Phenomenological and Neuropsychological Profile Across Motor Variants of Delirium in a Palliative-Care Unit
Abstract
Studies using composite measurement of cognition suggest that cognitive performance is similar across motor variants of delirium. The authors assessed neuropsychological and symptom profiles in 100 consecutive cases of DSM-IV delirium allocated to motor subtypes in a palliative-care unit: Hypoactive (N=33), Hyperactive (N=18), Mixed (N=26), and No-Alteration motor groups (N=23). The Mixed group had more severe delirium, with highest scores for DRS–R-98 sleep–wake cycle disturbance, hallucinations, delusions, and language abnormalities. Neither the total Cognitive Test for Delirium nor its five neuropsychological domains differed across Hyperactive, Mixed, and Hypoactive motor groups. Most patients (70%) with no motor alteration had DRS–R-98 scores in the mild or subsyndromal range even though they met DSM-IV criteria. Motor variants in delirium have similar cognitive profiles, but mixed cases differ in expression of several noncognitive features.
Delirium is an acute neuropsychiatric syndrome of impaired consciousness, comprising inattention, impaired higher-level thinking, and circadian disturbances. Despite a wide variety of etiologies, delirium has a characteristic constellation of symptoms that suggests a final common neural pathway. Motor disturbances are core symptoms of delirium, and they occur frequently, as do cognitive impairments and sleep–wake cycle disturbances.1
Disturbances of motor behavior are a highly visible and almost inevitable feature of delirium,2 and have been used to define clinical subtypes of delirium. A requirement of clinically meaningful subtypes of any disorder is that certain associated features clearly separate subtypes and that subtypes have predictive value for some underlying physiology or outcome. To date, studies of delirium motor subtypes have used many different assessment methods, not all of which have been focused on motor features specifically. Using these various classifications, motor variants have been reported to differ regarding non-motor symptoms,3 etiology,4,5 pathophysiology,6,7 detection rates,8 treatment response,9–11 duration of episode, and outcome.11–14 To date, studies have been conducted among heterogeneous patient populations, yielding inconsistent patterns. For example, better prognosis has been reported in some studies in hypoactive patients,14 whereas others report better prognosis in hyperactive patients.11,15 Studies of cognitive profile suggest that it is comparable across variants, as are EEG abnormalities,16 although those studies used a composite measure of cognition, rather than comparing individual neuropsychological domains.
Drawing conclusions from the existing literature is difficult because of inconsistent approaches to defining motor presentation, where many descriptions include psychomotor features that are not specific to delirium, such as singing, shouting, or laughing; difficult-to-manage behaviors or combativeness; and where the threshold for categorization might only require that a single symptom be present. Instruments vary in structure; long psychomotor checklists, “clinical impression,” visual-analog scales, and motor items taken from standardized delirium rating instruments.16 These issues are highlighted by a recent study of delirium finding only 34% concordance across four commonly-used motor subtype methods applied to the same study population (three of which were psychomotor checklists).2 More recently, motor variants were redefined17 in a controlled study by analyzing data using the 30-item Delirium Motor Checklist, which comprised all non-redundant items taken from combining three popular psychomotor schemas.11,18,19 The resultant new motor scale, is more concise, data-derived, focused on motor disturbances, and relatively specific for delirium patients, as compared with non-delirium control subjects in the same setting.17 Furthermore, this new scale has been validated against objective motor-activity measurements using accelerometry.20,21 Use of this validated motor-focused scale should enable more accuracy and clarity when applied to research in delirium patients to verify whether, indeed, motor subtypes exist and what constitutes their clinical meaningfulness. Much of the previous literature on motor subtypes in delirium may, in fact, need to be interpreted with some degree of skepticism.
We studied phenomenological and neuropsychological profiles in delirium patients categorized into groups defined by the new motor scale to determine whether cognitive and noncognitive features of delirium were different across motor groups (Hyperactive, Hypoactive, and Mixed) when compared with delirium control subjects without motor alterations.
METHODS
Subjects and Design
The work was conducted at Milford Care Hospice, where all patients are screened with the Confusion Assessment Method (CAM23) as part of the admission procedure and on daily rounds by the palliative-care team. Patients with an altered mental state as per the CAM were referred to Liaison Psychiatry and assessed within 24 hours to confirm DSM-IV delirium.22 Patients meeting DSM-IV criteria for delirium underwent a detailed delirium assessment of demographics, phenomenological profile, dementia status, and, of course, delirium (see below for assessment methods used). These procedures were developed as part of a validation study of the CAM in palliative-care settings.24 Patients diagnosed with delirium by the treating medical team were excluded if death was imminent or where circumstances were too difficult to allow assessment (as per opinion of the treating medical team), which resulted in the exclusion of 12 patients; 121 consecutive cases of DSM-IV delirium were assessed, of which 100 underwent at least two assessments and were included in a larger serial-analysis study. For the purpose of this article, we describe the phenomenological and neuropsychological profile across motor variants in the first assessment of these 100 patients.
Because of the noninvasive nature of the study, Limerick Regional Ethics Committee approval was given to augment patient assent with proxy consent from next-of-kin (where possible) or a responsible caregiver for all participants in accordance with Helsinki Guidelines for Medical research involving human subjects.25
Procedures
For each patient, we documented demographic profile, duration of delirium symptoms at referral, and possibility of underlying dementia (noted by history or investigation). All medication prescribed for the patient during the previous 24-hour period of study was noted, and dose-equivalents of opioids, antipsychotics, steroids, and benzodiazepines were calculated according to standard-equivalents.
We assessed delirium symptoms over the previous 3–4 day period with the Delirium Rating Scale–Revised-9826 and neuropsychological performance with the Cognitive Test for Delirium.27 Assessments were conducted by raters previously trained and highly experienced in the administration of these instruments (ML, DM). To further enhance interrater reliability, difficult ratings were discussed and rated by consensus between raters.
Delirium Rating Scale–Revised-98 (DRS–R-98(26))
The DRS–R-98 is a widely-used instrument used to measure symptom severity as well as diagnose delirium. It is a 16-item, clinician-rated scale, with 13 severity and 3 diagnostic items, and it is a valid measure of delirium severity over a broad range of symptoms. The 13-item Severity section can be scored separately from the 3-item Diagnostic section; their sum constitutes the Total scale score. The severity of individual items is rated from 0 to 3 points, and each item is anchored by text descriptions as guides for rating along a continuum from Normal to Severely Impaired. Thus, DRS–R-98 Severity scores range from 0 to 39, with higher scores indicating more severe delirium and a cutoff score >15 consistent with a diagnosis of delirium. The total scale can be scored initially to enhance differential diagnosis by capturing characteristic features of delirium, such as acute onset and fluctuation of symptom severity. Two items of the DRS–R-98 assess motor presentation (Item #7: Agitation, Item #8: Retardation). Although the instrument can be used to rate symptoms over variable periods from hours to weeks, it is ideally used to rate delirium over 24 hours, so as to improve recognition of intermittent symptoms, and, for the purposes of this study, was applied biweekly to encompass the previous 3–4 day period since the previous assessment. It has high interrater reliability, validity, sensitivity, and specificity for distinguishing delirium from mixed neuropsychiatric populations including dementia, depression, and schizophrenia.26
Cognitive Test for Delirium (CTD27)
This is a relatively brief (15–20 minutes) test of neuropsychological functioning that emphasizes visual abilities and is suitable for assessing a broad range of delirium patients, including those who are intubated or cannot speak. Originally validated in severely ill ICU patients, responses can be nonverbal (pointing, nodding head, raising hand). The CTD comprises five neuropsychological domains (orientation, attention, memory, comprehension, vigilance) and generates a score from 0 to 30, with higher scores indicating better cognitive functioning. An optimal cutoff score to discriminate delirium from other disorders is <19, however it can be used as a continuous, unidimensional measure of cognition in delirium. It distinguished delirium from dementia, schizophrenia, and depression.
Categorization Into Motor Groups
Staff nurses completed the 30-item Delirium Motor Checklist (DMC) independently of the psychiatrist for the same 3–4-day period for which the DRS–R-98 was rated. These results were used to categorize patients into Hyperactive, Mixed, Hypoactive, or No-Motor subtype groups, on the basis of the new data-based motor scale, the Delirium Motor Subtype Scale (DMSS).17 Presence of at least 2 of 4 Hyperactive items (increased quantity of motor activity, loss of control of activity, restlessness, and wandering) and/or 2 of 7 Hypoactive items (decreased amount of activity, decreased speed of actions, reduced awareness of surroundings, decreased speed and amount of speech, listlessness, reduced alertness, withdrawal) were required for classification. Mixed motor-subtype criteria were met if patients had evidence of both Hyperactive and Hypoactive subtype in the previous 3–4 days.
Assessment of Ease of Ward Management
Nurses completed a 4-point checklist outlining the ease with which patients can be nursed on the unit: the so-called Ease of Ward Management Scale (EOWM):
| 1. | The patient's behavior poses little or no difficulty; he or she is cooperative with treatment and requires only routine observation. | ||||
| 2. | Some problems exist with the patient's behavior, but the patient is generally compliant with treatment and manageable with observation or minimal sedation (e.g., once off low-dose medication). | ||||
| 3. | Significant problems exist in the management of the patient's behavior, necessitating more than minimal medication and/or special measures (e.g., close observation). | ||||
| 4. | The patient's behavior poses a major problem (e.g., is a significant risk to self or others), requiring sedation and/or restraints and/or special nursing care. | ||||
Etiological Assessment
The Delirium Etiology Rating Checklist1 allows for the multifactorial assessment of delirium etiology by rating 12 categories of contributing etiologies according to the likelihood of their being a cause for delirium (rated on a 5-point scale ranging from Ruled Out/Not Present/Not Relevant (0) to Definite Cause.4
Statistical Analyses
We conducted data analysis with SPSS for Windows 14. Continuous variables for demographic and rating-scale scores were expressed as means and standard deviations (SD). Continuous variables were compared by one-way ANOVA followed by pairwise comparisons. Non-normal data were compared with nonparametric tests by use of the Kruskal-Wallis test for all four groups and Mann-Whitney U tests for between-group comparisons. Statistical significance was set at p<0.05.
RESULTS
Demographic and clinical values by motor group are shown in Table 1. Mean age was 70.3 (SD: 10.5) years (range: 36–90), and 49% were women. There were no significant age or sex differences among the groups.
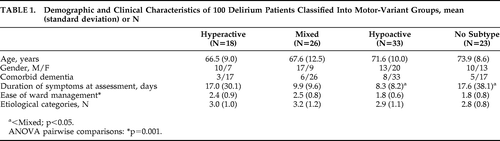 |
A total of 33 patients were classified as Hypoactive; 26 patients, Mixed; 18, Hyperactive; and 23 did not meet any criteria (No-Subtype). Hyperactive and Mixed groups had significantly higher mean EOWM scores than the other two groups. Groups were comparable for the number of contributing etiologies, with drug intoxication, metabolic-endocrine disturbances, and systemic infection the most commonly-implicated etiologies.
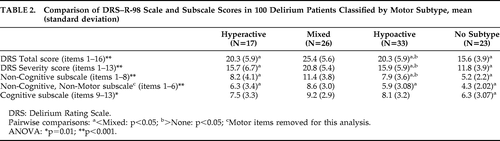
Table 2 lists DRS–R-98 scale and subscale scores for motor groups. Mean scores across all four groups were significantly different for all comparisons. This difference was primarily driven by the No-Subtype group, which was significantly less impaired than the other groups on all comparisons, scoring in the mild-to-subsyndromal range. Also, the Mixed group scored significantly higher than the Hypoactive and Hyperactive groups for overall severity of delirium and for the noncognitive subscale, including whether or not the motor items were removed (the so-called “noncognitive nonmotor” subscale). However, the three motor groups did not differ across the DRS–R-98 Cognitive subscale (Items #9–#13).
 |
Mean DRS–R-98 item scores are shown in Table 3. Attention, short-term memory, visuospatial ability, and thought-process abnormality were not significantly different across all four groups. Pairwise comparisons revealed some differences for other items among the motor groups. Mean scores in the Mixed group showed significantly more impairment than the Hyperactive group for language, motor retardation, orientation, and long-term memory, and significantly more impairment than the Hypoactive group for sleep–wake cycle, perceptual disturbances, and motor agitation. There were no differences for all other items across the three motor groups, except for the expected significant differences for more motor agitation in the Hyperactive group than the Hypoactive group, and vice versa.
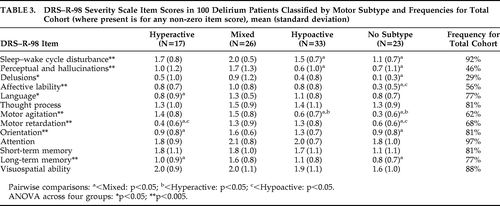 |
Mean item score for DRS–R-98 Motor Agitation was comparably impaired between Mixed and Hyperactive groups, and Motor Retardation comparable between the Mixed and Hypoactive groups, where the Mixed group would be expected to represent components of each motor presentation. These data provide some cross-validation between the DRS–R-98 and the new motor classification scale.
Frequencies for DRS–R-98 items across the whole cohort showed that inattention and sleep–wake cycle disturbance occurred in more than 90% of patients. Whereas 23% of the 100 patients did not reach motor subtype criteria according to the Meagher et al. method,30 many of these patients did have evidence of motor alterations as measured on the DRS–R-98, where 92% of the cohort scored ≥1 on Items #7 or #8, whereas only 45% scored ≥2 on either of these items. Also, 12 of the 23 patients who did not meet subtype criteria according to the Meagher et al. method did have at least some of the motor disturbances, albeit not at full subtype criteria levels.
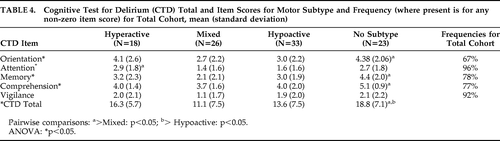
CTD scores for motor groups are shown in Table 4. Total CTD scores and all item scores except Vigilance were significantly different across all four groups. However, these differences were driven largely by the No-Subtype group. Cognitive functioning was not significantly different across motor groups for any neuropsychological domain, except that Attention was more impaired in Hyperactive than in Mixed patients.
 |
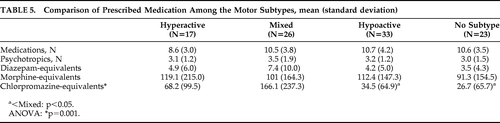
Medication exposure (including diazepam, morphine, and chlorpromazine-equivalents) is shown in Table 5. All delirium patients were receiving at least one psychotropic medication. Hypoactive and Mixed subtypes differed with regard to chlorpromazine-equivalent doses (p=0.002). There was no significant difference in morphine or diazepam-equivalent doses prescribed for each of the motor subtypes.
 |
DISCUSSION
Delirium occurs commonly in palliative-care settings, with rates of up to 85% reported;28 yet there is a relative paucity of literature addressing the assessment of motor subtypes of delirium in this setting. This may in part be explained by the considerable ethical challenges posed by studying such a frail and often seriously ill population, including those with terminal restlessness. In cases with refractory symptoms, deeper or “palliative” sedation may be required.29 The majority of these patients are receiving opiates for their underlying condition, and midazolam is widely prescribed. The degree to which these medications act as confounding factors in the study of motoric subtypes is unclear. In this study, it is noteworthy that no significant difference occurred in morphine-equivalents across the motor subtypes, suggesting that morphine does not play a significant role. Our population was younger, with less dementia and greater medical morbidity than perhaps in a general-hospital population. In an ongoing study, we aim to replicate this work in a general-hospital population, and this will allow direct comparison between the two populations.
This study investigated the phenomenology and neuropsychological domains across motor variants in delirium as defined by a new data-based motor scale focusing on motor symptoms that are relatively specific for delirium.17 This new motor scale has demonstrated concurrent20 and predictive validity,30 with recent validation against objective motor monitoring using electronically-measured accelerometry. This work is the first to compare specific cognitive and noncognitive features, with widely-used, standardized delirium instruments in three motor variant groups, as compared with a No Motor Variant delirium group. The pattern of DRS–R-98 motor item scores added another aspect of validation to the new motor subtyping scale because scores were comparable and higher for motor agitation in hyperactive and mixed groups than in hypoactive, and the converse for hypoactive and mixed groups for the motor retardation item.
Previous work has measured cognition as a single composite score and reported that cognitive impairment is comparable despite motor presentation of delirium.3 This finding is confirmed by our work and affirms that delirium is primarily a cognitive disorder. Unlike previous work, however, we found that the Mixed group was often more impaired for certain noncognitive symptoms of delirium and also had the worst overall level of impairment.
Other subtype scales11,18,19 include a variety of noncognitive symptoms (speech disturbances, combativeness, fear); however, in a recent study, Meagher et al.2 concluded that only 6 of 22 nonmotor symptoms differed, which suggests nonspecificity for delirium. There was greater similarity between Mixed subtype and Hyperactive than Hypoactive subtype for several noncognitive symptoms: sleep–wake cycle disturbances, hallucinations, and agitation, suggesting that these symptoms are particularly associated with Mixed and Hyperactive delirium. A recent factor analysis of the DRS–-R-98 in Colombian patients found two factors, cognitive and psychosis/agitation, where sleep–wake cycle, thought process, and attention loaded onto both factors, but the agitation factor loaded on motor agitation, along with delusions, perceptual disturbances, affective lability, and fluctuation of symptoms.31 It is likely that those symptoms are driven together by some underlying neuropathophysiological mechanism to co-occur in the motor-agitated state in delirium. Conversely, but consistent with these data for hyperactive symptoms, Meagher et al.3 found that their hypoactive group scored lower for sleep–wake cycle disturbances, delusions, variability of symptoms, and mood lability. Franco et al.31 found motor retardation to load on the factor with language and all cognitive items.
These data on different patterns of non-core, noncognitive symptoms have implications for understanding the neural underpinnings of delirium. The confirmation of the finding that cognitive impairment is essentially the same across all three subtypes also has important clinical implications. Hypoactive patients are commonly misdiagnosed or detected late32 and have poorer overall outcomes12,33 because they are less noticeable than hyperactive or mixed presentations of delirium, despite similar levels of cognitive disturbance. This strongly suggests the need for routine formal cognitive assessment of patients to achieve improved detection of delirium.
Twenty-three patients with DSM-IV delirium did not meet criteria for any motor subtype, according to the new method, raising concerns regarding its inclusiveness, given that previous work has indicated that motor activity disturbances are almost invariably present in delirium.2 However, these patients had significantly less severe delirium, rated according to the DRS–R-98, where only six of these patients had scores above the suggested diagnostic cutoff point of 15. These findings concur with those of de Jonghe et al.'s34 study of prodromal delirium, where non-motor features were prominent early indicators of delirium, and they suggest that motor disturbances are more prominent in full syndromal delirium than during subsyndromal phases.
Previous work, using the original 10-item Delirium Rating Scale,35 found that overall DRS scores were highest in the Hyperactive group, intermediate in the Mixed group, and lowest in the Hypoactive group.3 This present work found that patients with the Mixed subtype had greater overall severity of symptoms as measured on the DRS–R-98 and that this reflected more severe noncognitive than cognitive disturbances, and suggesting similar levels of cognitive dysfunction regardless of motor presentation. The relevance of these findings to prognosis remains uncertain, but some work has indicated poorer prognosis for the Mixed subtype,36,37 although most studies suggest that poorer prognosis is associated with Hypoactive presentations.16 It has been suggested that mixed presentations might reflect the varying impact of multiple etiologies for delirium and that a mixed subtype is associated with more complex etiological underpinnings, but our work indicates similar levels of etiological burden across motor subtypes.
Inattention, impaired vigilance, and sleep–wake cycle disturbance were the most consistently impaired ratings on the DRS–R-98 and/or the CTD, each occurring with a frequency of more than 90% of all patients. Language and thought-process abnormalities and impaired comprehension were in the next most common grouping, with a frequency of over 70%, along with the other cognitive items. These data support the proposal that there are three core domains of delirium: attention (plus other cognitive areas), circadian disturbance (sleep–wake cycle and possibly also motor alterations), and higher-level thinking (comprehension, language, and thinking processes).1,31 Motor disturbances were also highly prevalent by both DRS–R-98 (92%) and Meagher et al. subtype criteria (89%), which suggests that motor disturbances are very common and may be invariably present when measured over the course of a delirium episode, rather than cross-sectionally as per this work. Sleep and motor behavior are influenced by circadian rhythms and influenced by the hormone melatonin. Balan and colleagues6 found that levels of a melatonin metabolite (6-SMT) correlated closely with motor presentation during the delirium episode, with highest levels recorded in hypoactive patients. Further work exploring the relationship between circadian-rhythm disturbance and cognition may provide important insights into the pathobiology of delirium.
Similar to findings of previous reports,9,10 patients with both hyperactive and mixed subtypes received greater nursing attention and antipsychotic medication than their hypoactive counterparts. In part, this reflects the varying challenges that motor subtypes pose in real-world management, but it has also been suggested that hypoactive presentations are less energetically managed because their problems are perceived as less compelling. In support of this idea, studies have highlighted relatively less use of both drug and environmental manipulations to manage hypoactive patients,9,12 even though available evidence indicates that patients with a variety of motor presentations respond to antipsychotic treatment.16
A more detailed longitudinal study of delirium symptoms in a range of different populations (e.g., elderly, medical, postoperative, ICU) is needed to illuminate underlying etiologies and prognostic implications of motor subtypes of delirium. Also, studies need to clarify the stability of motor subtypes over the course of a delirium episode. The accuracy of the CAM is dependent on the skill of the administrator.8,24 Better detection of the different motor subtypes of delirium can occur with more routine systematic assessment of cognition, which, in turn, may be assisted by developments in human/computer interaction technology, allowing more reliable assessment of the cognitive domains most affected by delirium.38
1. : Oxford Textbook of Psychiatry, 2nd Edition. London, UK, Oxford University Press, 2009, chap. 4.1.1Google Scholar
2. : Motor symptoms in 100 patients with delirium versus control subjects: comparison of subtyping methods. Psychosomatics 2008; 49:300–308Crossref, Medline, Google Scholar
3. : Relationship between symptoms and motoric subtype of delirium. J Neuropsychiatry Clin Neurosci 2000; 12:51–56Link, Google Scholar
4. : Delirium: phenomenologic and etiologic subtypes. Int Psychogeriatr 1991; 3:135–147Crossref, Medline, Google Scholar
5. : Relationship between etiology and phenomenological profile in delirium. J Geriatr Psychiatry Neurol 1998b; 11:146–149Crossref, Medline, Google Scholar
6. : The relation between the clinical subtypes of delirium and the urinary level of 6-SMT. J Neuropsychiatry Clin Neurosci 2003; 15:363–366Link, Google Scholar
7. : Abnormal neurotransmitter metabolite levels in Alzheimer patients with a delirium. Int J Geriatr Psychiatry 2006; 21:838–843Crossref, Medline, Google Scholar
8. : Nurses' recognition of delirium and its symptoms: comparison of nurse and researcher ratings. Arch Intern Med 2001; 161:2467–2473Crossref, Medline, Google Scholar
9. : Use of environmental strategies and psychotropic medication in the management of delirium. Br J Psychiatry 1996; 168:512–515Crossref, Medline, Google Scholar
10. : An open trial of olanzapine for the treatment of delirium in hospitalized cancer patients. Psychosomatics 2002; 43:175–182Crossref, Medline, Google Scholar
11. : An empirical study of delirium subtypes. Br J Psychiatry 1992; 161:843–845Crossref, Medline, Google Scholar
12. : Clinical significance of delirium subtypes in older people. Age Ageing 1999; 28:115–119Crossref, Medline, Google Scholar
13. : Outcome of delirium: part 1. Int J Geriatr Psychol 1997; 12:609–613Crossref, Medline, Google Scholar
14. : Delirium severity and psychomotor types: their relationship with outcomes after hip-fracture repair. J Am Geriatr Soc 2002; 50:850–857Crossref, Medline, Google Scholar
15. : A retrospective study of the psychiatric management and outcome of delirium in the cancer patient. Support Care Cancer 1996; 4:351–357Crossref, Medline, Google Scholar
16. : Motor subtypes of delirium: past, present, and future. Int Rev Psychiatry 2009; 21:59–73Crossref, Medline, Google Scholar
17. : A new data-based motor subtype schema for delirium. J Neuropsychiatry Clin Neurosci 2008; 22:185–193Crossref, Google Scholar
18. : Transient cognitive disorder in the elderly. Am J Psychiatry 1983; 140:1426–1436Crossref, Medline, Google Scholar
19. : Clinical subtypes of delirium in the elderly. Dement Geriatr Cogn Disord 1999; 10:380–385Crossref, Medline, Google Scholar
20. : Motion analysis in delirium: a novel method of clarifying motoric subtypes. Neurocase 2007; 13:272–277Crossref, Medline, Google Scholar
21. : Direct measurement of human movement by accelerometry. Med Eng Physics 2008; 30:1364–1386Crossref, Medline, Google Scholar
22.
23. : Clarifying confusion: the Confusion Assessment Method. Ann Intern Med 1990; 113:941–948Crossref, Medline, Google Scholar
24. Validation of the Confusion Assessment Method in the palliative-care setting. Palliat Med 2009; 23:40–45Crossref, Medline, Google Scholar
25.
26. : Validation of the Delirium Rating Scale–Revised-98: Comparison to the Delirium Rating Scale and Cognitive Test for Delirium. J Neuropsychiatry Clin Neurosci 2001; 13:229–242Link, Google Scholar
27. : Validation of a cognitive test for delirium in medical ICU patients. Psychosomatics 1996; 37:533–546Crossref, Medline, Google Scholar
28. : Delirium in terminally ill cancer patients. Am J Psychiatry 1983; 140:1048–1050Crossref, Medline, Google Scholar
29. : Delirium issues in palliative care settings. J Psychosom Res 2008; 65:289–298Crossref, Medline, Google Scholar
30. : Testing the Predictive Ability of a New Motor-Based Subtyping Scheme for Delirium. New Abstract NR-5–050.
31. : Factor analysis of the Colombian Translation of the Delirium Rating Scale (DRS), Revised-98. Psychosomatics 2009; 50:255–262Crossref, Medline, Google Scholar
32. : Misdiagnosing delirium as depression in medically ill elderly patients. Arch Intern Med 1995; 155:2459–2464Crossref, Medline, Google Scholar
33. : Association between psychomotor activity delirium subtypes and mortality among newly admitted post-acute facility patients. J Gerontol Series A: Biol Sci 2007; 62:174–179Crossref, Medline, Google Scholar
34. : Early symptoms in the prodromal phase of delirium: a prospective cohort study in elderly patients undergoing hip surgery. Am J Geriatr Psychiatry 2007; 15:112–121Crossref, Medline, Google Scholar
35. : A symptom rating scale for delirium. Psychiatry Res 1988; 23:89–97Crossref, Medline, Google Scholar
36. : A retrospective study on delirium type. Japanese J Psychiatry Neurol 1992; 46:911–917Medline, Google Scholar
37. : Delirium in a general-hospital psychiatric consultation service. Int Med J 1997; 4:181–185Google Scholar
38. : Eye-tracking technology: a fresh approach in delirium assessment? Int Rev Psychiatry 2009; 21:8–14Crossref, Medline, Google Scholar



