Clock-Drawing Performance Predicts Inpatient Rehabilitation Outcomes After Traumatic Brain Injury
Abstract
The authors used clock-drawing performance to assess cognition and predict inpatient rehabilitation outcomes among persons with traumatic brain injury. Clock-drawing performance, as assessed with the Clock Drawing Interpretation Scale, predicts rehabilitation length of stay as well as Functional Independence Measure scores at the time of neurobehavioral assessment and rehabilitation discharge.
Physicians working in inpatient neurorehabilitation settings are often asked to evaluate the cognitive status of persons with recent traumatic brain injury (TBI) and to give opinions on likely rehabilitation outcomes. Glasgow Coma Scale (GCS)1 scores and/or estimates of posttraumatic amnesia duration, when available, may be useful toward these ends.2,3 Unfortunately, these assessments are not consistently present in medical records.4 Formal neuropsychological assessment also may be used for these purposes.5 However, access to neuropsychological assessment services in the inpatient neurorehabilitation setting varies widely.6 Also, the timing, content, and advisability of performing of such assessments during acute inpatient TBI neurorehabilitation are matters of debate.5 Physicians therefore often rely on assessments such as the Mini-Mental State Exam (MMSE)7 or clock-drawing tests8 when performing bedside cognitive evaluations and developing preliminary TBI neurorehabilitation outcome predictions.
Clock-drawing is a relatively time-efficient cognitive screening assessment that is useful in the evaluation of patients with a broad range of cognitive abilities and is easily performed by physicians.9 For these reasons, clock-drawing is generally included in the Behavioral Neurology & Neuropsychiatry (BNNP) consultations we perform on our inpatient TBI neurorehabilitation service. To our knowledge, however, clock-drawing has not been described as a cognitive assessment or outcome predictor among persons receiving inpatient neurorehabilitation after TBI.
This retrospective study therefore explored clock-drawing performance in this population and its relationship with acute rehabilitation outcomes. The hypotheses tested were that clock-drawing performance predicts 1) total Functional Independence Measure (FIM)10 score proximate to the time at which clock-drawing is performed; 2) total FIM score at the time of inpatient rehabilitation discharge; 3) total FIM efficiency; and 4) rehabilitation length of stay.
METHOD
This study was approved by the Colorado Multiple Institutional Review Board and the HCA-HealthONE Institutional Review Board.
Subjects
We reviewed medical records of 83 patients consecutively admitted to an acute inpatient neurorehabilitation unit after TBI. Inclusion criteria for this study were 1) clinical diagnosis of TBI by American Congress of Rehabilitation Medicine definition;11 2) non-penetrating TBI; 3) age 20–89 years; 4) English as the primary language; 5) not aphasic; 6) BNNP consultation performed, including cognitive examinations required to address the study hypotheses; 7) intracranial abnormality on computed tomography (CT) or MRI of the brain consistent with recent TBI (i.e., intracranial hemorrhage, focal contusion, diffuse axonal injury); and 8) medical records containing all relevant assessment and rehabilitation outcome measures.
Among participants meeting these inclusion criteria, data extracted from medical records included: subject age, gender, and education; mechanism of injury; Galveston Orientation and Amnesia Test (GOAT)12 scores at the time of rehabilitation admission and BNNP consultation; neurological examination findings; clock-drawings performed during BNNP consultation; FIM scores at time of rehabilitation admission, proximate (±3 days) to BNNP consultation, and at discharge; and acute hospital and rehabilitation lengths of stay.
Clock-Drawing Test Administration and Scoring
Instructions for the clock-drawing task were as follows: “I'd like you to draw a large circle and fill it in with numbers as if it were the face of a clock. Make it large enough so that a child could read it. (After clock is drawn) Now place the hands on the clock so that it reads 10 after 11.” Clock-drawing performance was scored by one investigator, using Mendez' Clock-Drawing Interpretation Scale (CDIS).13 Approximately 3 years later, a subset (N=7) of these clock-drawings were re-scored by that investigator, who was blinded to other subject data and previous CDIS scores; intra-rater reliability was 0.95.
Functional Independence Measure Assessments
The FIM10 is an 18-item scale that assesses the type and amount of assistance required by an individual to perform basic activities of daily living (“Motor” FIM) and to apply cognition functionally (“Cognitive” FIM). The 13 items comprising the Motor subscale focus principally on self-care (e.g., continence, dressing, eating, hygiene), locomotion (e.g., walking or wheelchair use), and mobility (e.g., transfers). The 5 items comprising the Cognitive subscale assess the effective use of memory, communication, social interaction, and problem-solving skills in daily life. Functional ability on each item is rated using a 7-point Likert scale, on which higher scores reflect greater functional independence. The FIM assessments were performed by physical therapists, occupational therapists, speech-language pathologists, and nursing staff assigned to the acute inpatient rehabilitation unit, using the method described in the Guide for Uniform Data Set for Medical Rehabilitation, Version 5.1.10 FIM efficiency was calculated as (discharge total FIM score – admission total FIM score)÷RLOS.
Statistical Analyses
Statistica 8.0 (StatSoft, Inc., Tulsa, OK) was used for all descriptive data analyses as well as for regression analyses to test the study hypotheses. Multiple-regression modeling was used to investigate the proportion of variance in FIM scores, FIM efficiency, and RLOS accounted for by CDIS score, controlling for the effects of age. This method was also used to perform exploratory analyses of the proportion of variance in motor and cognitive FIM subscale scores accounted for by CDIS score.
RESULTS
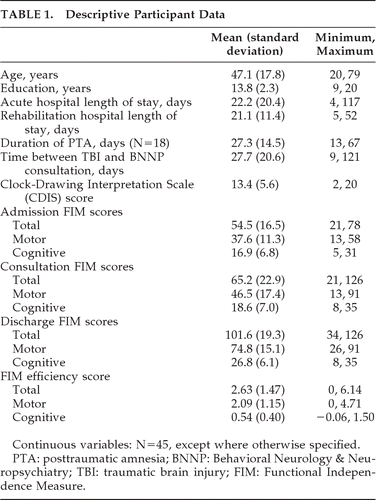
A total of 45 participants (10 women) met study inclusion criteria; these patients are described in Table 1 and examples of the clock drawings produced by this patient group are presented in Figure 1. Among these patients, causes of TBI included motor vehicle accidents (44.4%), falls (35.6%), sports/recreation (13.3%), and assaults (6.7%). At the time of rehabilitation admission, 18 patients (45.0%) were in posttraumatic amnesia; 11 (24.4%) were still in posttraumatic amnesia at the time of BNNP consultation (usually 5–6 days after rehabilitation admission). Mean CDIS score was 13.4 (standard deviation [SD]: 5.6), with a range of 2 to 20 (with a possible range of 0 to 20); these scores were normally distributed (Kolmogorov-Smirnov d=0.19, NS).
 |
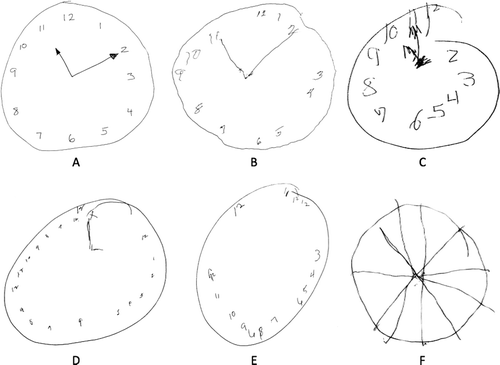
Clock-Drawing Interpretation Scale13 scores for these examples are A: 19; B: 17; C: 15, D: 12, E: 7; and F: 1.
Regression Modeling of FIM Scores and RLOS
CDIS score inversely correlated with patients' age (r = –0.41; p<0.005) but not education (r = –0.06; NS). After controlling for age, CDIS score predicted total FIM at consultation (adjusted R2=0.31; β=0.62; p<0.001) and discharge (adjusted R2=0.19; β=0.41; p<0.005) as well as Cognitive and Motor FIM subscale scores at consultation (adjusted R2=0.27; β=0.56; p<0.001 and adjusted R2=0.27; β=0.60; p<0.001, respectively) and discharge (adjusted R2=0.28; β=0.36; p<0.001 and adjusted R2=0.12; β=0.37; p<0.03, respectively). The combination of CDIS score and age predicts total FIM efficiency (adjusted R2=0.10; p<0.05). In this model, CDIS score contributed more strongly (β=0.30; p=0.06) than did Age (β = –0.13; NS). This combination predicts Motor, but not Cognitive, FIM efficiency (adjusted R2=0.11; p<0.04); CDIS score, but not age, contributed significantly to this model (β=0.34; p<0.04). Also, CDIS score predicts rehabilitation length of stay (adjusted R2=0.22; β = –0.53; p<0.002).
DISCUSSION
The CDIS performs well as a cognitive assessment in this population, yielding a broad and normally distributed set of values. CDIS predicts functional independence at the time of BNNP consultation as well as rehabilitation discharge, accounting for 31% and 19% of the variance in total FIM scores at these time-points, respectively. CDIS score accounted for 27% of the variance in both Motor and Cognitive FIM scores at the time of BNNP consultation as well as 12% of Motor FIM scores and 28% of Cognitive FIM scores at rehabilitation discharge. CDIS also predicts duration of inpatient rehabilitation hospitalization, accounting for 22% of the variance in rehabilitation length of stay. To our knowledge, this is the first report describing clock-drawing performance (and the CDIS, specifically) as a cognitive assessment and an outcome predictor among inpatients receiving neurorehabilitation after TBI.
The combination of CDIS and age predicts FIM efficiency—the rate of improvement in functional independence, defined as change in FIM score per day of rehabilitation—accounting for 10% of the variance in FIM efficiency score. In this model, CDIS trends toward independent prediction of FIM efficiency and independently predicts Motor FIM efficiency. It is likely that its failure to serve as an independent predictor of Cognitive FIM efficiency reflects inadequate power to do so as a result of this study's relatively modest sample size and the narrow distribution of Cognitive FIM efficiency scores.
Clock-drawing tests are used most often as qualitative assessments of cognition. However, this study suggests that there may be value to standardized administration and scoring of this test when assessing cognition and predicting functional status among persons with TBI in the inpatient rehabilitation setting. As reviewed and discussed by Shulman,9 clock-drawing possesses many of the qualities of an ideal cognitive screening test. Among the most relevant of these qualities to the present study are ease of administration and scoring, tolerability and acceptability to patients, relative independence from the effects of culture, language, and education, and, with respect to functional status and rehabilitation lengths of stay, predictive validity. The clock-drawing test used in our neuropsychiatric consultations adheres strictly to a script,8 in order to minimize examiner-specific influences on patient performance. When selecting a method for quantitative interpretation of clock-drawing performance, we sought one that provided easily-applied objective scoring anchors and, in light of our previous observations in this population,14,15 emphasized assessment of impairments in frontally-mediated cognition. Among the measures we considered,9 the CDIS13 appeared to meet these needs. This characteristic, we believe, also offers the most likely explanation for the predictive relationship between CDIS score and inpatient rehabilitation outcomes observed in this group of subjects with TBI.
Limitations of the present study include its retrospective design and use of data collected originally for clinical rather than research purposes. The measure used to interpret clock-drawings in this clinical sample, the CDIS, was not developed originally as an assessment of posttraumatic cognitive impairment. We also did not perform formal data-driven comparison of the CDIS with other clock-drawing scoring methods before performing the study described here. However, the psychometric properties of the CDIS are well-established,9 and its preliminary performance as a variable with which to test our study hypotheses was productive.
Although the CDIS was used in this study to predict functional status and rehabilitation outcomes among persons with TBI, we do not believe that this predictive relationship is specific to this population. Based on more than a decade of performing BNNP consultations in inpatient neurorehabilitation settings, we hypothesize that CDIS scores also will predict functional status and acute inpatient neurorehabilitation outcomes among persons with other recent-onset neurological disorders (e.g., stroke, hypoxic-ischemic brain injury, cerebral neoplasms). Testing this hypothesis will require prospective studies on these populations, the results of which will inform usefully on the merits of routinely incorporating the clock-drawing test into BNNP consultations performed in such settings.
The present study also does not inform fully on the cognitive and noncognitive contributors to clock-drawing performance in this population or their contributions to the variance in functional status and rehabilitation outcomes not accounted for by CDIS scores. Identification of the cognitive contributors to clock-drawing performance will require concurrent evaluation with a neuropsychological testing battery that is appropriate for use in an inpatient neurorehabilitation setting.5,16 Identification of the noncognitive contributors (e.g., disinhibition, perseveration, depression, anxiety, apathy) to clock-drawing performance will require use of a measure such as the Neuropsychiatric Inventory–Nursing Home version,17 which facilitates neuropsychiatric symptom-identification among patients with limited capacity for accurate self-report. Pending the performance of prospective studies that address these issues, our suggestion that the relationship between clock-drawing performance and functional status reflects frontally-mediated cognition remains speculative.
With these limitations in mind, translation of the statistical relationships identified here between the CDIS and our rehabilitation outcome variables is premature. On the other hand, the ability of the CDIS to predict rehabilitation outcomes in a data set derived from everyday clinical practice suggests that these observations may be more readily applied to clinical populations than those derived from a rarified research population. Further study of these issues in a manner that overcomes the limitations of the present study is warranted.
1. : Assessment of coma and impaired consciousness: a practical scale. Lancet 1974; 2:81–84Crossref, Medline, Google Scholar
2. : A review of the predictive ability of Glasgow Coma Scale scores in head-injured patients. J Neurosci Nurs 2007; 39:68–75Crossref, Medline, Google Scholar
3. : Classification schema of posttraumatic amnesia duration-based injury severity relative to 1-year outcome: analysis of individuals with moderate and severe traumatic brain injury. Arch Phys Med Rehabil 2009; 90:17–19Crossref, Medline, Google Scholar
4. : Prognosis of six-month functioning after moderate-to-severe traumatic brain injury: a systematic review of prospective cohort studies. J Rehabil Med 2010; 42:425–436Crossref, Medline, Google Scholar
5. : Feasibility of a brief neuropsychologic test battery during acute inpatient rehabilitation after traumatic brain injury. Arch Phys Med Rehabil 2008; 89:942–949Crossref, Medline, Google Scholar
6. : Concussion management by primary care providers. Br J Sports Med 2006; 401:e2; DiscussionCrossref, Google Scholar
7. : “Mini-Mental State:” a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198Crossref, Medline, Google Scholar
8. : Neuropsychiatry: An Introductory Approach. Cambridge, UK, New York, Cambridge University Press, 2001Google Scholar
9. : Clock-drawing: is it the ideal cognitive screening test? Int J Geriatr Psychiatry 2000; 15:548–561Crossref, Medline, Google Scholar
10. Guide for Uniform Data Set for Medical Rehabilitation (including the Functional Independence Measure Instrument), Version 5.1. Buffalo, NY, State University of New York at Buffalo; 1997Google Scholar
11. : Definition of mild traumatic brain injury: report from the Mild Traumatic Brain Injury Committee of the Head Injury Interdisciplinary Special Interest Group of the American Congress of Rehabilitation Medicine. J Head Trauma Rehabil 1993; 8:86–87Crossref, Google Scholar
12. : The Galveston Orientation and Amnesia Test: a practical scale to assess cognition after head injury. J Nerv Ment Dis 1979; 167:675–684Crossref, Medline, Google Scholar
13. : Development of scoring criteria for the clock-drawing task in Alzheimer's disease. J Am Geriatr Soc 1992; 40:1095–1099Crossref, Medline, Google Scholar
14. : Subtle neurological signs predict the severity of subacute cognitive and functional impairments after traumatic brain injury. J Neuropsychiatry Clin Neurosci 2009; 21:463–466Link, Google Scholar
15. : Comparison of the O-Log and GOAT as measures of posttraumatic amnesia. Brain Inj 2007; 21:513–520Crossref, Medline, Google Scholar
16. : The predictive validity of a brief inpatient neuropsychologic battery for persons with traumatic brain injury. Arch Phys Med Rehabil 2008; 89:950–957Crossref, Medline, Google Scholar
17. : The use of the Neuropsychiatric Inventory in nursing home residents: characterization and measurement. Am J Geriatr Psychiatry 2000; 8:75–83Crossref, Medline, Google Scholar



