CSF Thyrotropin-Releasing Hormone Gender Difference
Abstract
In light of the postulated role of thyrotropin-releasing hormone (TRH) as an endogenous antidepressant, 56 refractory mood-disordered patients and 34 healthy adult control subjects underwent lumbar puncture for cerebrospinal fluid (CSF) TRH analysis. By two-way analysis of variance, there was no difference between CSF TRH in patients (as a group or by diagnostic subtype) and control subjects (n=90, F=0.91, df= 2,84, P=0.41). There was, however, a CSF TRH gender difference (females, 2.95 pg/ml; males, 3.98 pg/ml; n=90, F=4.11, df= 1,84, P<0.05). A post hoc t-test revealed the greatest gender difference in the bipolar group (t=2.52, P<0.02). There was no significant difference in CSF TRH in “ill” vs. “well” state (n=20, P=0.41). The role of elevated levels of CSF TRH in affectively ill men—or the role of decreased levels of CSF TRH in affectively ill women—remains to be investigated but could be of pathophysiological relevance.
Many neuropeptides, through direct cerebrospinal fluid (CSF) measurement, have been implicated in the pathogenesis of affective illness.1–3 In the case of thyrotropin-releasing hormone (TRH), two clinical studies4,5 have reported elevated levels of CSF TRH in patients with acute depression, while a third study6 was negative. Of particular interest is the question of whether this TRH elevation or “hypersecretion” should be conceptualized as primary pathology—i.e., related to the mood disorder itself—or as a compensatory response to maintain homeostasis with potential therapeutic benefit.1
In addition to its role in the hypothalamic-pituitary-thyroid axis (HPT), many preclinical studies implicate TRH in a number of extra-HPT neuromodulatory processes. These processes have been compartmentalized into TRH “subsystems,” including limbic system-amygdala-hippocampus,7–14 preoptic anterior hypothalamus–suprachiasmatic nucleus,15 and brainstem–dorsal raphe.16
Neuroendocrine circadian rhythms must be taken into account when measuring TRH secretion into cerebrospinal fluid. Basic studies in rodents have identified diurnal rhythms in TRH content in both hypothalamic and extrahypothalamic locations.17 Specific rhythms in brainstem18 and cortex19 TRH levels have been identified that show periods separate and distinct from rhythms expressed in the hypothalamus. Clinical studies involving TRH measurements in human CSF have not addressed the question of circadian fluctuations in peptide concentrations. A single study in rhesus monkey,20 however, has examined this issue. TRH levels measured in the monkey were similar to those measured in humans, showing afternoon increases of approximately 33% of baseline levels. CSF TRH levels in the rhesus monkey were at baseline levels between 0800 and 1000 h. Although direct extrapolation cannot be made to humans, the observed nyctohemeral rhythm of human TSH secretion3 would be consistent with the TRH pattern observed in monkey. Given that lumbar punctures were performed at 0800–0900 h in the present study, TRH levels were presumably at or near baseline.
TRH administration in a number of animal models of depression has been reported to exert antidepressant properties,2,21 and in many clinical studies, as recently reviewed by Callahan et al.,22 TRH has shown antidepressant efficacy. In light of the postulated role of TRH as an endogenous antidepressant, this investigation was conducted to further assess the CSF levels of TRH in a refractory mood-disorder population.
METHODS
Fifty-six depressed inpatients (35 female, 21 male; 28 bipolar, 28 unipolar; average age 39.8±12.7 years) were studied in the Biological Psychiatry Branch, National Institute of Mental Health (NIMH). Their mood disorder was confirmed with the Structured Clinical Interview for DSM-III-R or DSM-IV shortly after hospital admission. They were otherwise healthy; specifically, there was no history of endocrinological illness, thyroid supplementation, or current alcohol use. Eleven of the patients had exposure to lithium in the 3 months prior to the lumbar puncture. Thirty-four medication-free control subjects (12 female, 22 male) with an average age of 32.5±10 years were used as a comparison group. Written informed consent was obtained after the lumbar puncture procedure had been explained to the patients and the control subjects.
Patients were in a double-blind, medication-free period for at least 2 weeks (mean=31.5±2.54 days). All of the patients remained symptomatic of their illness during the placebo washout. Both patients and control subjects adhered to a monoamine-restricted, caffeine-limited diet for the 2 weeks preceding the lumbar puncture. CSF was collected according to a standardized method that included an overnight bed rest and fast, with the procedure completed in a lateral decubitus position between 0800 and 0900 h (TRH levels presumably at or near baseline). To evaluate the effect of medications on mood and CSF TRH, a second lumbar puncture for patients was often obtained after 6 weeks of a blinded study medication (carbamazepine, nimodipine, lamotrigine, gabapentin, venlafaxine, or bupropion). Mood at the time of lumbar puncture was assessed by using a modified version (28 items) of the Hamilton Rating Scale for Depression (Ham-D).23
An aliquot of the 26th–27th cc of CSF fluid removed was placed in a –70°C freezer until the time of TRH radioimmunoassay. Human CSF aliquots of 100 μl were assayed in duplicate according to the method of Bassiri and Utiger.24 Assay sensitivity was 0.49 pg per tube, and the inhibitory concentration (IC50) was 30 pg. The interassay variation was 2.89%. All immunoassays were performed blind with regard to mood state and diagnosis.
To review the patient's past medical history, we used the retrospective life chart method NIMH-LCM™.25 The LCM™ allows for systematic quantification of a number of retrospective course-of-illness variables, including duration of illness, age at first symptoms, and number of prior episodes and hospitalizations. The mean length of acute episode and the duration of illness for this patient group were 10.6±2.7 weeks and 24.55±2.26 years, respectively.
In order to test previous reports of elevated CSF TRH, we used a two-factor analysis of variance (ANOVA) with post hoc t-tests, where indicated, to assess TRH differences by gender and diagnosis. Paired t-tests were used to assess differences in CSF TRH between conditions: on/off medications and “ill”/“well” (i.e., Ham-D score ≥18/<18). Finally, retrospective life chart measures were analyzed by Pearson correlation. Means plus or minus standard deviations are reported.
RESULTS
As presented in Figure 1, two-way ANOVA found no difference between CSF TRH in patients (as a group or by diagnostic subtype) and control subjects (bipolar, mean=3.70 pg/ml; unipolar, mean=2.99 pg/ml; control, mean=3.61 pg/ml; n=90, F=0.91, df=2,84, P=0.41). There was, however, a CSF TRH gender difference (females, 2.95 pg/ml; males, 3.98 pg/ml; n=90, F=4.11, df=1,84, P<0.05). A post hoc t-test revealed the greatest gender difference for the bipolar group (t=2.52, P=0.02) compared with the unipolar (t=0.74, P=0.50) and control groups (t=0.024, P=0.98). There was no significant CSF TRH difference between patients who had exposure to lithium in the 3 months prior to the lumbar puncture (n=11, mean=2.93 pg/ml) and patients who did not have lithium exposure within the 3 months (n=44, mean=3.45 pg/ml, t=0.652, df=53, P=0.52).
There was no correlation between CSF TRH and age (n=90, r=0.03, P=0.78). There was a correlation between CSF TRH and height (n=71, r=0.25, P=0.038) that was not significant when broken down by gender (n=38 female, r=0.17, P=0.30, n=33 male, r=0.05, P=0.78). There was no correlation between CSF TRH and length of time from lithium discontinuation (n=11, r=–0.34, P=0.31).
There was no correlation between CSF TRH and mean Ham-D score (P=0.63), length of acute depressive episode (n=42, r=–0.10, P=0.95), or duration of affective illness (n=41, r=–0.25, P=0.12). When CSF TRH was analyzed on/off lamotrigine or gabapentin (n=10), nimodipine (n=16), carbamazepine (n=15), or the antidepressants venlafaxine or bupropion (n=13), no significant changes were noted. Similarly, there was no significant difference in CSF TRH in “ill” versus “well” state (n=20, P=0.41).
There was no relationship between CSF TRH and age at first symptoms (P=0.28), number of prior episodes (P=0.58), or hospitalizations (P=0.73). Analysis by gender, however, revealed that males tended to have a positive correlation between CSF TRH and prior episodes (n=17, r=0.50, P=0.04) and hospitalizations (n=17, r=0.42, P=0.093).
DISCUSSION
These data in a refractory mood-disorder population with prolonged course of illness (approximately 25 years) do not confirm previous reports of elevated CSF TRH in acute depression.4–6 Furthermore, the lack of a state-dependent correlation (i.e., correlation with the Ham-D scale or “ill”/“well” state) replicates two5,6 of the three previous studies. (Kirkegaard et al.4 did not report severity of illness.) Additionally, our study did not find a difference in levels of CSF TRH on/off various agents, including carbamazepine, and thus does not replicate the previous report of Marangell et al.26
Notably, among these studies Banki et al.5 had the shortest acute episode length (3 weeks) associated with CSF TRH elevation; this finding contrasts with the present study and that of Roy et al.,6 where acute episode lengths of 2.5 months and 7.8 months, respectively, were not associated with a CSF TRH elevation. In addition to the prolonged length of acute episode in the present study, the lack of CSF TRH elevation could be related to a longer duration of illness (24.5 years) when compared with the only previously documented duration of illness (7 years).5 Kirkegaard et al. 4 did not report duration of acute episode or course of illness. Chronicity of illness could thus possibly contribute to a neuroendocrine “burnout,” which could theoretically erase a neuroendocrine perturbation, pathologic or compensatory, that was present earlier in an acute episode or course of illness. Although this hypothesis is quite speculative, it is supported by animal studies that show central TRH receptor downregulation in various brain regions following chronic exposure to TRH.27 The positive correlation in our study between CSF TRH and prior episodes and hospitalizations in males, however, does not support this hypothesis.
The CSF TRH gender difference in the present study appears not to be related to age, height, or, for patients, current severity of depression, duration of acute episode, or duration of illness. This study, comprising 90 subjects (47 female), provides greater power to detect a gender difference in comparison to the three previous studies,4–6 which comprised 46 patients (35 identified as female). This gender difference is similar to that found in the study by Fossey et al.,28 where male subjects had higher CSF TRH levels than female subjects (2.30±0.95 pg/ml vs. 1.69±0.80 pg/ml; t=2.61, df=54, P<0.02). It may be that the gender difference reported in the present study should be conceptualized as a relative increase in CSF TRH levels in males. Thus, the positive correlation between number of prior episodes and CSF TRH in affectively ill males could suggest a residual-state measure of prior episodes.
Alternatively, the gender difference could be conceptualized as a relative decrease in CSF TRH levels in women. This difference does have important implications, given epidemiological studies that indicate a higher lifetime prevalence rate of major depression in women.29 Additionally, as reviewed by Leibenluft,30 females with bipolar disorder have a greater propensity than bipolar males for rapid cycling, perhaps related to a “labile switch mechanism.” With the greatest gender difference appearing in the bipolar group, it is interesting to speculate whether the bipolar female depressed population has a TRH deficit that, when corrected, is associated with mood stabilization or switch “quiescence.” No formal test of this hypothesis has appeared in the literature.
The clinical literature for TRH as a somatic treatment for depression is extensive and complex.22 These complexities stem from evolving diagnostic criteria over 20 years of clinical experience, as well as differences in study design (case reports vs. open and double-blind studies), study duration, route of TRH administration (intravenous, subcutaneous, intrathecal), and criteria for response. Recently, Marangell et al.31 reported a significant reduction in depressive symptoms with intrathecal TRH (500 μg) in patients with severe refractory depression; further analysis of baseline CSF TRH (n=11) did not show a difference between intrathecal responders and nonresponders (Post et al., 1998, unpublished observations).
These data, although not consistent with previous reports of elevated CSF TRH in depression, do suggest important gender differences. The role of elevated levels of CSF TRH in affectively ill men or the role of decreased levels of CSF TRH in affectively ill women remains to be investigated but could be of pathophysiological relevance. Further study should be encouraged to clarify whether this gender difference has any potential implications for treatment of refractory mood disorder.
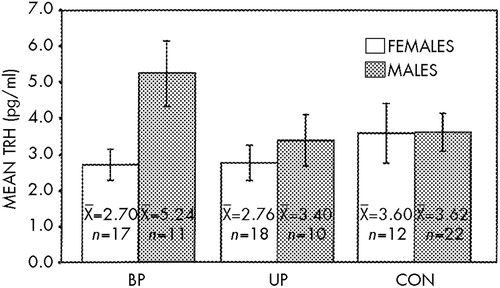
FIGURE 1. Gender differences in levels of thyrotropin-releasing hormone (TRH) in cerebrospinal fluid in bipolar (BP), unipolar (UP), and control (CON) subjectsAnalysis of variance: significant for Gender, P<0.05; nonsignificant for Diagnosis and Diagnosis×Gender. Post hoc t-tests, gender difference: BP, t=2.52, P=0.02; UP, t=0.74, P=0.50; CON, t=0.024, P=0.98
1 Post RM, Weiss SR: Endogenous biochemical abnormalities in affective illness: therapeutic versus pathogenic. Biol Psychiatry 1992; 32:469–484Crossref, Medline, Google Scholar
2 Nemeroff CB, Loosen PT, Bissette G, et al: Pharmacobehavioral effects of hypothalamic peptides in animals and man: focus on thyrotropin-releasing hormone and neurotensin. Psychoneuroendocrinology 1978; 3:279–310Crossref, Medline, Google Scholar
3 Winokur A: Neuroendocrinology of mood and anxiety disorders. Current Review of Mood and Anxiety Disorders 1997; 1:127–138Google Scholar
4 Kirkegaard C, Faber J, Hummer L, et al: Increased level of TRH in cerebrospinal fluid from patients with endogenous depression. Psychoneuroendocrinology 1979; 4:227–235Crossref, Medline, Google Scholar
5 Banki CM, Bissette G, Arato M, et al: Elevation of immunoreactive CSF TRH in depressed patients. Am J Psychiatry 1988; 145:1526–1531Google Scholar
6 Roy A, Wolkowitz OM, Bissette G, et al: Differences in CSF concentrations of thyrotropin-releasing hormone in depressed patients and normal subjects: negative findings. Am J Psychiatry 1994; 151:600–602Crossref, Medline, Google Scholar
7 Manaker S, Eichen A, Winokur A, et al: Autoradiographic localization of thyrotropin releasing hormone receptors in human brain. Neurology 1986;36:641–646Google Scholar
8 Sattin A, Pekary AE, Lloyd RL: TRH gene products are implicated in the antidepressant mechanisms of seizures. Ann NY Acad Sci 1994; 739:135–153Crossref, Medline, Google Scholar
9 Kubek MJ, Knoblach SM, Sharif NA, et al: Thyrotropin-releasing hormone gene expression and receptors are differentially modified in limbic foci by seizures. Ann Neurol 1993; 33:70–76Crossref, Medline, Google Scholar
10 Rosen JB, Cain CJ, Weiss SR, et al: Alterations in mRNA of enkephalin, dynorphin and thyrotropin-releasing hormone during amygdala kindling: an in situ hybridization study. Brain Res Mol Brain Res 1992; 15:247–255Crossref, Medline, Google Scholar
11 Kim SY, Post RM, Rosen JB: Differential regulation of basal and kindling induced TRH mRNA expression by thyroid hormone in the hypothalamic and limbic structures. Neuroendocrinology 1996; 63:297–304Crossref, Medline, Google Scholar
12 Winokur A, Utiger RD: Thyrotropin-releasing hormone: regional distribution in rat brain. Science 1974;185:265–267Google Scholar
13 Wan RQ, Noguera EC, Weiss SRB: Anticonvulsant effects of intrahippocampal injection of TRH in amygdala kindled rats. Neuroreport 1998; 9:677–682Crossref, Medline, Google Scholar
14 Stanton TL, Beckman AL, Winokur A: Thyrotropin-releasing hormone effects in the central nervous system: dependence on arousal state. Science 1981; 214:678–681Crossref, Medline, Google Scholar
15 Gary KA, Sollars PJ, Lexow N, et al: Thyrotropin releasing hormone phase shifts circadian rhythms in hamsters. Neuroreport 1996; 7:1631–1634Google Scholar
16 Funk D, Hall FS, Post RM, et al: Role of central dopaminergic and 5-hydroxytryptaminergic projections in the behavioral responses elicited by thyrotropin-releasing hormone in rats. Psychopharmacology 1997; 133:356–362Crossref, Medline, Google Scholar
17 Kerdelhue B, Palkovits M, Karteszi M, et al: Circadian variations in substance P, lutherin (LH-RH), and thyroliberin (TRH) contents in hypothalamic and extrahypothalamic brain nuclei of adult male rats. Brain Res 1981; 206: 405–413Google Scholar
18 Koivusalo F, Leppaluoto J: Brain TRF immunoreactivity during various physiological and stress conditions in the rat. Neuroendocrinology 1979; 29: 231–236Google Scholar
19 Martino E, Bambini G, Vaudagna G, et al: Effects of continuous light and dark exposure on hypothalamic thyrotropin-releasing hormone in rats. J Endocrin Invest 1985; 8: 31–33Google Scholar
20 Berelowitz M, Perlow MJ, Hoffman JH, et al: The diurnal variation of immunoreactive thyrotropin-releasing hormone and somatostatin in the cerebrospinal fluid of the rhesus monkey. Endocrinology 1981; 109:2102–2109Google Scholar
21 Metcalf G: Regulatory peptides as a source of new drugs: the clinical prospects for analogues of TRH which are resistant to metabolic degradation. Brain Res Brain Res Rev 1982; 4:389–408Crossref, Google Scholar
22 Callahan AM, Frye MA, Marangell LB, et al: Comparative antidepressant effects between parenteral and intrathecal TRH: confounding effects of tolerance and implications for therapeutics. Biol Psychiatry 1997; 41:264–272Crossref, Medline, Google Scholar
23 Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1978; 35:837–844Google Scholar
24 Bassiri RM, Utiger RD: The preparation and specificity of antibody to thyrotropin-releasing hormone. Endocrinology 1972; 90:722–727Crossref, Medline, Google Scholar
25 Leverich GL, Post RM: Life charting the course of bipolar disorder. Current Review of Mood and Anxiety Disorders 1996; 1:48–61Google Scholar
26 Marangell LB, George MS, Bissette G, et al: Carbamazepine increases cerebrospinal fluid thyrotropin-releasing hormone levels in affectively ill patients. Arch Gen Psychiatry 1994; 51:625–628Crossref, Medline, Google Scholar
27 Simasko S, Horita A: Treatment of rats with the TRH analog MK–771. Neuropharmacology 1985; 24:157–165Crossref, Medline, Google Scholar
28 Fossey MD, Lydiard RB, Ballenger JC, et al: Cerebrospinal fluid thyrotropin-releasing hormone concentrations in patients with anxiety disorders. J Neuropsychiatry Clin Neurosci 1993; 5:335–337Link, Google Scholar
29 American Psychiatric Association: Practice Guidelines for Major Depressive Disorder in Adults. Am J Psychiatry 1993; 150(suppl)Google Scholar
30 Leibenluft E: Women with bipolar illness: clinical and research issues. Am J Psychiatry 1996; 153:163–173Crossref, Medline, Google Scholar
31 Marangell LB, George MS, Callahan AM, et al: Effects of intrathecal protirelin (thyrotropin releasing hormone) in refractory depressed patients. Arch Gen Psychiatry 1997; 54:214–222Crossref, Medline, Google Scholar



