Latent Toxoplasmosis gondii: Emerging Evidence for Influences on Neuropsychiatric Disorders
Abstract
Toxoplasmosis gondii (T. gondii), an intracellular parasite discovered in 1908, is present in approximately one-third of the earth’s population.9,10 Its presence in humans was first recognized in 1923.11 Once infected, humans remain seropositive for life. Studies focused on likely transmission vectors and likely subpopulations more at-risk have confirmed previously-known exposure risks of feline feces, eating raw or undercooked meats, and drinking unpasteurized goat’s milk.12 Other exposure risks include eating raw oysters, clams, or mussels, and unwashed raw fruits and vegetables.13–17 There are well-documented effects of acute infection in immunocompromised patients (i.e., encephalitis and large intracranial cysts) and in infants with congenital ocular disease and/or mental retardation secondary to in-utero exposure. Latent chronic infection in immunocompetent (healthy) adult persons was thought to be benign. However, recent evidence has challenged this assumption. Within healthy immunocompetent populations, there have been increasing numbers of reported associations between T. gondii seropositivity and suicide, schizophrenia, psychiatric hospitalizations, depression, personality changes, and even, in very preliminary investigations, Alzheimer’s disease (AD).
In brief, T. gondii has three infectious stages: sporozoites (in oocysts), bradyzoites (in tissue cysts), and tachyzoites.9,10,14,17 The transmission life-cycle for humans usually begins with ingestion of the oocysts (found in soil, water, or feline feces) or tissue cysts (from undercooked meat). In either case, cysts open, and tachyzoites are quickly formed. These cross the gastrointestinal barrier, rapidly divide, and disseminate widely (can cross both placental and blood–brain barriers). In immunocompetent individuals, tachyzoites transform into the slow-growing bradyzoite stage, forming microscopic intracellular cysts in brain and muscle.11,14,15 Key for the parasite to continue in this latent, asymptomatic stage is to achieve a balance between coexistence in its host cells without destruction of the host’s tissues. The immune cascade is necessary for this coexistence and includes release of many immune components, including macrophages, interleukins, cytokines, and other factors.11,15,18
The feline (including the domestic cat) is the definite host for this parasite to undergo gametogenesis and sexual reproduction.9,10,15,19 Interestingly, T. gondii can use many mammalian species (e.g., livestock, rodents, humans, sea otters) as intermediate hosts in which it undergoes asexual reproduction.20 Long-standing evidence suggests a remarkable ability to manipulate a current host’s behavior to advance survivability and promote its propagation. This theory is termed “behavioral manipulation.”4,21–24 There are some highly specific behavioral changes consistently found over decades of studies in various laboratory and naturalistic experimental conditions in T. gondii-infected rodents. These include increased approach/decreased avoidance to feline urinary odors; decreased defensive behaviors, including spending more time in vulnerable open areas and decreased fear of novel stimuli; increased psychomotor activity; decreased climbing and rearing; and increased rate of entrapment.4,24–27 As noted by many authors, these are all behaviors that could lead to increased probability of predation. Studies indicate that the behaviors are not due to generalized illness/sickness. Many areas of behavior, learning, and social interactions are not affected, indicating the very focused nature of the behavioral changes. A multifaceted study that included magnetic resonance imaging (MRI), neuropathologic, immunologic, neurologic, behavioral, and appearance measures of mice infected at the equivalent of early or mid-adult years and then evaluated 1 year later (equivalent to middle-to-early elderly human years), reported significant differences in grooming and body position, increased urination/defecation while being held, decreased exploratory movements, and impairments of sensorimotor function.28 The seropositive group had many changes that echo human neurodegenerative disease patterns, including significantly enlarged ventricles on MRI (particularly near the aqueduct of Sylvius), contrast uptake asymmetries, increased expression of genes that can modify immune responses, and microscopic presence of inflammatory cells (particularly perivascular, hippocampal, and leptomeningeal, and in proximity to the aqueduct of Sylvius).
In view of the rodent evidence, scientists are beginning to examine whether the latent stage of T. gondii infection in healthy persons is as asymptomatic as previously thought. Although there has been no suggestion that the parasite by itself is the direct and single cause of neuropsychiatric illness in immunocompetent individuals, there are several significant correlations that are noteworthy. A series of 16 studies over 15 years elucidated T. gondii-related personality and psychomotor differences in healthy Czech citizens and military.29,30 In total, over 3,700 subjects had antibody testing and completed personality inventory examinations. Nine of eleven studies using the Cattell’s 16-Personality Factor self-report questionnaire found significant and consistent results for both genders. Seropositive men overall had lower regard for rules and higher vigilance (suspicious, jealous, rigid/inflexible) than seronegative men. In contrast, seropositive women had greater regard for rules and higher warmth than seronegative women. Both seropositive genders were more anxious than matched healthy-comparison subjects. Three of five studies using the Cloninger Temperament and Character Inventory found both seropositive women and men to have lower novelty-seeking behavior scores than healthy-comparison subjects. Behavioral observations and interviews were completed to ascertain whether the gender differences found in self-report measures were replicated by objective measures. Seropositive men scored significantly lower than seronegative men on Self-Control, Clothes Tidiness, and Relationships. The differences were less impressive for the seropositive women, with only trends toward higher scores on Self-Control and Clothes Tidiness as compared with seronegative women. The authors view the study results as objective confirmation that T. gondii presence can change a human host’s behaviors. In a later study, they presented evidence linking the observed differences to gender-specific coping styles (individualistic versus prosocial) under stress.31 This group also found slightly slower reaction times and decreased concentration in T. gondii-positive subjects and a higher relative risk for an at-fault traffic accident.29,30 In a later prospective study (3,890 male military conscripts), they confirmed a higher risk for traffic accidents in the seropositive group and found that risk was mitigated by RhD positivity.32 Such higher risk for traffic accidents has also been reported in Turkish populations.33,34
Higher rates of T. gondii seropositivity have been reported for several psychiatric conditions in multiple parts of the world. A series of studies comparing the prevalence rates of the parasite with the national suicide rates in 20 European countries found that suicide rates were higher in countries with greater T. gondii prevalence, with no effect of wealth/gross domestic product.17,35,36 Both presence of the Finno-Ugrian gene (previously associated with suicide) and T. gondii predicted higher suicide rates in men and women. When the data for women were stratified by age, they found strong statistical significance for higher suicide rates in T. gondii-seropositive postmenopausal women. A study from Turkey of emergency room patients with suicide attempts found that 41% of the patient group was seropositive, as compared with 28% of the controls.37 In the United States, significantly higher T. gondii antibody titers were found in mood-disorder patients with previous suicide attempts than in mood-disorder patients without previous suicide attempts or in controls.38 The same group reported that seropositivity was associated with suicide attempts in patients with schizophrenia younger than 38 years of age, but not in those older than 38.39 Several groups have reported elevated rates of seropositivity in inpatient psychiatric hospitals, as compared with community controls. One group examined 15 surveys across different areas of China, comparing rates of seropositivity in psychotic inpatients versus healthy individuals from the same provinces; 14 of the 15 reports indicated significantly higher rates of T. gondii antibodies in the psychotic inpatients.40,41 A study of German psychiatric inpatients reported a significant association between comorbid personality disorder and antibody titer.42 A study from Mexico found 18.2% of 137 inpatients to be seropositive, as compared with 8.9% of controls.13 The rate for patients with schizophrenia was 26.3%.
There have been a multitude of studies over decades looking for underlying causes of chronic schizophrenia, including many theories of infection; T. gondii has been considered since 1953.16 A very recent metaanalysis reviewing 38 published studies of T. gondii antibody seropositivity in patients previously diagnosed with schizophrenia compared with healthy controls (total: 6,058 patients; 8,715 controls) found that the patients were more likely to be seropositive (odds ratio: 2.71), confirming their previous study.43,44 As compared with other risk factors for development of the illness (including having first-degree relatives with the condition), T. gondii antibody positivity was considered an intermediate risk factor. To begin to ascertain whether having schizophrenia predisposes patients to intake of T. gondii oocytes (i.e., poor hygiene habits) or T. gondii is present before schizophrenia diagnosis, one group examined banked blood specimens from the U.S. military to determine rates of previous infection in new-onset schizophrenia within the military.45 Although it was a small study (180 patients, 8% positive; 532 controls, 7% positive), there was a significant association between antibody level and risk of schizophrenia (hazard ratio: 1.24), indicating that T. gondii could be considered one factor contributing to the illness.
A related line of study suggests the possible antiparasitic effects of select antipsychotic medications. The in-vitro antiprotozoan effects of phenothiazine-related compounds have been documented since the late 1800s.46 More recently, several studies have used human fibroblast cells infected with T. gondii tachyzoites to assess in-vitro anti-parasitic activity of psychiatric medications. One study reported modest anti-parasitic activity for sodium valproate; the other reported more robust activity for haloperidol and valproic acid.46,47 The second study also reported modest results for seven other antipsychotic compounds. A third study of five antipsychotics produced mixed results.48 Fluphenazine and thioridazine had a robust effect; trifluoperazine a modest effect; and haloperidol and clozapine, no effect. In-vivo studies have also produced mixed results. One study reported that haloperidol and valproic acid protected T. gondii-infected rats from developing detrimental behavioral changes (equal to the effects of standard anti-parasitic agents), whereas another found no anti-parasitic advantages on either preventing acute infection or reducing cyst burden in chronic infection.49,50
A very new area of possible linkage is that of T. gondii to Alzheimer’s disease (AD). In the inpatient study from Mexico (above), the authors mention that both patients in the study hospitalized for AD were seropositive for T. gondii.13 A study from Turkey compared rates of T. gondii IgG seropositivity in 34 patients with AD versus well-matched healthy individuals.51 The rates were significantly different: 44.1% in the AD group and 24.3% in the healthy-comparison subjects. Efforts to examine this linkage have been undertaken in a rodent model, using mice bred to carry the rodent equivalent of AD, with and without T. gondii infection.52 Mice infected with T. gondii had significantly lower rates of β-amyloid plaque formation in cortex and hippocampus, and maintained learning and memory skills that declined in the T. gondii seronegative group. The authors felt that these results were due to the increased presence of some anti-inflammatory processes in the seropositive group. They reaffirmed the importance of inflammation in the cascade of events necessary for AD progression and echoed a previous theme in the literature of a host–parasite balance with T. gondii. These early studies and observations may open new lines of study in neurodegenerative diseases.
The neuropathology of chronic latent T gondii infection is quite different from acute cerebral toxoplasmosis, in which necrosis and inflammation are widespread; infecting organisms may be present outside of host cells; and multiple types of brain cells may harbor parasites.53–56 Most studies are in animal models, but a case report of incidental findings at autopsy of an immunocompetent patient indicated a similar pattern in humans.56 An early study of chronic latent infection in mice (examined at 3, 6, and 12 months post-infection) reported that infected brains appeared normal on gross inspection.57 Parasite-containing cysts were found primarily in gray matter (90%). Ultrastructural examination of 50 cysts established that all were contained within intact host cells, most of which were positively identified as neurons by presence of synapses. Cysts were present in all parts (dendrite, axon, soma) of neurons, some of which were severely distended by the presence of a large cyst (Figure 1). A study of latent congenital infection in mice also reported that all cysts were contained within intact host cells.58 In that study, all host cells were identified as neurons on the basis of immunohistochemical staining of neurofilament protein. Later studies have consistently reported that cysts were found only in neurons in animals with chronic infection, although astrocytic processes could be in close association.2,5,28 Studies that assessed cyst burden at two times after infection (1 month versus 2 months; 2 months versus 6 months) reported an overall decrease (approximately half in both studies) at the later time-point, indicating that some clearance of cysts can occur (Figure 2).2,5 Although both studies noted areas in which the burden of cysts did not decline, the areas were not the same. Several studies have quantified the relative burden of cysts across brain areas at various times post-infection (Figure 2). At 1–2 months post-infection, there is a general pattern of a higher burden of cysts in subcortical areas than in cerebral cortex or cerebellum.2,4,5,7 However, there were considerable differences across studies in the relative ranking of the most commonly involved structures (e.g., amygdala, hippocampus, hypothalamus, striatum, thalamus). At 4–6 months post-infection, the general pattern was a higher burden of cysts in areas of cerebral cortex than in subcortical areas.5,6 The significance of these findings is difficult to assess, as studies varied considerably in many aspects of methodology, and most also reported considerable individual differences. As noted in one study, the finding of major differences across individuals does not support the presence of preferential targeting of specific areas (e.g., amygdala), as has been suggested as the basis for the induced behavioral changes.6
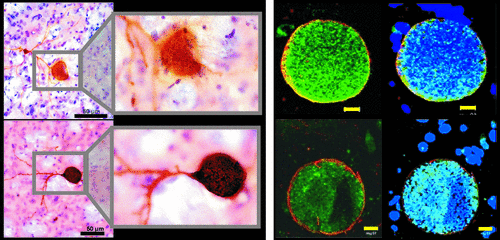
Cover and FIGURE 1. A coronal section from the Mouse Brain Library is shown on the cover.1Toxoplasmosis gondi cysts can be present in any part of a host neuron in the brains of mice with chronic latent infection. Shown on the lefta are representative brain sections immune-stained for T. gondii antibody (brown, black scale bar: 50 um).2 The upper set show a cyst within a dendrite of a striatal neuron; the lower set show a cyst within the soma of a cortical neuron (two levels of magnification). Note that T. gondii antigen is also present within the cytosol of the host neurons. Cysts can contain dopamine (or its immediate precursor), as illustrated on the right (yellow scale bar: 10 μm) for two different areas (upper and lower rows) immune-stained for anti-dopamine antibody (green) and TRITC-lectin (red, cyst wall).3 Addition of immune-staining with DAPI (blue, right column) provides visualization of neural cells and individual bradyzoites.
aReproduced under the terms of the Creative Commons Attribution License.
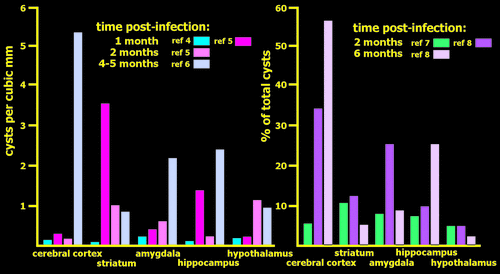
FIGURE 2. Studies quantifying T. gondii cyst burden in multiple brain areas in rodents with chronic infection found differences in relative burden across regions, but the patterns vary considerably.2,4–7 Results for a few commonly reported regions are illustrated, with longer times post-infection indicated by lighter colors. Studies are separated into two histograms, based on method of quantification used. Note that every anatomic area had the heaviest burden of cysts in at least one study.
The neurobiological origins of the behavioral changes in rodents induced by chronic latent infection with T gondii are presently not known, but several intriguing possibilities have been identified. The possible role of dopamine has been actively considered since a study found an increased level of dopamine, but not serotonin or norepinephrine, in brains of mice with chronic latent infection at 5 weeks post-infection.59 In contrast, dopamine level was not found to be affected in a study of congenitally-infected mice.60 Recent studies indicate that T gondii cysts may be able to directly influence synthesis of dopamine, as this parasite has two genes encoding tyrosine hydroxylases (the rate-limiting step in dopamine synthesis).61 Although they differed in other regions, both genes were very similar to the catalytic domain of mammalian tyrosine and phenylalanine hydroxylases. Tests demonstrated that expressed proteins were able to catabolize both tyrosine and phenylalanine, with a two-to-threefold preference for tyrosine. In cell cultures, expression of one of these genes was present at similar levels during all stages of infection, whereas the other increased greatly during the cyst-forming stage.61 In a follow-up study, immunofluorescence assay of brain sections from mice at 6–8 weeks post-infection showed intense staining within cysts for antibodies to both dopamine and T gondii tyrosine hydroxylase (Figure 1).3 Dopamine release was much higher (350%) in cultures of dopaminergic neurons that were infected, and the level correlated with parasite burden.3 These results raise the possibility that dopamine signaling may be altered in the vicinity of T gondii cysts, with both synaptic and volume transmission potentially affected.62 Direct impairment of the functioning of neurons containing cysts is another potential mechanism of action recently identified in a study in mice that compared behavioral, neuropathological, and functional measures at 30 days and 60 days post-infection.2 Although the burden of cysts was lower at the later time-point, changes in behavior were present at 60 days but not at 30 days. Also, at 60 days, more neurons had reduced or absent uptake of thallium (which acts as a potassium analog), indicating impaired functioning. As noted by the authors, these results indicate that duration of chronic infection may be an important factor and functional silencing of neurons may contribute to behavioral changes. There is also evidence for alterations in both focal brain volume and evoked activity in limbic areas in male rats infected with this parasite.63 Of potentially high relevance to the known changes in behavior associated with chronic infection, brain activation evoked by exposure to cat urine (assessed by c-Fos) was found not only in the expected areas important for defensive behaviors, but also in nearby areas associated with reproductive behaviors.
Unlike chronic latent infection, imaging in acute toxoplasma encephalitis has been well documented over the years. Gadolinium (Gad)-enhanced T1 MRI is the standard imaging procedure to identify the “target sign” of a ring-enhancing lesion containing a small area of hyperintense signal in acute toxoplasma encephalitis in immunocompromised patients.64,65 Gad contrast highlights breaks in the blood–brain barrier, with hyperintensities in abnormal areas. It may not be positive in “preclinical” or early-stage infections.65,66 Ultra-small superparamagnetic particles of iron oxide (USPIO), a new contrast agent for MRI, has been used recently to study neuroinflammatory diseases. Circulating monocytes in the bloodstream internalize these particles, which travel within the inflammatory cells into areas of phagocytosis. A study of 30 mice acutely infected with T. gondii (by intracerebral injection of tachyzoites) were imaged with Gad and/or USPIO at various time-periods in the acute (1 week) infectious process, followed by microscopic examination.66 The USPIO imaging (post-contrast T2 and T2* weighted images, decreased signal) identified several T. Gondii lesions not visualized on either unenhanced T2 and T2*, or Gad-enhanced T1-weighted MRI. However, the Gad imaging identified breaks in the blood–brain barrier more effectively than did the USPIO. The authors suggest that USPIO might be an additional imaging contrast to use in combination with Gad for earlier detection of subclinical neuroinflammatory processes. Perhaps, in the future, USPIO could be used to identify latent toxoplasma, as well.
Voxel-based morphometry (VBM), a method of measuring volumes of brain areas, was used to compare patients with schizophrenia (12 T. gondii seropositive; 32 seronegative) to a control group of matched (age, sociodemographic) individuals (13 T. gondii seropositive; 43 seronegative).8 As previously seen in multiple studies, the patients with schizophrenia had reduced overall gray-matter volume in cortical areas, hippocampus, and caudate. The novel aspect of this study is that they went on to examine the influence of T. gondii status. Patients with schizophrenia who were seropositive had significantly reduced gray-matter volume in left cerebellum and bilaterally in the caudate, thalamus, occipital cortex, and medial cingulate cortex, as compared with seronegative patients. When each patient group was compared with their respective controls, gray-matter reductions were much greater in the seropositive group (Figure 3). No differences related to T. gondii status were found in the control group. There were no differences in white-matter volume among any of the comparison groups. The authors proposed that T. gondii triggered release of immune factors leading to downstream changes in glutamate NMDA, and/or that effects on dopamine (described above) were possible contributing factors to the imaging differences.
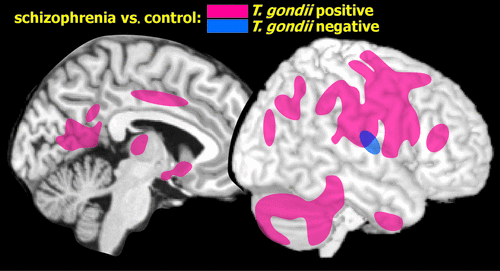
FIGURE 3. A recent voxel-based morphometry study assessed T. gondii status as well as average gray-matter volume in patients with schizophrenia [R] and matched healthy controls [L].8 As compared with controls, the group with schizophrenia had reduced gray-matter volume in multiple areas of cortex, hippocampus, and caudate (not illustrated). Both groups were split based on T. gondii status, and comparisons were repeated. Major areas of significantly reduced gray matter in the groups with schizophrenia are approximated on representative magnetic resonance images. Note the much greater number and extent of areas in the group of patients with schizophrenia that were positive (pink) than negative (blue) for T. gondii when compared with their respective healthy controls.
Conclusion
Toxoplasmosis gondii has been recognized as a human parasite for almost a century, yet much is still unknown about host reactions to its chronic presence. Recent evidence from both human and animal studies has led to new theories regarding how this parasite may change or manipulate a host’s behavior to gain advantage. Particularly fascinating are the possible links to neuropsychiatric disorders and/or personality changes that might occur or be exacerbated in individuals with chronic latent infection. These require more study to understand the possible interplay of this parasite with genetic predisposition and other factors resulting in neuropsychiatric illness.
1 : Mapping genes that modulate mouse brain development: a quantitative genetic approach. Results Probl Cell Differ 2000; 21–49Google Scholar
2 : Toxoplasma gondii activity inhibits neuronal function in chronically infected mice. PLoS ONE 2012; 7:e35516SCrossref, Google Scholar
3 : The neurotropic parasite Toxoplasma gondii increases dopamine metabolism. PLoS ONE 2011; 6:e23866Crossref, Medline, Google Scholar
4 : Behavioral changes induced by Toxoplasma infection of rodents are highly specific to aversion of cat odors. Proc Natl Acad Sci U S A 2007; 104:6442–6447Crossref, Medline, Google Scholar
5 : Host cell preference of Toxoplasma gondii in murine brain: a confocal study. J Neuroparasitol 2010; 1Crossref, Medline, Google Scholar
6 : The distribution of Toxoplasma gondii cysts in the brain of a mouse with latent toxoplasmosis: implications for the behavioral manipulation hypothesis. PLoS ONE 2011; 6:e28925Crossref, Medline, Google Scholar
7 : Chronic Toxoplasma infection modifies the structure and the risk of host behavior. PLoS ONE 2012; 7:e32489Crossref, Medline, Google Scholar
8 : Latent toxoplasmosis reduces gray matter density in schizophrenia but not in controls: voxel-based-morphometry (VBM) study. World J Biol Psychiatry 2012; 13:501–509Crossref, Medline, Google Scholar
9 : A brief history and overview of Toxoplasma gondii. Zoonoses Public Health 2010; 57:1–7Crossref, Medline, Google Scholar
10 : Mechanisms of Toxoplasma gondii persistence and latency. FEMS Microbiol Rev 2012; 36:717–733Crossref, Medline, Google Scholar
11 : Neuropsychiatric disease and Toxoplasma gondii infection. Neuroimmunomodulation 2009; 16:122–133Crossref, Medline, Google Scholar
12 : Risk factors for Toxoplasma gondii infection in the United States. Clin Infect Dis 2009; 49:878–884Crossref, Medline, Google Scholar
13 : Seroepidemiology of Toxoplasma gondii infection in psychiatric inpatients in a northern Mexican city. BMC Infect Dis 2006; 6:178Crossref, Medline, Google Scholar
14 : Effects of Toxoplasma gondii infection on the brain. Schizophr Bull 2007; 33:745–751Crossref, Medline, Google Scholar
15 : Toxoplasma gondii: host–parasite interaction and behavior manipulation. Parasitol Res 2009; 105:893–898Crossref, Medline, Google Scholar
16 : Toxoplasma and schizophrenia. Parasite Immunol 2009; 31:706–715Crossref, Medline, Google Scholar
17 : Toxoplasma gondii seropositivity and suicide rates in women. J Nerv Ment Dis 2011; 199:440–444Crossref, Medline, Google Scholar
18 : Potential immunomodulatory effects of latent toxoplasmosis in humans. BMC Infect Dis 2011; 11:274Crossref, Medline, Google Scholar
19 : Life cycle of toxoplasma gondii. BMJ 1969; 4:806Crossref, Medline, Google Scholar
20 : Patterns of mortality in southern sea otters (Enhydra lutris nereis) from 1998-2001. J Wildl Dis 2003; 39:495–509Crossref, Medline, Google Scholar
21 : Chronic Toxoplasma infections and familiarity–novelty discrimination in the mouse. Ann Trop Med Parasitol 1980; 74:145–150Crossref, Medline, Google Scholar
22 : Behavioural abnormalities in Toxoplasma-infected mice. Ann Trop Med Parasitol 1980; 74:337–345Crossref, Medline, Google Scholar
23 : Chronic Toxoplasma infections and motor performance in the mouse. Ann Trop Med Parasitol 1980; 74:507–510Crossref, Medline, Google Scholar
24 : The role of parasites and pathogens in influencing generalised anxiety and predation-related fear in the mammalian central nervous system. Horm Behav 2012; 62:191–201 [epub ahead of print]Crossref, Medline, Google Scholar
25 : Rats, cats, people, and parasites: the impact of latent toxoplasmosis on behaviour. Microbes Infect 2001; 3:1037–1045Crossref, Medline, Google Scholar
26 : The effect of Toxoplasma gondii on animal behavior: playing cat and mouse. Schizophr Bull 2007; 33:752–756Crossref, Medline, Google Scholar
27 : Behavioral changes in mice caused by Toxoplasma gondii invasion of brain. Parasitol Res 2012; 111:53–58Crossref, Medline, Google Scholar
28 : Neurological and behavioral abnormalities, ventricular dilatation, altered cellular functions, inflammation, and neuronal injury in brains of mice due to common, persistent, parasitic infection. J Neuroinflammation 2008; 5:48Crossref, Medline, Google Scholar
29 : Gender differences in behavioural changes induced by latent toxoplasmosis. Int J Parasitol 2006; 36:1485–1492Crossref, Medline, Google Scholar
30 : Effects of toxoplasma on human behavior. Schizophr Bull 2007; 33:757–760Crossref, Medline, Google Scholar
31 : Pattern of money allocation in experimental games supports the stress hypothesis of gender differences in Toxoplasma gondii-induced behavioural changes. Folia Parasitol (Praha) 2010; 57:136–142Crossref, Medline, Google Scholar
32 : Increased incidence of traffic accidents in Toxoplasma-infected military drivers and protective effect of RhD molecule revealed by a large-scale prospective cohort study. BMC Infect Dis 2009; 9:72Crossref, Medline, Google Scholar
33 : Is Toxoplasma gondii a potential risk for traffic accidents in Turkey? Forensic Sci Int 2006; 163:34–37Crossref, Medline, Google Scholar
34 : Higher prevalence of toxoplasmosis in victims of traffic accidents suggest increased risk of traffic accident in Toxoplasma-infected inhabitants of Istanbul and its suburbs. Forensic Sci Int 2009; 187:103–108Crossref, Medline, Google Scholar
35 : Brain parasites and suicide. Psychol Rep 2010; 107:424Crossref, Medline, Google Scholar
36 : Predicting European suicide rates with physiological indices. Psychol Rep 2010; 107:713–714Crossref, Medline, Google Scholar
37 : May Toxoplasma gondii increase suicide attempt: preliminary results in Turkish subjects? Forensic Sci Int 2010; 199:15–17Crossref, Medline, Google Scholar
38 : Toxoplasma gondii antibody titers and history of suicide attempts in patients with recurrent mood disorders. J Nerv Ment Dis 2009; 197:905–908Crossref, Medline, Google Scholar
39 : Toxoplasma gondii antibody titers and history of suicide attempts in patients with schizophrenia. Schizophr Res 2011; 133:150–155Crossref, Medline, Google Scholar
40 : Epidemiological evidences from China assume that psychiatric-related diseases may be associated with Toxoplasma gondii infection. Neuroendocrinol Lett 2007; 28:115–120Medline, Google Scholar
41 : Psychosis may be associated with toxoplasmosis. Med Hypotheses 2009; 73:799–801Crossref, Medline, Google Scholar
42 : The diagnosis of a personality disorder increases the likelihood for seropositivity to Toxoplasma gondii in psychiatric patients. Folia Parasitol (Praha) 2010; 57:129–135Crossref, Medline, Google Scholar
43 : Antibodies to Toxoplasma gondii in patients with schizophrenia: a meta-analysis. Schizophr Bull 2007; 33:729–736Crossref, Medline, Google Scholar
44 : Toxoplasma gondii and other risk factors for schizophrenia: an update. Schizophr Bull 2012; 38:642–647Crossref, Medline, Google Scholar
45 : Selected infectious agents and risk of schizophrenia among U.S. military personnel. Am J Psychiatry 2008; 165:99–106Crossref, Medline, Google Scholar
46 : Drugs used in the treatment of schizophrenia and bipolar disorder inhibit the replication of Toxoplasma gondii. Schizophr Res 2003; 62:237–244Crossref, Medline, Google Scholar
47 : Scriptaid and suberoylanilide hydroxamic acid are histone deacetylase inhibitors with potent anti-Toxoplasma gondii activity in vitro. J Parasitol 2007; 93:694–700Crossref, Medline, Google Scholar
48 : Evaluation of five anti-schizophrenic agents against Toxoplasma gondii in human cell cultures. J Parasitol 2011; 97:148–151Crossref, Medline, Google Scholar
49 : Parasites as causative agents of human affective disorders? the impact of anti-psychotic, mood-stabilizer, and anti-parasite medication on Toxoplasma gondii’s ability to alter host behaviour. Proc Biol Sci 2006; 273:1023–1030Crossref, Medline, Google Scholar
50 : Evaluation of the mood-stabilizing agent valproic acid as a preventative for toxoplasmosis in mice and activity against tissue cysts in mice. J Parasitol 2008; 94:555–557Crossref, Medline, Google Scholar
51 : Could Toxoplasma gondii have any role in Alzheimer disease? Alzheimer Dis Assoc Disord 2011; 25:1–3Crossref, Medline, Google Scholar
52 : Toxoplasma gondii infection in the brain inhibits neuronal degeneration and learning and memory impairments in a murine model of Alzheimer’s disease. PLoS ONE 2012; 7:e33312Crossref, Medline, Google Scholar
53 : Toxoplasmosis of the central nervous system in the adult: electron microscopic observations. Acta Neuropathol 1978; 41:211–216Crossref, Medline, Google Scholar
54 : Histopathology of cerebral toxoplasmosis in human immunodeficiency virus infection: a comparison between patients with early-onset and late-onset acquired immunodeficiency syndrome. Hum Pathol 1994; 25:1091–1097Crossref, Medline, Google Scholar
55 : Best cases from the AFIP: cerebral toxoplasmosis. Radiographics 2009; 29:1200–1205Crossref, Medline, Google Scholar
56 : Persistent toxoplasma bradyzoite cysts in the brain: incidental finding in an immunocompetent patient without evidence of a toxoplasmosis. Clin Neuropathol 2009; 28:210–212Crossref, Medline, Google Scholar
57 : The host–parasite relationship of Toxoplasma gondii in the brains of chronically infected mice. Virchows Arch A Pathol Anat Histopathol 1987; 411:39–43Crossref, Medline, Google Scholar
58 : An electron microscope and immunohistochemical study of the intracellular location of Toxoplasma tissue cysts within the brains of mice with congenital toxoplasmosis. Br J Exp Pathol 1989; 70:317–325Medline, Google Scholar
59 : Changes in brain concentrations of catecholamines and indoleamines in Toxoplasma gondii-infected mice. Ann Trop Med Parasitol 1985; 79:153–157Crossref, Medline, Google Scholar
60 : Congenital infection of mice with Toxoplasma gondii induces minimal change in behavior and no change in neurotransmitter concentrations. J Parasitol 2012; 98:706–712Crossref, Medline, Google Scholar
61 : A unique dual-activity amino acid hydroxylase in Toxoplasma gondii. PLoS ONE 2009; 4:e4801Crossref, Medline, Google Scholar
62 : The discovery of central monoamine neurons gave volume transmission to the wired brain. Prog Neurobiol 2010; 90:82–100Crossref, Medline, Google Scholar
63 : Predator cat odors activate sexual arousal pathways in brains of Toxoplasma gondii infected rats. PLoS ONE 2011; 6:e23277Crossref, Medline, Google Scholar
64 : High-intensity signals in the basal ganglia from gadolinium-enhanced T1-weighted MRI as an early change in toxoplasma encephalitis in an AIDS patient. J Infect Chemother 2010; 16:135–138Crossref, Medline, Google Scholar
65 : Eccentric target sign in cerebral toxoplasmosis: neuropathological correlate to the imaging feature. J Magn Reson Imaging 2010; 31:1469–1472Crossref, Medline, Google Scholar
66 : Detection of toxoplasmic lesions in mouse brain by USPIO-enhanced magnetic resonance imaging. Magn Reson Imaging 2007; 25:1442–1448Crossref, Medline, Google Scholar



