Transcutaneous Electrical Stimulation (TENS) for Psychogenic Movement Disorders
Abstract
Psychogenic movement disorders (PMDs) often result in disability and diminished quality of life, yet medical therapies are presently limited and largely ineffective. On the basis of previous reports that transcutaneous electrical nerve stimulation (TENS) is helpful for certain patients with organic movement disorders, the authors studied the effects of TENS in 19 patients with PMDs, utilizing the Psychogenic Movement Disorder Rating Scale (PMDRS) as well as patient-rated assessments of PMD magnitude, persistence, and disability. The PMDRS Severity score significantly improved after a mean follow-up of 6.9 months, and short duration of PMD was found to be the only identifiable predictor of a favorable outcome. Although the tingling sensation produced by TENS makes it poorly suited for a controlled clinical trial, the device has a favorable side-effect profile and is an acceptable palliative treatment for a subset of PMD patients.
Psychogenic movement disorders (PMDs) include various abnormalities of motor functioning that are not explained by an identifiable neurological disease and that appear to result from an underlying psychological illness. Although PMDs are not included within APA Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR),1 many patients with PMDs meet diagnostic criteria for a somatoform disorder, such as conversion or somatization disorder. About 3%–4% of patients seen in subspecialty movement disorder clinics have a PMD;2–4 yet, little is known regarding their pathophysiology, and the absence of a defined neurobiology has slowed progress in PMD diagnosis, prognostication, and treatment. Indeed, at present, there are no proven therapies for PMDs, and patients often suffer morbidity related to unnecessary and costly medical and surgical interventions targeted toward “organic” disorders.5,6 Since PMDs result in levels of disability and impaired quality of life similar to neurodegenerative disease,7 more effective therapies are needed.
Transcutaneous electrical nerve stimulation (TENS) devices emit low-voltage electrical currents to the skin and are widely used to treat acute and chronic pain related to various conditions, including musculoskeletal diseases, neuropathy, surgery, and childbirth.8,9 Although the mechanism of action and optimal parameters of TENS are not established, some data suggest that TENS modulates both sensory and motor transmission within the CNS.10–12 In light of its potential to modify motor-cortical excitability, TENS has been investigated in patients with several movement disorders, including idiopathic dystonia,13–15 tremor,16 propriospinal myoclonus,17,18 posttraumatic dystonia,19 and “belly dancer's dyskinesia.”20 Although not all patients benefited from TENS,16 some, particularly those with dystonia, did improve.13–15,17,19,20 In the present study, we assessed the efficacy of TENS in patients with probable or clinically-established PMDs.21 The rationale for the study was partly based on the fact that, in several patients with TENS-responsive movement disorders, a psychogenic etiology was considered;17,19,20 these include two recently reported patients who meet diagnostic criteria for a PMD.18 Furthermore, since transcranial magnetic stimulation (TMS) studies have shown that psychogenic and organic dystonia exhibit similar neurophysiological abnormalities, as compared with controls,22,23 it is plausible that the neuromodulatory effects of TENS might alleviate both disorders. Also, patients with PMDs often have associated pain that can be disabling and difficult to treat with standard pain medications, and the analgesic effects of TENS may be helpful for this symptom, as well. Finally, TENS has not been associated with significant adverse effects, making the anticipated risk-to-benefit ratio favorable.
METHOD
Participants
We enrolled 19 patients, age 18 or older, with a probable or clinically-established PMD, defined according to the Fahn and Williams criteria.21 Exclusion criteria were unwillingness to comply with the study requirements, lactation or lack of a safe contraceptive method in women, and presence of any implanted electrical device. All patients signed an informed consent before entering the study, and the study protocol was approved by the Baylor College of Medicine's Internal Review Board for Human Research. Of patients eligible to enroll in the study, four patients declined participation, citing geographical constraints (two patients) and personal reasons (two patients), presumably, lack of confidence in their diagnosis. One participant who was enrolled had used TENS previously for pain.
Rating Scales and Evaluation Procedures
All patients underwent a semistructured interview to assess clinical features, precipitating events, alleviating and aggravating factors, clinical course, treatment responses, and medical comorbidities associated with their PMD. PMD severity was prospectively assessed via video examinations, which were later scored by a blinded rater using the Psychogenic Movement Disorders Rating Scale (PMDRS).24 Patients also ranked the magnitude, persistence, and disability caused by their movement disorder over the past 30 days, using a 10-point numerical rating scale, with 10 being the most severe. The following psychological measures were also completed at baseline: We used the Beck Anxiety Inventory (BAI)25 and Beck Depression Inventory–II (BDI–II),26 to gauge anxiety and depression. Both inventories are validated, consisting of 21-self-report questions, with possible scores ranging between 0 and 63. Scores on the BAI of >7, 15, and 25 correspond with mild, moderate, and severe anxiety, respectively; and scores on the BDI–II of >13, 19, and 28 correspond with mild, moderate, and severe depression.
Using the Multidimensional Health Locus of Control Form C (MHLC),27 we evaluated the extent to which patients believe that they can control their own health. The MHLC is a validated 18-item self-report instrument with five subscales (Internal, Chance, Physicians, Others, and a Total score for the examined external variables), reflecting how strongly patients attribute health outcomes to Internal factors (i.e., healthy behaviors) or External factors (i.e., chance, other people, and physicians). Scores on the Internal and Chance subscales range from 6 to 36, with higher scores indicating a greater belief in that type of control. Scores on the Physician and Other subscale range from 3 to 18, and, as previously stated, the External subscale is a total score for Chance, Physicians, and Others.
Because psychological dissociation may be relevant to PMDs,28 patients completed the Dissociative Experiences Scale (DES).29 The DES is a validated 28-item self-report screening questionnaire in which patients rate the frequency of various dissociative experiences (unrelated to alcohol or drug use); questions are framed in a normative manner, attempting not to stigmatize the respondent for positive responses. For this measure, patients were asked to mark a line anchored at 0% on the left and 100% on the right, indicating the percentage of time they experience the proposed statement. This measure contains dissociative instances which are judged to be within normal limits in day-to-day experiences; cumulative scores >30 reflect a high likelihood of pathological dissociation.30
We screened for posttraumatic stress disorder using the Posttraumatic Stress Disorder Checklist (PCL).31,32 The PCL is a validated 17-item self-report rating scale based upon Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) criteria. Summary scores range between 17 and 85. Recent research studies have revealed variable cutoff scores, depending on the population under investigation and the type of trauma that has been encountered. A cutoff of 30 points was used for this study on the basis of previous research33 and our sample's high incidence of reported abuse. This cutoff score has been shown to provide optimal efficiency in identifying individuals who could benefit from a clinical intervention.32
After the baseline assessments, a trial of TENS was performed in the clinic via a dual-channel electrical generator (Empi, EPIX Vt, St. Paul, MN) using 5 cm×5 cm self-adhesive electrodes. Electrodes were placed 5 cm apart over muscles that were maximally affected by the PMD. TENS was delivered in 2-sec. trains, separated by 2-sec. pauses, administered at a frequency of 150 Hz, similar to what has been reported in previous studies of TENS for organic focal hand dystonia.11 Stimulus strength was titrated to produce a tingling sensation in the stimulated area without muscle twitching or pain. Thirty minutes after initiating TENS, we performed a second video examination. All patients who subjectively concluded that short-term treatment with TENS was effective in reducing their PMD were offered outpatient TENS therapy, administered daily for 30 minutes per day with the aforementioned settings. All patients were asked to return to the clinic within 4 months for follow-up, at which time a third video examination was performed. At this time, patients also reassessed PMD magnitude, persistence, and disability over the past 30 days, using the same 10-point numerical rating scale. Patients who were unable or unwilling to return to the clinic were asked to complete the rating scale by phone. Adverse events were prospectively recorded at each point of contact (in the clinic or by phone).
Data Analysis
All rating scales were administered and scored according to their published guidelines. The primary outcome measure was the change from baseline in the PMDRS score after outpatient TENS therapy. Secondary outcome measures included change from baseline in self-report scales of PMD magnitude, persistence, and disability. Change from baseline was analyzed by repeated-measures analysis of variance (ANOVA). We performed a linear regression to identify factors that predicted a favorable outcome at follow-up. The criterion for statistical significance was set at p≤0.05. All analyses were performed by SPSS 17.0.
RESULTS
Sociodemographic and baseline clinical characteristics are provided in Table 1. On psychometric testing, 47% met the clinical cutoff score for depression on the BDI–II; 17% were classified as mild, 5% as moderate, and 28% as severe. One patient with severe symptoms of depression endorsed the item, “I would like to kill myself;” three patients (one with moderate and two with severe symptoms of depression) reported having thoughts about the possibility of suicide, but never being able to carry out the act. Mild, moderate, and severe symptoms of anxiety were reported by 22%, 33%, and 28% of patients, respectively. Few dissociation-type experiences were endorsed by patients, and only one patient met clinical criteria for dissociation on the basis of the DES. In contrast, 47% of the sample met the cutoff score for PTSD on the PCL; 22% of the sample reported a history of abuse, which was positively correlated with meeting the clinical cutoff score on the PCL (p<0.001). Results of the MHLC showed that patients perceived internal factors as having a larger influence on their movement than did chance (p<0.03) and others (p<0.04).
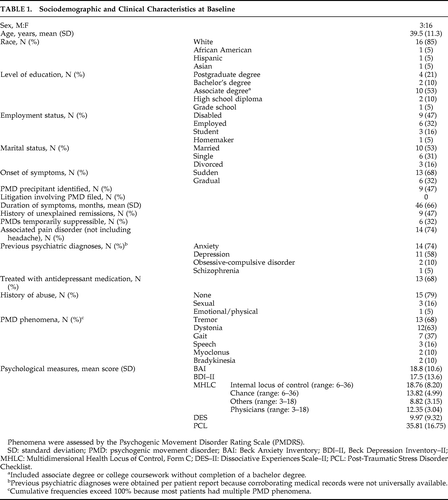 |
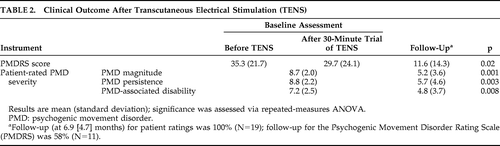
Although 15 patients (79%) elected to continue TENS as outpatients, only 5 demonstrated a robust (>50%) improvement in PMDRS score during the initial trial of TENS in the clinic. Two patients had a 20%–30% improvement, and two had a transient >25% worsening in PMDS score. In the remaining 10 patients, the PMDRS score changed <5% from baseline during the initial trial of TENS in the clinic. Eight (42%) participants failed to return for a follow-up assessment in the clinic, including all four patients who had elected not to continue outpatient TENS. However, all patients who did not return provided follow-up ratings of PMD magnitude, persistence, and disability by phone. At a mean follow-up of 6.9 (SD: 4.7) months, there was a significant improvement in both PMDRS scores and all self-rated outcome measures compared with baseline (Table 2). All five patients who experienced a >50% improvement during their first session of TENS had no visible abnormal movements at follow-up. Linear-regression analysis to determine which demographic, clinical, and psychometric variables correlated with PMD prognosis in this patient cohort found that a short PMD duration best predicted a favorable outcome (β=0.211 and p=0.001). Apart from the transient exacerbation in PMD severity noted above, no adverse effects were reported during the study.
 |
DISCUSSION
Conventional therapies for organic movement disorders are usually ineffective for PMDs, and published treatment data for PMDs are regrettably sparse.5 Modest evidence supports psychodynamic psychotherapy,34 and antidepressants appear to reduce PMD severity in patients with a comorbid mood or anxiety disorder.35 Cognitive-behavioral therapy has proven helpful for patients with other “medically unexplained symptoms” but has not been adequately studied for PMDs specifically.36 Some centers advocate a combination of psychotherapy and physiotherapy.37 In this pilot study, we evaluated TENS both as a short-term (clinic-based) intervention and as a continued (outpatient) treatment for PMDs. We found a statistically significant improvement in PMDRS severity scores at follow-up, attributable largely to a complete PMD remission in approximately one-fourth of patients. Patient-rated assessments of PMD magnitude, persistence, and disability likewise improved. Similar to previous studies of TENS for other indications, we did not observe serious or permanent adverse effects related to the therapy. Symptoms of two patients, however, temporarily worsened during their initial TENS trial, and further work is needed to confirm the safety of TENS for PMDs.
The course of PMDs is more variable than most movement disorders, and improvements in PMD severity generally are infrequent and modest,38–41 with remission rates in the range of 5% to 15%.40,42,43 A more favorable (but certainly not benign) disease course has been reported at some movement-disorder centers, but what might account for this variation still awaits clarification.2,21,44 Previous studies suggest a better PMD outcome in patients with a comorbid DSM Axis I disorder39 and in those with a positive perception of their social situation and physician.44 Outcomes are less favorable in patients who have a personality disorder,45 experience secondary gain,39 and in those who are suspected to have a factitious disorder or malingering.21,35 Age at onset46 and pending litigation39 also may influence PMD outcome, but data are conflicting.21,44,47 In agreement with several previous studies,2,39,40,43–45,48 we found that PMD prognosis was more favorable in patients with a shorter disease duration. Accordingly, clinicians should aim to lessen the interval between symptom onset and diagnosis, so that counseling and treatment can be implemented as early as possible in the disease course.
PMDs have been associated with depression, anxiety, and personality disorders,2,21,40,44 but surprisingly little else has been published regarding psychological comorbidities. Literature on conversion disorder in general has found associations with previous trauma, and common psychobiological systems may underlie conversion disorder and posttraumatic dissociative symptoms.28 In our cohort, one-fifth of patients reported a history of abuse, and half met the cutoff score for PTSD on the PCL. We did not, however, find evidence of significant dissociation on the DES. An earlier study of patients with fixed dystonia, a condition that frequently has a psychogenic basis, also failed to find notable changes on the DES,42 whereas DES scores are elevated in persons with psychogenic seizures.49 As psychogenic seizures and PMDs are similar in terms of phenomenology, the lesser degree of dissociation in PMD patients may be related to the more continuous, less episodic nature of their illness. Health locus-of-control refers to the degree of control that patients believe various factors possess over their personal health. Health locus-of-control has prognostic relevance in many illnesses, in that a greater sense of self-efficacy (internal locus-of-control) facilitates effective coping and treatment strategies.50 For example, a greater belief in personal control over one's illness has been shown to predict a favorable prognosis in patients with chronic fatigue syndrome and fibromyalgia, two syndromes that often coexist with PMDs.51,52 Some studies also have found that psychogenic or functional disorders may be associated with a lower internal locus-of-control when compared with healthy subjects.49 In this study, locus-of-control scores were similar to those of a previous study of patients with organic dystonia50 and in line with other normative data for patients with other chronic illnesses.27 We did not find that MHLC scores were of prognostic significance in this sample of PMD patients, but further work in a larger population is warranted.
We recognize limitations of this study, including the small sample size, heterogeneous population, relatively short duration of follow-up, lack of a control population. and high drop-out rate (42% for the PMDRS). We attempted to maximize objectivity by utilizing PMDRS scores as our primary outcome, which were assessed by an independent, blinded, rater. Adequate patient blinding of TENS versus a placebo (sham) device would have been inherently problematic because of the tingling sensation that is generated by the electrical current.53 Furthermore, although a placebo-controlled study would be preferable, the effect of placebo in PMDs may be more complex than in other neurological disorders.54 One goal of conducting a placebo-controlled trial is to clarify whether a perceived treatment effect is the result of suggestion versus a physiological effect on the specific disorder being studied. However, the placebo response and other forms of suggestion, such as hypnosis, are themselves routed in a neurobiology. Physicians have long postulated mechanistic similarities between psychogenic disorders and hypnotic states, a view that is now being explored with functional brain imaging and other techniques.55–58 If the PMDs and suggestion share common neural systems, it is feasible that placebo might correct (perhaps temporarily) the reversible neurophysiologic disturbances that underlie some psychogenic disorders, thereby clouding the conceptual distinction between a placebo and a disease-modifying effect.
When counseling patients regarding the use of TENS (or other possible treatments) for PMDs, we would urge clinicians to discuss the gaps that exist in our current understanding of PMD neurobiology and acknowledge the regrettable fact that no therapies are proven effective in placebo-controlled studies. Despite these limitations, this study confirms that patients with a shorter PMD duration have a more favorable prognosis and that early intervention in the context of a supportive physician–patient relationship is desirable, even when treatment strategies lack a firm evidence-base.
1.
2. : Psychogenic movement disorders: frequency, clinical profile, and characteristics. J Neurol Neurosurg Psychiatry 1995; 59:406–412Crossref, Medline, Google Scholar
3. : Psychogenic movement disorders: diagnosis and management. CNS Drugs 2004; 18:437–452Crossref, Medline, Google Scholar
4. : Movement Disorders Fellowship Training Program at Columbia University Medical Center, 2001–2002. Mov Disord 2006; 21:479–485Crossref, Medline, Google Scholar
5. : Therapeutic approaches to psychogenic movement disorders, in: Psychogenic Movement Disorders: Neurology and Neuropsychiatry. Edited by Hallett MFahn SJankovic J. Philadelphia, PA, AAN Enterprises and Lippincott Williams & Wilkins, 2006, pp 323–328Google Scholar
6. : Psychogenic movement disorders in children. Mov Disord 2008; 23:1875–1881Crossref, Medline, Google Scholar
7. : Impact of psychogenic movement disorders versus Parkinson's on disability, quality of life, and psychopathology. Mov Disord 2007; 22:2204–2209Crossref, Medline, Google Scholar
8. : Transcutaneous electrical nerve stimulation (TENS) for chronic pain. Cochrane Database of Systematic Reviews 2008; 3:CD003222. DOI:https://doi.org/10.1002/14651858.CD003222.pub2Medline, Google Scholar
9. : Assessment: efficacy of transcutaneous electric nerve stimulation in the treatment of pain in neurologic disorders (an evidence-based review): Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2010; 74:173–176Crossref, Medline, Google Scholar
10. : Short-term, high-frequency transcutaneous electrical nerve stimulation decreases human motor cortex excitability. Neurosci Lett 2004; 355:85–88Crossref, Medline, Google Scholar
11. : Effects of transcutaneous electrical nerve stimulation on motor cortex excitability in writer's cramp: neurophysiological and clinical correlations. Mov Disord 2006; 21:1908–1913Crossref, Medline, Google Scholar
12. : How repeatable are the physiological effects of TENS? Clin Neurophysiol 2008; 119:1834–1839Crossref, Medline, Google Scholar
13. : Treatment of myoclonic dystonia with transcutaneous electrical nerve stimulation. Ital J Neurol Sci 1985; 6:75–78Crossref, Medline, Google Scholar
14. : Effect of electrical nerve stimulation on dystonic tremor. Lancet 1990; 336:1385–1386Crossref, Medline, Google Scholar
15. : TENS for the treatment of writer's cramp dystonia: a randomized, placebo-controlled study. Neurology 2005; 64:1946–1948Crossref, Medline, Google Scholar
16. : Acute effect of transcutaneous electrical nerve stimulation on tremor. Mov Disord 2003; 18:191–194Crossref, Medline, Google Scholar
17. : TENS for the treatment of propriospinal myoclonus. Mov Disord 2008; 23:2256–2257Crossref, Medline, Google Scholar
18. : Transient improvement of psychogenic (proprio-)spinal-like myoclonus to electrical nerve stimulation. Mov Disord 2009; 24:2024–2025Crossref, Medline, Google Scholar
19. : Post-whiplash dystonia well controlled by transcutaneous electrical nervous stimulation (TENS): case report. J Trauma 1990; 30:909–910Crossref, Medline, Google Scholar
20. : Etiological and therapeutic observations in a case of belly dancer's dyskinesia. Mov Disord 2005; 20:251–253Crossref, Medline, Google Scholar
21. : Phenomenology and psychopathology related to psychogenic movement disorders. Adv Neurol 1995; 65:231–257Medline, Google Scholar
22. : Cortical and spinal abnormalities in psychogenic dystonia. Ann Neurol 2006; 59:825–834Crossref, Medline, Google Scholar
23. : Cortical excitability is abnormal in patients with the “fixed dystonia” syndrome. Mov Disord 2008; 23:646–652Crossref, Medline, Google Scholar
24. : Rating Scale for Psychogenic Movement Disorders: scale development and clinimetric testing. Mov Disord 2005; 20:1592–1597Crossref, Medline, Google Scholar
25. : Manual for the Beck Anxiety Inventory. San Antonio, TX, The Psychological Corporation; 1990Google Scholar
26. : Manual for the Beck Depression Inventory, 2nd Edition. San Antonio, TX, The Psychological Corporation; 1996Google Scholar
27. : Form C of the MHLC scales: a condition-specific measure of locus of control. J Pers Assess 1994; 63:534–553Crossref, Medline, Google Scholar
28. : Should conversion disorder be reclassified as a dissociative disorder in DSM-V? Psychosomatics 2007; 48:369–378Crossref, Medline, Google Scholar
29. : Development, reliability, and validity of a dissociation scale. J Nerv Ment Dis 1986; 174:727–735Crossref, Medline, Google Scholar
30. : Patterns of dissociation in clinical and nonclinical samples. J Nerv Ment Dis 1996; 184:673–679Crossref, Medline, Google Scholar
31. : The PTSD Checklist (PCL): Reliability, Validity, and Diagnostic Utility.
32. : Estimating population prevalence of posttraumatic stress disorder: an example using the PTSD Checklist. J Trauma Stress 2008; 21:290–300Crossref, Medline, Google Scholar
33. : Validating the Primary Care Posttraumatic Stress Disorder Screen and the Posttraumatic Stress Disorder Checklist with soldiers returning from combat. J Consult Clin Psychol 2008; 76:272–281Crossref, Medline, Google Scholar
34. : Single-blind clinical trial of psychotherapy for treatment of psychogenic movement disorders. Parkinson Relat Disord 2006; 12:177–180Crossref, Medline, Google Scholar
35. : Antidepressant treatment outcomes of psychogenic movement disorder. J Clin Psychiatry 2005; 66:1529–1534Crossref, Medline, Google Scholar
36. : What is the evidence for the efficacy of treatments for somatoform disorders? a critical review of previous intervention studies. Psychosom Med 2007; 69:889–900Crossref, Medline, Google Scholar
37. : A randomised, controlled clinical trial on the additional effect of hypnosis in a comprehensive treatment programme for inpatients with conversion disorder of the motor type. Psychother Psychosom 2002; 71:66–76Crossref, Medline, Google Scholar
38. : Diagnostic and pathophysiological aspects of psychogenic tremors. Mov Disord 1998; 13:294–302Crossref, Medline, Google Scholar
39. : Slater revisited: 6-year follow-up study of patients with medically unexplained motor symptoms. BMJ 1998; 316:582–586Crossref, Medline, Google Scholar
40. : Psychiatric outcome in patients with a psychogenic movement disorder: a prospective study. Neuropsychiatry Neuropsychol Behav Neurol 2001; 14:169–176Medline, Google Scholar
41. : The 12-year prognosis of unilateral functional weakness and sensory disturbance. J Neurol Neurosurg Psychiatry 2003; 74:591–596Crossref, Medline, Google Scholar
42. : The prognosis of fixed dystonia: a follow-up study. Parkinson Relat Disord 2009; 15:592–597Crossref, Medline, Google Scholar
43. : Psychogenic tremor: long-term prognosis in patients with electrophysiologically-confirmed disease. Mov Disord 2009; 24:72–76Crossref, Medline, Google Scholar
44. : Long-term prognosis of patients with psychogenic movement disorders. Parkinson Relat Disord 2006; 12:382–387Crossref, Medline, Google Scholar
45. : Motor conversion disorder: a prospective 2- to 5-year follow-up study Psychosomatics 1998; 39:519–527Crossref, Medline, Google Scholar
46. : Ten-year prognosis of conversion disorder. Br J Psychiatry 1996; 169:282–288Crossref, Medline, Google Scholar
47. : Post-traumatic movement disorders: effect of the legal system on outcome. J Forensic Sci 1998; 43:334–339Crossref, Medline, Google Scholar
48. : Psychogenic movement disorders in children: a report of 15 cases and a review of the literature. Mov Disord 2008; 23:1882–1888Crossref, Medline, Google Scholar
49. : Dissociation, hypnotizability, coping styles, and health locus of control: characteristics of pseudoseizure patients. Seizure 2000; 9:314–322Crossref, Medline, Google Scholar
50. : Comparing health locus of control in patients with spasmodic dysphonia, functional dysphonia, and nonlaryngeal dystonia. J Voice 2009; 23:699–706Crossref, Medline, Google Scholar
51. : Coping and adaptive outcome in chronic fatigue syndrome: importance of illness cognitions. J Psychosom Res 1998; 45:39–51Crossref, Medline, Google Scholar
52. : Pain locus of control predicts return to work among Spanish fibromyalgia patients after completion of a multidisciplinary pain program. Gen Hosp Psychiatry 2009; 31:137–145Crossref, Medline, Google Scholar
53. : Transcutaneous electrical nerve stimulation for acute pain. Cochrane Database Syst Rev 2009; 2:CD006142. DOI:https://doi.org/10.1002/14651858.CD006142.pub2Medline, Google Scholar
54. : Psychogenic parkinsonism: use of placebo. Mov Disord 2010; (in press)Google Scholar
55. : Hypnosis and conversion hysteria: a unifying model. Cogn Neuropsychiatry 1999; 4:243–265Crossref, Medline, Google Scholar
56. : Imaging hypnotic paralysis: implications for conversion hysteria. Lancet 2000; 355:986–987Crossref, Medline, Google Scholar
57. : Are patients with somatization disorder highly suggestible? Acta Psychiatr Scand 2008; 117:232–235Crossref, Medline, Google Scholar
58. : The involuntary nature of conversion disorder. Neurology 2010; 74:223–228Crossref, Medline, Google Scholar



