 – 26
There are multiple factors that potentially complicate research in this area. On average, males have larger bodies. Body mass index (BMI) is also a very important variable. For accurate dose comparisons, consumption must be corrected for distribution volume. A growing body of studies have reported gender-based differences in many aspects of neurobiology (e.g., brain structure, regional neurotransmitter receptor levels, task-related brain activations [including differences by phase of menstrual cycle]), making gender-matched comparisons essential. 27 , 28 An added complication is that males generally have larger brains than females, and scaling of structures may not be linear. 29 , 30 Thus, it may be necessary to match brain size to confirm gender-based differences. The normal aging process is associated with changes in both structure and function that may differ by gender. 31 – 35 Thus age is a potentially confounding variable when examining the impact of substance abuse on brain structure and neuropsychological functioning. It has also been suggested that significant substance use and aging may interact to produce more profound changes in brain structure and functioning. 1 Most studies utilize treatment-seeking populations, although few individuals with substance use disorders enroll in treatment. It has been suggested that significant differences exist between individuals who seek treatment and those who do not (e.g., more severe disorders, greater psychological distress, more significant legal consequences). 36 Additionally, individuals with substance use disorders are likely to have other conditions (e.g., tobacco use disorder, comorbid psychological disorder, head trauma, illness such as HIV/AIDS, disruption of normal sleep cycle, poor nutrition, trauma history) that might alter brain functioning. It is important to not attribute differences in brain anatomy and functioning between individuals with and without substance use disorders solely to the substances used. Many studies exclude individuals with comorbid psychological disorders or dependence on other substances, so findings may not apply to the wider population. Lastly, it is possible that individuals with a family history of substance use disorder have preexisting differences (e.g., smaller overall brain volume, structural differences) compared to individuals without a family history of substance use disorder. 37
The following discussion of the differences between males and females in vulnerability to development of brain changes due to substance use/abuse will focus on alcohol, as this is the most studied substance.
STRUCTURAL IMAGING
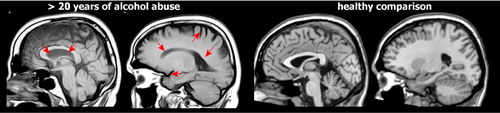
Multiple studies have found reductions in overall volume of both gray matter and white matter as well as increased volume of CSF in individuals with alcohol dependence when compared to individuals without a history of alcohol dependence or abuse ( Figure 1 ). 38 , 39 While these changes are often described as atrophy, sustained periods of sobriety can result in at least partial recovery (e.g., ∼25% over 6 weeks in one study) of brain volumes. 38 – 41 Older studies suggested that females were more susceptible to the deleterious effects of alcohol on the brain than males, with decreased brain volume occurring more quickly and at a lower alcohol consumption. 38 , 39 More recent studies provide insight into some of the factors that may be influential.
Studies in community samples allow investigation of the effects of lower levels of alcohol exposure. A study utilizing the Framingham Offspring cohort (ages 33–88) concluded that even modest alcohol intake was associated with reduced brain volume (adjusted brain volume ∼78.5 for abstainers versus ∼77.2 for group consuming >14 drinks per week), with females more vulnerable than males. 42 A study using older adults (ages 60–64) randomly selected from a community who varied in their use of alcohol found that woman showed similar consumption-related (drinks per week) increases in ventricular volume relative to men, but greater decreases in white matter volume. 43 Neither study indicated the number of participants that identified themselves as having an alcohol use disorder (AUD) or included information about participants’ substance abuse treatment history. In contrast, a study of individuals with moderate alcohol use that excluded any with a personal or family history of AUD found that neither current nor lifetime intake was related to reduced brain or increased CSF volumes in either males or females. 44 Mean lifetime alcohol intake (lifetime intake adjusted for duration of use) was related only to increased white matter volume (mainly frontal) and only in males. As noted by the authors, these results suggest moderate alcohol use may have quite different effects from abuse.
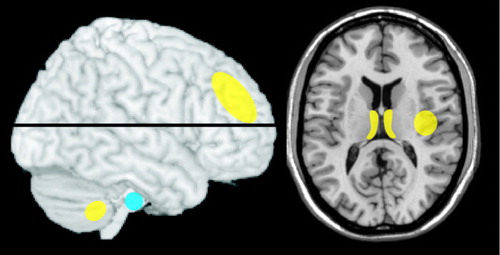
Recent studies of individuals with AUD have utilized a wider variety of populations. One group reported greater reductions in gray matter volume in females than males with AUD (inpatient, abstinent ∼3 weeks) relative to matched (age, sex) healthy comparison subjects, although the female group had a much shorter duration of abuse (8.5±5.7 years versus 14.3±7.5 years) ( Figure 2 ). 2 In a later study the authors controlled for alcohol distribution volume and found that the female group had a higher recent alcohol exposure, suggesting that dose may at least partially account for the differences. 45 Another group reported similar levels of brain atrophy in male and female individuals (global atrophy index, males 87.5% versus females 87.8%) with AUD (inpatient, abstinent median 9 days) relative to matched healthy comparison subjects (global atrophy index, males 91.5% versus females 91.7%). 40 Although the alcohol exposure was similar when adjusted for weight, the female group had a shorter duration of abuse (5.5±4.2 years versus 10.4±5.0), supporting presence of greater vulnerability. A study of non-treatment-seeking individuals found decreased gray matter volume in the heavy (>100 drinks per month, males 39.8% versus females 40.9%) compared to light (<45 drinks per month, males 40.4% versus females 44.6%) alcohol use group, with greater decreases in females, although duration of use and consumption were similar to males. 37 This study did not correct for alcohol distribution volume, so the actual alcohol exposure was likely higher for the females. A subgroup analysis comparing tobacco users to nonusers found significant decreases in gray matter volume only in the group that combined alcohol and tobacco use. 46 A later study from these researchers of non-treatment-seeking individuals with AUD relative to healthy comparison subjects that controlled for both weight and age reported similar decreases in gray matter volume in males and females. 47 In contrast, another research group found significant decreases in gray matter and white matter volumes in male but not female individuals with AUD (outpatient, abstinent 2–15 months) relative to matched healthy comparison subjects ( Figure 2 ). 3 In a later study of infratentorial structures (pons, cerebellum) both males and females with AUD had reduced volume relative to matched healthy comparison subjects, with greater reductions in males than females, although functional deficits (ataxia measures) were similar. 48 A study in adolescents and young adults found decreased cerebellar volume in males but not females with AUD relative to matched (age, sex, handedness) healthy comparison subjects (cerebellar volume: AUD males 141.3 versus healthy comparison males 150.7, AUD females 141.7 versus healthy comparison females 134.7). 49 As noted by the authors, presence of attention-deficit hyperactivity disorder may have contributed to this gender difference. The only other regional changes were decreased prefrontal cortex volumes (gray matter and white matter) in both males and females with AUD relative to matched healthy comparison subjects. In contrast, a study of prefrontal cortex in adolescents found decreased volume in females and increased volume in males with AUD relative to demographically similar healthy comparison subjects. 50 These studies differ on several factors including age range of participants and comorbid conditions. As noted in both studies, disentangling the influences of preexisting vulnerabilities, maturational influences, and toxic effects is not yet possible. These results do suggest that age of onset may be another important factor to consider in adult studies.

Voxel-based morphometry (VBM) has been used to identify focal changes in gray and white matter density related to alcohol exposure. A study comparing females and males with AUD (inpatient, abstinent >10 days) to matched (sex, age) healthy comparison subjects reported multiple areas of decreased gray matter density ( Figure 3 ), but no significant differences by gender. 4 The estimated lifetime doses of alcohol were not indicated, so it is not clear if men and women had comparable drinking histories. As the authors noted, an additional possible limitation was the small number of women included. Several studies have utilized VBM in nonpatient populations, with very mixed results. Two studies investigated individuals with no history of AUD. 51 , 52 One found that mean lifetime alcohol intake was related to focally (Brodmann’s areas 6 and 40) increased white matter and decreased gray matter density in males, but not females. 51 The other found no relationship for either males or females between lifetime alcohol consumption and either global or regional changes in gray matter or white matter density. 52 VBM was used to further examine a cohort of older adults (ages 60–64) randomly selected from a community. 53 A linear association was found for men between current alcohol consumption and focal increases in gray matter density (multiple frontal, parietal, occipital, and temporal lobe areas) as well as decreases in white matter density (superior temporal and parahippocampal areas). Greater vulnerability for women was not supported, as there were no significant associations in women.
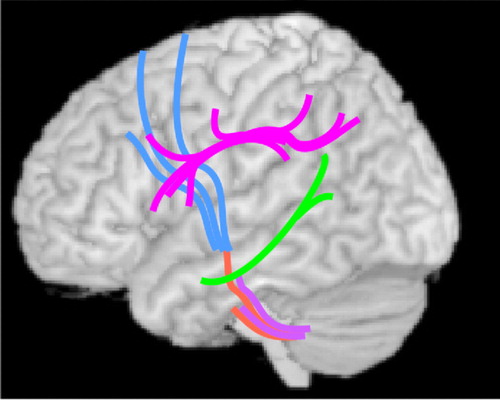
Recently, diffusion tensor imaging (DTI) has been utilized to assess changes in focal white matter integrity related to alcohol exposure. A series of studies from one group indicated that both males and females with AUD have widespread decreases in white matter fractional anisotropy (FA; decreases considered indicative of microstructural injury in adults) and increases in radial diffusivity (indicative of myelin disruption) relative to matched healthy comparison subjects, with anterior and superior areas most affected. 5 , 54 , 55 One study utilized quantitative fiber tracking to assess 11 major white matter bundles. 5 Comparison of subgroups matched on several important variables (alcohol consumption, age, duration of sobriety) indicated greater fractional anisotropy reductions in females than males in portions of multiple fiber tracts ( Figure 4 ). As noted by the authors of this study, healthy comparison females had reduced fractional anisotropy relative to healthy comparison males in many of these areas, suggesting the possibility of a sex-based higher risk for alcohol-related injury. A region of interest study from the same group found that most areas of the corpus callosum had reduced fractional anisotropy and increased radial diffusivity in the AUD group relative to the healthy comparison group. However, no area was differentially affected in matched (alcohol consumption, age, duration of sobriety) subgroups of males and females. 55 This contrasts with a similar study in adolescents with AUD, which found areas with increased fractional anisotropy (rostral body, isthmus) relative to healthy comparison subjects. 56 The authors suggested that this unexpected finding may indicate faster than normal myelination in the prefrontal and temporo-parietal cortex as a risk factor for substance use disorders. However, another study utilizing tract-based spatial statistics did not report differences in the corpus callosum between healthy comparison subjects and adolescents with AUD but identified an area of decreased fractional anisotropy in the superior longitudinal fasciculus. 57
FUNCTIONAL IMAGING
A series of studies from one group has used MRI approaches to explore the influence of gender and alcohol use on the brain. 58 – 60 In a functional MRI (fMRI) study, young adult women (18–25 years) with histories of alcohol dependence (all recruited several years previously from treatment programs) were compared to demographically similar healthy women (nondrinkers or weekend social alcohol use). 58 At the time of imaging, most (7/10) were currently alcohol dependent (primarily weekend binge drinking) and the remainder had been abstinent for longer than 6 months. Between-group comparisons indicated that performance level and task-related activations were quite similar for a simple vigilance task. Performance on a spatial working memory task was impaired in the AUD group (83% versus 91% correct), and there was less task-related activation of parietal and prefrontal regions. Although a lower level of performance can indicate failure to engage resources, a similar difference in activations was also present in subgroups matched on performance. Of note, a measure related to past exposure (experiencing alcohol withdrawal symptoms) correlated with both spatial working memory task performance and neurocognitive testing, whereas measures related to present use (drinks per month, recent use) did not. In a later fMRI study, they compared male and female adolescents with AUD to demographically similar healthy comparison subjects using the same set of tasks. 59 There were no differences in performance accuracy between the groups, although the AUD groups were faster. There were differences in task-related activation by both gender and diagnosis. Overall, a more abnormal activation pattern was found in females with AUD than males, suggesting greater vulnerability to deleterious effects. However, as noted by the authors, the female group also had higher alcohol exposure. Interestingly, in a later study they found decreased resting state perfusion (single slice arterial spin labeling MRI) in parietal and prefrontal regions in a group of young adult women (18–25 years old) with histories of alcohol dependence compared to demographically similar healthy females. 60 Perfusion was not predicted by days since last heavy drinking, suggesting that chronic exposure to alcohol can cause prolonged alteration in regulation of cerebral blood flow. As noted by the authors, this possibility will need to be taken into consideration in interpretation of functional imaging studies that involve blood flow.
There is some evidence that chronic exposure to alcohol impacts GABA-benzodiazepine receptors differently in males and females. 61 Compared with females without a history of heavy alcohol use, abstinent (3 months–14 years) alcohol-dependent females had reduced GABA-benzodiazepine receptor binding in the parietal and occipital cortex and cerebellum. In contrast, alcohol-dependent males had reduced binding in the parietal, the right frontal, and medial frontal cortex when compared with males without a history of heavy alcohol use. As noted by the authors, these findings only reached the trend level, perhaps due to the small sample size.
One study assessed the influence of several factors (e.g., consumption level, consumption pattern, family history) on regional levels of brain metabolites using magnetic resonance spectroscopy (MRS) in nontreatment seeking individuals. 62N -acetylaspartate (NAA, indicator for neurons) was reduced in frontal white matter in heavy compared to light drinkers, suggesting presence of axonal injury. Subgroup analysis indicated the greatest decreases were in females. In contrast, several metabolites indicative of glial or general metabolism (creatinine, choline, myoinositol) were increased in parietal gray matter in heavy compared to light drinkers, suggesting the presence of either gliosis or osmotic changes. Subgroup analysis indicated the greatest increases were in binge drinkers. Metabolite levels correlated with several functional and electrophysiological measures in heavy but not light drinkers. As noted by the authors, these changes are milder than previously found in individuals recovering from AUD, indicating the importance of studying different populations.
The possibility that a binge drinking pattern is injurious even in the short term has been investigated in one longitudinal study that measured emotional valence judgment task-related brain activation as indicated by the auditory event-related potential. 63 First-year college students with no history of drinking (matched on psychological, behavioral, and electrophysiological measures) were paired based on their prediction of alcohol use (high versus low consumption) during the upcoming school year. Retesting 9 months later showed no behavioral differences but delayed latencies for all event-related potential components in the binge drinking group compared to the low consumption group, with no differences by gender. These findings suggest that even short-term binge drinking can result in processing impairments and are consistent with a diffusion tensor imaging study in adolescent subclinical binge drinkers that found multiple small areas in the white matter of reduced fractional anisotropy. 64
CONCLUSION
There appear to be important differences between men and women with regard to substance use disorders, with women exhibiting more overall negative effects than men. Additionally, men and women appear to benefit from substance abuse treatment that is tailored to the specific areas of difficulty they are experiencing. Women in particular appear to have treatment gains when other psychosocial issues are addressed as well as substance abuse. Given the growing evidence for neurobiological differences between men and women, it is not surprising that substances tend to have differential effects. However, the challenges and potential limitations of this research must always be kept in mind. Most studies utilize treatment-seeking populations, so results may not generalize. Studies have also varied considerably in their inclusion of potentially important variables such as age at onset, duration of use, duration of sobriety, present age, alcohol distribution volume, lifetime consumption, tobacco use, psychiatric comorbidities, and medical conditions.
1. Fein G, Shimotsu R, Di Sclafani V, et al: Increased white matter signal hyperintensities in long-term abstinent alcoholics compared with nonalcoholic controls. Alcohol Clin Exp Res 2009; 33:70–78Google Scholar
2. Hommer DW, Momenan R, Kaiser E, et al: Evidence for a gender-related effect of alcoholism on brain volumes. Am J Psychiatry 2001; 158:198–204Google Scholar
3. Pfefferbaum A, Rosenbloom M, Deshmukh A, et al: Sex differences in the effects of alcohol on brain structure. Am J Psychiatry 2001; 158:188–197Google Scholar
4. Mechtcheriakov S, Brenneis C, Egger K, et al: A widespread distinct pattern of cerebral atrophy in patients with alcohol addiction revealed by voxel-based morphometry. J Neurol Neurosurg Psychiatry 2007; 78:610–614Google Scholar
5. Pfefferbaum A, Rosenbloom M, Rohlfing T, et al: Degradation of association and projection white matter systems in alcoholism detected with quantitative fiber tracking. Biol Psychiatry 2009; 65:680–690Google Scholar
6. National Institute on Drug Abuse, National Institutes of Health, US Department of Health and Human Services: Principles of Drug Addiction Treatment: A Research-Based Guide, 2nd ed, revised. Bethesda, Md, NIH, 2009Google Scholar
7. Grant BF, Stinson FS, Dawson DA, et al: Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry 2004; 61:807–816Google Scholar
8. Office of National Drug Control Policy: The Economic Costs of Drug Abuse in the United States, 1992–2002. Washington, DC, Executive Office of the President, 2004Google Scholar
9. Greenfield SF, Brooks AJ, Gordon SM, et al: Substance abuse treatment entry, retention, and outcome in women: a review of the literature. Drug Alcohol Depend 2007; 86:1–21Google Scholar
10. Grella CE: From generic to gender-responsive treatment: changes in social policies, treatment services, and outcomes of women in substance abuse treatment. J Psychoactive Drugs 2008; (suppl 5):327–343Google Scholar
11. Greenfield SF, Back SE, Lawson K, et al: Substance abuse in women. Psychiatr Clin North Am 2010; 33:339–355Google Scholar
12. American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC, American Psychiatric Publishing, 2000Google Scholar
13. Johnston LD, O’Malley PM, Bachman JG, et al: Monitoring the Future: National Results On Adolescent Drug Use. Overview of Key Findings. Bethesda, Md, NIH, 2008Google Scholar
14. Keyes KM, Grant BF, Hasin DS: Evidence for a closing gender gap in alcohol use, abuse, and dependence in the United States population. Drug Alcohol Depend 2008; 93:21–29Google Scholar
15. Keyes KM, Martins SS, Blanco C, et al: Telescoping and gender differences in alcohol dependence: new evidence from two national surveys. Am J Psychiatry 2010 May 3. [Epub ahead of print]Google Scholar
16. Diehl A, Croissant B, Batra A, et al: Alcoholism in women: is it different in onset and outcome compared to men? Eur Arch Psychiatry Clin Neurosci 2007; 257:344–351Google Scholar
17. Hernandez-Avila CA, Rounsaville BJ, Kranzler HR: Opioid-, cannabis- and alcohol-dependent women show more rapid progression to substance abuse treatment. Drug Alcohol Depend 2004; 74:265–272Google Scholar
18. Ashley OS, Marsden ME, Brady TM: Effectiveness of substance abuse treatment programming for women: a review. Am J Drug Alcohol Abuse 2003; 29:19–53Google Scholar
19. Green CA, Polen MR, Lynch FL, et al: Gender differences in outcomes in an HMO-based substance abuse treatment program. J Addict Dis 2004; 23:47–70Google Scholar
20. Darke S, Ross J, Mills KL, et al: Patterns of sustained heroin abstinence amongst long-term dependent heroin users: 36 months findings from the Australian Treatment Outcome Study (ATOS). Addict Behav 2007; 32:1897–1906Google Scholar
21. Suh JJ, Pettinati HM, Kampman KM, et al: Gender differences in predictors of treatment attrition with high dose naltrexone in cocaine and alcohol dependence. Am J Addict 2008; 17:463–468Google Scholar
22. Haver B, Dahlgren L, Willander A: A 2-year follow-up of 120 Swedish female alcoholics treated early in their drinking career: prediction of drinking outcome. Alcohol Clin Exp Res 2001; 25:1586–1593Google Scholar
23. Campbell CI, Alexander JA, Lemak CH: Organizational determinants of outpatient substance abuse treatment duration in women. J Subst Abuse Treat 2009; 37:64–72Google Scholar
24. McCrady BS, Epstein EE, Cook S, et al: A randomized trial of individual and couple behavioral alcohol treatment for women. J Consult Clin Psychol 2009; 77:243–256Google Scholar
25. Greif GL: One dozen considerations when working with men in substance abuse groups. J Psychoactive Drugs 2009; 41:387–390Google Scholar
26. Golder S, Logan TK: Lifetime victimization and psychological distress: cluster profiles of out of treatment drug-involved women. Violence Vict 2010; 25:62–83Google Scholar
27. Cahill L: Why sex matters for neuroscience. Nat Rev Neurosci 2006; 7:477–484Google Scholar
28. Cosgrove KP, Mazure CM, Staley JK: Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry 2007; 62:847–855Google Scholar
29. Leonard CM, Towler S, Welcome S, et al: Size matters: cerebral volume influences sex differences in neuroanatomy. Cereb Cortex 2008; 18:2920–2931Google Scholar
30. Luders E, Gaser C, Narr KL, et al: Why sex matters: brain size independent differences in gray matter distributions between men and women. J Neurosci 2009; 29:14265–14270Google Scholar
31. Sowell ER, Peterson BS, Kan E, et al: Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cereb Cortex 2007; 17:1550–1560Google Scholar
32. Kim I-J, Kim S-J, Kim Y-K: Age- and sex-associated changes in cerebral glucose metabolism in normal healthy subjects: statistical parametric mapping analysis of F-18 fluorodeoxyglucose brain positron emission tomography. Acta Radiol 2009; 50:1169–1174Google Scholar
33. Salat DH, Lee SY, van der Kouwe AJ, et al: Age-associated alterations in cortical gray and white matter signal intensity and gray to white matter contrast. Neuroimage 2009; 48:21–28Google Scholar
34. Taki Y, Kinomura S, Sato K, et al: A longitudinal study of gray matter volume decline with age and modifying factors. Neurobiol Aging 2009 Jun 2. [Epub ahead of print]Google Scholar
35. Riddle WR, DonLevy SC, Lee H: Modeling brain tissue volumes over the lifespan: quantitative analysis of postmortem weights and in vivo MR images. Magn Reson Imaging 2010; 28:716–720Google Scholar
36. Fein G, Di Sclafani V, Cardenas VA, et al: Cortical gray matter loss in treatment-naive alcohol dependent individuals. Alcohol Clin Exp Res 2002; 26:558–564Google Scholar
37. Cardenas VA, Studholme C, Meyerhoff DJ, et al: Chronic active heavy drinking and family history of problem drinking modulate regional brain tissue volumes. Psychiat Res: Neuroimaging 2005; 138:115–130Google Scholar
38. Moselhy HF, Georgiou G, Kahn A: Frontal lobe changes in alcoholism: a review of the literature. Alcohol Alcohol 2001; 36:357–368Google Scholar
39. Hommer DW: Male and female sensitivity to alcohol-induced brain damage. Alcohol Res Health 2003; 27:181–185Google Scholar
40. Mann K, Ackermann K, Croissant B, et al: Neuroimaging of gender differences in alcohol dependence: are women more vulnerable? Alcohol Clin Exp Res 2005; 29:896–901Google Scholar
41. Cardenas VA, Studholme C, Gazdzinski S, et al: Deformation-based morphometry of brain changes in alcohol dependence and abstinence. Neuroimage 2007; 34:879–887Google Scholar
42. Paul CA, Au R, Fredman L, et al: Association of alcohol consumption with brain volume in the Framingham study. Arch Neurol 2008; 65:1363–1367Google Scholar
43. Anstey KJ, Jorm AF, Reglade-Meslin C, et al: Weekly alcohol consumption, brain atrophy, and white matter hyperintensities in a community-based sample aged 60 to 64 years. Psychosom Med 2006; 68:778–785Google Scholar
44. de Bruin EA, Pol HEH, Bijl S, et al: Associations between alcohol intake and brain volumes in male and female moderate drinkers. Alcohol Clin Exp Res 2005; 29:656–663Google Scholar
45. Agartz I, Shoaf S, Rawlings RR, et al: CSF monoamine metabolites and MRI brain volumes in alcohol dependence. Psychiat Res: Neuroimaging 2003; 122:21–35Google Scholar
46. Durazzo TC, Cardenas VA, Studholme C, et al: Non-treatment-seeking heavy drinkers: effects of chronic cigarette smoking on brain structure. Drug Alcohol Depend 2007; 87:76–82Google Scholar
47. Fein G, Shimotsu R, Barakos J: Age-related gray matter shrinkage in a treatment naive actively drinking alcohol-dependent sample. Alcohol Clin Exp Res 2010; 34:175–182Google Scholar
48. Sullivan EV, Rohlfing T, Pfefferbaum A: Pontocerebellar volume deficits and ataxia in alcoholic men and women: no evidence for “telescoping.” Psychopharmacology 2010; 208:279–290Google Scholar
49. De Bellis MD, Narasimhan A, Thatcher DL, et al: Prefrontal cortex, thalamus, and cerebellar volumes in adolescents and young adults with adolescent-onset alcohol use disorders and comorbid mental disorders. Alcohol Clin Exp Res 2005; 29:1590–1600Google Scholar
50. Medina KL, McQueeny T, Nagel BJ, et al: Prefrontal cortex volumes in adolescents with alcohol use disorders: unique gender effects. Alcohol Clin Exp Res 2008; 32:386–394Google Scholar
51. de Bruin EA, Pol HEH, Schnack HG, et al: Focal brain matter differences associated with lifetime alcohol intake and visual attention in male but not female non-alcohol-dependent drinkers. Neuroimage 2005; 26:536–545Google Scholar
52. Sasaki H, Abe O, Yamasue H, et al: Structural and diffusional brain abnormality related to relatively low level alcohol consumption. Neuroimage 2009; 46:505–510Google Scholar
53. Sachdev PS, Chen X, Wen W, et al: Light to moderate alcohol use is associated with increased cortical gray matter in middle-aged men: a voxel-based morphometric study. Psychiatry Res 2008; 163:61–69Google Scholar
54. Pfefferbaum A, Adalsteinsson E, Sullivan EV: Supratentorial profile of white matter microstructural integrity in recovering alcoholic men and women. Biol Psychiatry 2006; 59:364–372Google Scholar
55. Pfefferbaum A, Rosenbloom MJ, Fama R, et al: Transcallosal white matter degradation detected with quantitative fiber tracking in alcoholic men and women: selective relations to dissociable functions. Alcohol Clin Exp Res 2010; 34:1–11Google Scholar
56. De Bellis MD, Van Voorhees E, Hoooper SR, et al: Diffusion tensor measures of the corpus callosum in adolescents with adolescent-onset alcohol use disorders. Alcohol Clin Exp Res 2008; 32:395–404Google Scholar
57. Thatcher DL, Pajtek S, Chung T, et al: Gender differences in the relationship between white matter organization and adolescent substance use disorders. Drug Alcohol Depend 2010; 110:55–61Google Scholar
58. Tapert SF, Brown GG, Kindermann SS, et al: fMRI measurement of brain dysfunction in alcohol-dependent young women. Alcohol Clin Exp Res 2001; 25:236–245Google Scholar
59. Caldwell LC, Schweinsburg AD, Nagel BJ, et al: Gender and adolescent alcohol use disorders on BOLD (blood oxygen level dependent) response to spatial working memory. Alcohol Alcohol 2005; 40:194–200Google Scholar
60. Clark CP, Brown GG, Eyler LT, et al: Decreased perfusion in young alcohol-dependent women as compared with age-matched controls. Am J Drug Alcohol Abuse 2007; 33:13–19Google Scholar
61. Lingford-Hughes AR, Acton PD, Gacinovic S, et al: Levels of gamma-aminobutyric acid-benzodiazepine receptors in abstinent, alcohol-dependent women: preliminary findings from a 123I-iomazenil single photon emission tomography study. Alcohol Clin Exp Res 2000; 24:1449–1455Google Scholar
62. Meyerhoff DJ, Blumenfeld R, Truran D, et al: Effects of heavy drinking, binge drinking, and family history of alcoholism on regional brain metabolites. Alcohol Clin Exp Res 2004; 28:650–661Google Scholar
63. Maurage P, Pesenti M, Philippot P, et al: Latent deleterious effects of binge drinking over a short period of time revealed only by electrophysiological measures. J Psychiatry Neurosci 2009; 34:111–118Google Scholar
64. McQueeny T, Schweinsburg BC, Schweinsburg AD, et al: Altered white matter integrity in adolescent binge drinkers. Alcohol Clin Exp Res 2009; 33:1278–1285Google Scholar