The Clock Drawing Task: Common Errors and Functional Neuroanatomy
Abstract
Clock-drawing is a simple and effective test to include in the neuropsychiatric assessment of patients.4 Reviews of the research literature support its use as a reliable screening tool for cognitive dysfunction, particularly for dementia.5,6 Although in use since the 1960s, it was made popular in 1983, when Goodglass and Kaplan incorporated it into the Boston Aphasia Battery.7–9 Although the majority of studies utilize the clock-drawing test to assess cognition in the context of screening for dementia, other conditions have also been evaluated. For example, recent studies have reported the use of a clock-drawing test for diagnosing and grading the severity of hepatic encephalopathy, predicting rehabilitation outcomes after traumatic brain injury (TBI), and assessing functional status in veterans with deployment-related mild TBI.10–12 At least 13 scoring systems have been introduced over the years. Shulman’s review, concluding that clock-drawing tests are complementary to the Mini-Mental State Exam (MMSE) and provide a significant advance in the early detection of dementia and monitoring cognitive change, also noted that a simple scoring system with emphasis on the qualitative aspects of clock-drawing should maximize its utility.5 Documenting the type of clock-drawing errors can contribute to the clinical evaluation of patients with suspected neuropsychiatric disorders and syndromes, both in the initial and subsequent assessments. It also has great educational value for patients’ families and in teaching trainees, as it provides an easily understood demonstration of subtle cognitive deficits sometimes challenging to identify in a routine bedside exam. The focus of this review is to present the major qualitative clock-drawing errors and the evidence linking performance with neuroanatomy. The wide variability in how clock-drawing tests are administered and scored is beyond the scope of this review.5
Clock-Drawing Errors
The most commonly utilized system of qualitative clock-drawing errors was described by Rouleau.1 He categorized five types of errors in addition to size of the clock (Figure 1): 1) graphic difficulties; 2) stimulus-bound response; 3) conceptual deficit; 4) spatial and/or planning deficit; and 5) perseveration. It is important to note that many publications do not include all six qualitative errors. There is greater emphasis on comparing errors due to stimulus-bound responses, conceptual deficit, spatial and/or planning difficulties, or perseveration. As noted above, there is also variability in how clock-drawing tests are administered. Some tests provide a predrawn circle and different time-settings. Most formal tests first instruct the patient to draw a clock (i.e., draw-to-command) and then copy a clock (i.e., copy-to-command). There is evidence that patients with Alzheimer’s disease (AD) make fewer errors on clock-drawing when asked to copy a clock, presumably because there is less utilization of executive functions and semantic memory.1

FIGURE 1. Representative illustration of common types of clock-drawing errors.1
Size of the Clock (not illustrated)
A clock-drawing is considered small if it measures less than 1.5 inches, and large if it measures more than 5 inches. Patients with Huntington’s disease (HD) have a higher incidence of small clocks, whereas patients with AD have a higher incidence of large clocks.1 The small clocks seen in HD may be due to the micrographia seen in disorders that involve the basal ganglia. The large clocks seen in AD may be a result of poor visuospatial planning due to impairment of executive (frontal lobe) and visuospatial (right parietal lobe) functioning.
Graphical Difficulties
Graphical difficulties are present when the lines are not precise, resulting either in distortions of the clock face or in numbers that are difficult to read. The hands are not straight and sometimes fail to connect in the middle. The overall performance may appear inaccurate and clumsy, but the drawing is still usually recognizable as a clock. Graphical errors are more common in HD and in moderate vascular dementias than in AD.1,13 Graphical errors were found to worsen in vascular dementia as the disease progressed.13 Graphical difficulties seen in both HD and vascular dementias are likely a result of secondary disruption of frontostriatal circuits necessary for coordinating fine motor control and planning.
Stimulus-Bound Response
A stimulus-bound response is the tendency of the drawing to be dominated or guided by a single stimulus, most often related to the time-setting instructions. Usually, the instructions are to set the time at “10 after 11.” In one error, the hands are set for “10 ‘til 11” instead of “10 after 11.” The patient fails to re-code the “10” in “10 after 11” as a “2” in order to set the minute hand. The patient is “attracted” to the strong stimulus source (i.e., “10”) rather than the appropriate response that involves a more complex operation (i.e., setting the minute hand at “2”). In the second type of stimulus-bound error, the “1” is written near the “11” or between “10 and 11” on the clock. The hands may be absent or pointed toward “10” and/or “11.” This second type of stimulus-bound error can also be rated as a conceptual error. Several studies have reported stimulus-bound errors to be more common in AD than in HD, Parkinson’s disease (PD), or frontotemporal dementia.1,14,15 However, contrary results have also been reported, with stimulus-bound errors more common in PD with dementia than in AD.16 One possible way to reconcile these findings is that frontostriatal circuits may be greatly impaired by the time PD progresses to dementia, resulting in executive-functioning deficits.
Conceptual Deficits
Conceptual deficits are defined as a loss or impairment in accessing knowledge of the attributes, features, and meaning of a clock. This category encompasses a wide variety of errors. Conceptual deficits in clock-drawing can be due to a drawing that does not look like a clock (i.e., misrepresentation of the clock) or drawing with hands that do not communicate a time (i.e., misrepresentation of time). Misrepresentation of the clock suggests the unavailability of a correct graphic representation of a clock. Misrepresentation of the time (e.g., hands absent, hands inadequately represented, the time written on the clock) suggests a deficit in the knowledge of the feature that confers most of the meaning of a clock, which is to communicate time.
Several studies have found that conceptual deficits in clock-drawing are more common and occur earlier in the course of disease-progression in AD than in HD, PD, vascular dementia, or frontotemporal dementia.14,16 Deficits may be evident even in mild cases and were more frequently seen as AD became more severe.1,17–19 There is evidence that conceptual errors are more common in patients with mild cognitive impairment (MCI) than in normal subjects, but clock-drawing is not considered a single screening tool for MCI.19–21 A lesion/deficit study found that injuries in the left inferior frontal-parietal opercular cortices were associated with time-setting errors.22 Conceptual deficits are likely due to impairment in semantic memory, a primary function of the lateral temporal lobes.23 Semantic memory refers to our store of conceptual and factual knowledge that is not related to any specific memory (i.e., episodic memory).
Spatial and/or Planning Deficits
Spatial and planning deficits are due to errors in the layout of numbers on the clock-drawing (e.g., neglect of the left hemispace, deficit in planning resulting in gaps in number spacing, deficit in spatial layout of numbers in absence of a specific pattern in spatial disorganization, numbers written outside the clock face, numbers written counter-clockwise). Spatial/planning deficits have been found to be more common in AD than in frontotemporal dementia or schizophrenia.14,24 This is likely due to greater parietal lobe involvement in AD. More spatial/planning deficits are present in PD with dementia, Diffuse Lewy Body (DLB) disease, or vascular dementia than AD.13,25 These findings are likely due to the subcortical involvement in these conditions. Clock-drawing errors due to spatial/planning deficits are due to impairment in the nondominant right hemisphere, especially the right parietal lobe.22 Perhaps the most commonly-referenced clock-drawing error is seen in the patient with a right parietal lesion that results in left hemispace neglect (the clock may have all the numbers aligned on the right side). In the case of stroke, this tends to be more evident in the acute phase. Patients may even have anosognosia and deny any errors or problems with the clock face. Furthermore, circuits that communicate between the parietal lobe and the frontal lobe (frontoparietal circuits) and between the frontal lobe and subcortical area (frontostriatal circuits) are needed to plan and accurately draw a clock face. Frontoparietal circuits likely play a role in coordinating the visuospatial understanding of a clock and frontostriatal circuits the executive functions that result in an accurate clock face.
Perseveration
Perseveration is defined as the continuation or recurrence of activity without an appropriate stimulus. In clock-drawing, this can be due to perseveration of hands (e.g., presence of more than two hands, reflecting a failure to terminate the ongoing set of tracing the hands) or perseveration of numbers (e.g., abnormal prolongation of numbers, such as writing beyond 12 or inappropriate recurrence of the same numbers). Perseveration errors are more common in AD than in normal subjects or in patients with schizophrenia.17,24 Perseveration clock-drawing errors are likely due to impairment of executive function in the prefrontal area of the frontal lobe, which is found in many dementia disorders.
Imaging
Several types of imaging evidence support the participation of both cortical and subcortical regions in performance of the clock-drawing task. An fMRI study in normal, healthy individuals performing a task that required drawing clock hands to indicate time found activations in both frontal and parietal regions (Figure 2).2 A study in patients with MCI, using resting-state fMRI to assess functional connectivity of dorsolateral prefrontal cortex (DLPFC), reported that connectivity to multiple brain regions (e.g., inferior parietal lobule, superior frontal gyrus, medial frontal gyrus, putamen) was reduced, but only the reduction in connectivity between left DLPFC and left thalamus correlated with performance impairment on the clock-drawing task (Figure 3).3 A study using voxel-based relaxometry to identify structural injury found that the clock-drawing task was more sensitive than several other commonly-used tests (e.g., MMSE, Trailmaking test Part A, Trailmaking test Part B) to the presence of small areas of brain injury, but that the injuries were to a wide variety of cortical (insula, temporal, frontal, posterior parietal) and subcortical (corpus callosum, caudate) locations.26 Another study found that white-matter abnormalities in the periventricular region were more strongly associated with impaired performance than were white-matter abnormalities in other areas, implicating fronto-subcortical connections.27 Several studies have utilized positron emission tomography (PET) and single photon emission computed tomography (SPECT) to examine the relationship between localized reductions in cerebral blood flow or cerebral metabolic rate and impaired performance on the clock-drawing task in various disorders. The most commonly-associated areas in patients with AD or MCI have been parietal and posterior cingulate cortices and the hippocampal region.28–30 Areas implicated in patients with DLB dementia include both cortical (frontal, temporoparietal) and subcortical (putamen, thalamus) regions.31,32 Studies utilizing voxel-based morphometry in patients with AD or MCI to assess the relationship between local gray-matter density and clock-drawing performance have reported associations with numerous cortical (temporal, frontal, parietal, cerebellum) and subcortical (thalamus, caudate) areas.33–35 Of note, a study comparing several scoring systems found that all correlated with regional gray-matter volume in the right parietal lobe, but they differed for other areas, indicating that choice of scoring system will influence results.34
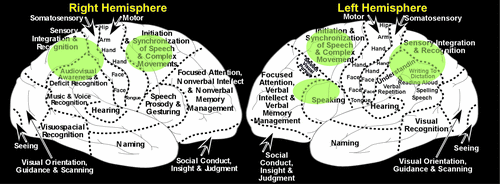
FIGURE 2. Illustrations of the left and right hemispheres, summarizing the major functions of the cortical regions.
A study using functional magnetic resonance imaging (fMRI) in normal, healthy individuals mapped the areas activated by the hand-placing portion of the clock-drawing task (approximate areas indicated in green), providing a general guide to the cortical networks involved.2
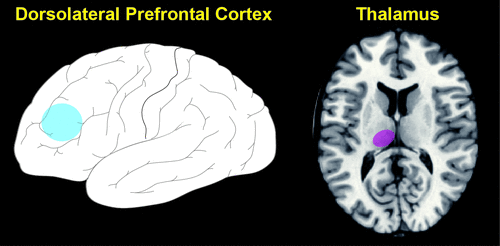
FIGURE 3. A recent study used resting-state fMRI to compare the functional connectivity of the dorsolateral area of prefrontal cortex (approximate location indicated in blue, DLPFC, BA 46) in patients with mild cognitive impairment (MCI) and healthy-controls matched for age and gender.3
In the MCI group, DLPFC had reduced connectivity with multiple cortical (inferior parietal lobule, superior and medial frontal gyri) and subcortical (putamen, thalamus) areas, indicating changes in both the fronto-parietal and fronto-striatal-thalamic circuits. Impaired performance on the clock-drawing task in the MCI group correlated with reduced connectivity between left DLPFC and an area in the left thalamus (approximate location indicated in purple). As noted by the authors, this finding suggests that fronto-striatal-thalamic disconnection may underlie, at least in part, the executive deficits found in these patients.
Only a handful of studies have examined the anatomic correlates of specific types of errors. A lesion/deficit study in patients with focal brain injury identified two predominant patterns of errors on the clock-drawing task.22 Problems with spatial organization and proper placement of numbers was more common in patients with right-hemisphere lesions, whereas problems with proper placement of clock hands (time-setting) were more common in patients with left-hemisphere lesions. The authors of this study noted that these findings are consistent with impaired visuospatial processing in the first group, and impaired language processing in the second. A study assessing the impact of high versus low load of periventricular white-matter abnormalities (WMA) on performance of clock-drawing in patients with dementia compared patients with either AD or vascular dementia to those with PD on total score and score by functional domain (gross motor, time, perseveration/pull, spatial layout).36 The Low WMA group had better performance than the other two groups (PD; High WMA), which did not differ on either total score or domain scores. The Low WMA group performed better than high-WMA on the gross motor domain and better than PD on time and spatial layout domains. As noted by the authors, these results support the participation of both cortical and subcortical areas in the performance of this task.
Conclusion
The neural correlates of clock-drawing performance have been studied primarily in clinical populations with dementias, and few studies have examined specific errors. Thus, there is much still to be learned. Although more research is needed to better understand the functional neuroanatomy of clock-drawing, it is clear that multiple brain regions, in cortical and subcortical areas, and the circuits that connect them, play a role. Impaired clock-drawing can provide a signal of cognitive impairment that guides the clinician to better understanding of the patient and the context of the presenting symptoms. The clinician can also use clock-drawing as a means of monitoring cognitive changes and providing a teaching tool for family and students.
1 : Quantitative and qualitative analyses of clock drawings in Alzheimer’s and Huntington’s disease. Brain Cogn 1992; 18:70–87Crossref, Medline, Google Scholar
2 : Parieto-frontal networks for clock-drawing revealed with fMRI. Neurosci Res 2003; 45:71–77Crossref, Medline, Google Scholar
3 : Functional disconnection and compensation in mild cognitive impairment: evidence from DLPFC connectivity using resting-state fMRI. PLoS ONE 2011; 6:e22153Crossref, Medline, Google Scholar
4 : The process approach to neuropsychological assessment of psychiatric patients. J Neuropsychiatry Clin Neurosci 1990; 2:72–87Link, Google Scholar
5 : Clock-drawing: is it the ideal cognitive screening test? Int J Geriatr Psychiatry 2000; 15:548–561Crossref, Medline, Google Scholar
6 : Literature review of the Clock Drawing Test as a tool for cognitive screening. Dement Geriatr Cogn Disord 2009; 27:201–213Crossref, Medline, Google Scholar
7 : The Parietal Lobes. Oxford, UK, Williams & Wilkins, 1966Google Scholar
8 : The Assessment of Aphasia and Related Disorders. Osford, UK, Williams & Wilkins, 1972, pp vii–51Google Scholar
9 : The Assessment of Aphasia and Related Disorders, 2nd Edition. Oxford, UK, Williams & Wilkins, 1983, pp vii–70Google Scholar
10 : Relationship between clock- and star-drawing and the degree of hepatic encephalopathy. Postgrad Med J 2011; 87:605–611Crossref, Medline, Google Scholar
11 : Clock-drawing performance predicts inpatient rehabilitation outcomes after traumatic brain injury. J Neuropsychiatry Clin Neurosci 2011; 23:449–453Link, Google Scholar
12 : Executive clock-drawing correlates with performance-based functional status in people with combat-related mild traumatic brain injury and comorbid posttraumatic stress disorder. J Rehabil Res Dev 2010; 47:841–850Crossref, Medline, Google Scholar
13 : Qualitative analyses of clock-drawings in Alzheimer’s disease and vascular dementia. Psychiatry Clin Neurosci 2001; 55:485–491Crossref, Medline, Google Scholar
14 : Quantitative and qualitative analyses of clock-drawing in frontotemporal dementia and Alzheimer’s disease. J Int Neuropsychol Soc 2006; 12:159–165Crossref, Medline, Google Scholar
15 : Base rates of “10 to 11” clocks in Alzheimer’s and Parkinson’s disease. Int J Neurosci 2009; 119:1261–1266Crossref, Medline, Google Scholar
16 : Characteristics of Clock-Drawing Test (CDT) errors by dementia type: quantitative and qualitative analyses. Arch Gerontol Geriatr 2009; 48:58–60Crossref, Medline, Google Scholar
17 : Screening for dementia of the Alzheimer type in the community: the utility of the Clock-Drawing Test. Arch Clin Neuropsychol 1996; 11:529–539Crossref, Medline, Google Scholar
18 : Longitudinal analysis of clock drawing in Alzheimer’s disease patients. Brain Cogn 1996; 31:17–34Crossref, Medline, Google Scholar
19 : Longitudinal changes in clock-drawing test (CDT) performance according to dementia subtypes and severity. Arch Gerontol Geriatr 2011; 53:e179–e182Crossref, Medline, Google Scholar
20 : Clock-drawing test in mild cognitive impairment: quantitative analysis of four scoring methods and qualitative analysis. Dement Geriatr Cogn Disord 2008; 26:483–489Crossref, Medline, Google Scholar
21 : Quantitative and qualitative analyses of The Clock-Drawing Test in mild cognitive impairment and Alzheimer disease: evaluation of a modified scoring system. J Geriatr Psychiatry Neurol 2011; 24:108–118Crossref, Medline, Google Scholar
22 : Does the Clock Drawing Test have focal neuroanatomical correlates? Neuropsychology 2008; 22:553–562Crossref, Medline, Google Scholar
23 : Understanding memory dysfunction. Neurologist 2009; 15:71–79Crossref, Medline, Google Scholar
24 : Comparison of a clock-drawing test in elderly schizophrenia and Alzheimer’s disease patients: a preliminary study. Int J Geriatr Psychiatry 2000; 15:638–643Crossref, Medline, Google Scholar
25 : Discrimination of dementia with Lewy bodies from Alzheimer disease and Parkinson disease using the clock-drawing test. Cogn Behav Neurol 2003; 16:85–92Crossref, Medline, Google Scholar
26 : Cognitive test performance and brain pathology. Nurs Res 2008; 57:75–83Crossref, Medline, Google Scholar
27 : Relation between the Clock-Drawing Test (CDT) and structural changes of brain in dementia. Arch Gerontol Geriatr 2009; 48:218–221Crossref, Medline, Google Scholar
28 : Neural correlates of impaired performance on the clock-drawing test in Alzheimer’s disease. Dement Geriatr Cogn Disord 2005; 19:390–396Crossref, Medline, Google Scholar
29 : Neural correlates of the Clock-Drawing Test performance in Alzheimer’s disease: a FDG-PET study. Dement Geriatr Cogn Disord 2008; 26:306–313Crossref, Medline, Google Scholar
30 : Poor performance in Clock-Drawing Test associated with visual memory deficit and reduced bilateral hippocampal and left temporoparietal regional blood flows in Alzheimer’s disease patients. Psychiatry Clin Neurosci 2008; 62:167–173Crossref, Medline, Google Scholar
31 : Cerebral substrates related to impaired performance in the clock-drawing test in dementia with Lewy bodies. Dement Geriatr Cogn Disord 2008; 25:524–530Crossref, Medline, Google Scholar
32 : Metabolic alterations associated with impaired clock-drawing in Lewy body dementia. Psychiatry Res 2010; 181:85–89Crossref, Medline, Google Scholar
33 : Clock-drawing performance and brain morphology in mild cognitive impairment and Alzheimer’s disease. Brain Cogn 2008; 67:88–93Crossref, Medline, Google Scholar
34 : Neural correlates of performance on the different scoring systems of the clock-drawing test. Neurosci Lett 2011; 487:421–425Crossref, Medline, Google Scholar
35 : Anatomical and functional deficits in patients with amnestic mild cognitive impairment. PLoS ONE 2012; 7:e28664Crossref, Medline, Google Scholar
36 : Clock-drawing errors in dementia: neuropsychological and neuroanatomical considerations. Cogn Behav Neurol 2004; 17:74–84Crossref, Medline, Google Scholar



