The Three-Dimensional Approach to Neuropsychiatric Assessment
Abstract
Brain–behavior relationships form the foundation for clinical assessment in neuropsychiatry and behavioral neurology. The complexity of the brain and its clinical disorders makes it important to have a systematic and useful way to apply them. This article introduces the three-dimensional approach to neuropsychiatric assessment (3DA), a process-based approach to integrating brain–behavior relationships into clinical activity. The 3DA is a simple, four-step process for teaching these relationships and their clinical use. The four steps are 1) Explain the principle of localization; first, as applied in general neurology; then, as applied to behavioral neurology and neuropsychiatry; 2) Review brain–behavior relationships in three dimensions: laterality (left–right), anteriority (anterior–posterior), and verticality (cortical–subcortical); 3) Introduce the “frontal–subcortical paradox” (subcortical dysfunction may cause what many know as “frontal lobe” signs) and its explanation (the neurobehavioral correlates of the frontal–subcortical circuits); 4) Present model disorders for the three dimensions. The presentation describes the rationale and approach for using the 3DA to teaching neuropsychiatry and behavioral neurology.
The brain is organized in three dimensions. We all know this. Our knowledge of brain–behavioral relationships associates signs and symptoms of brain disease with dysfunction of neural systems, each with its own location and function. Students learning neuropsychiatry and behavioral neurology develop their clinical approach by learning these relationships. But the nervous system is complex, and it is easy to feel overwhelmed by the wealth of facts and relationships. Consensus recommendations for neuropsychiatry and behavioral neurology1,2 tell us much of what we should teach, but little of how. In other words, the content curriculum of neuropsychiatry and behavioral neurology is relatively well defined. The process for teaching it is not.
The three-dimensional approach to neuropsychiatric assessment (3DA) is a simple, four-step process for introducing students to these relationships and their clinical use. The four steps are 1) Explain the principle of localization; first, as applied in general neurology; then, as applied to behavioral neurology and neuropsychiatry; 2) Review brain–behavior relationships in three dimensions: laterality (left–right), anteriority (anterior–posterior), and verticality (cortical–subcortical); 3) Introduce the frontal–subcortical paradox (subcortical dysfunction may cause what many know as “frontal lobe” signs) and its explanation (the neurobehavioral correlates of the frontal–subcortical circuits); 4) Present model disorders for the three dimensions, one each for laterality and verticality, and two for anteriority, one for anterior and one for posterior.
A word is in order about the teaching atmosphere for the 3DA. Many of the details that make up the 3DA are familiar to students and residents: they usually remember that localization is important in clinical neurology; they are able to work through basic examples of localization in two dimensions; they have heard about anosognosia, frontal lobe syndromes, aphasia and the like; they remember a little about Phineas Gage and, sometimes, the frontal–subcortical circuits. Since familiarity fosters a sense of competence, their motivation to go more deeply into neuropsychiatry and behavioral neurology is aided by presenting the material interactively with clear as well as tacit indications that “a lot of what we’ll be talking about is already familiar to you.” Then, as new material is presented, students are more inclined to offer their best guess at an answer. In fact, it is advisable to encourage them to guess or speak their first thoughts because we will be clearer then about what they do and do not know and how best to fit the new information to their current understanding. This speeds up learning.
Now, the 3DA’s four steps, each step presented as a question:
Step 1: Where Is the Lesion?
The first step in the 3DA is reminding students of general neurology’s clinical dictum: localize the lesion. Neurology students learn that “Where is the lesion?” is the first question to answer in assessing new cases. Localizing the region of dysfunction is a crucial first step in clinical evaluation because it orients us to the fundamental organizing principle of clinical assessment of neurological disease: as a first approximation, the signs and symptoms of neurological disease are usually best accounted for by identifying the site and, thereby, the neural system affected. Localization is also valuable because the number and location of lesions has implications for diagnosis and treatment.
The 3DA is based on three dimensions; however, general neurology usually uses just two. Why three dimensions for the 3DA? In general neurology, laterality and verticality—left/right, up/down—suffice for most clinical questions, and certainly suffice to introduce the basics of clinical neurology. Refined questions may benefit from a three-dimensional approach. For introductory purposes though, a two-dimensional approach usually suffices. For example, it is helpful to know that the association of a left hemiparesis with right facial weakness suggests a right-sided pontine lesion.
In neuropsychiatry, a two-dimensional approach is helpful, too. For example, a dense, right hemiparesis associated with aphasia suggests dysfunction of the left hemisphere, and this relationship has psychiatric implications: left anterior hemisphere dysfunction is associated with depression and with executive cognitive dysfunction. Left hemiparesis associated with the same nonfluent aphasia calls for a different explanation. We may delve further into the history, perhaps to find a history of previous stroke,3 left-handedness, or a diagnosis of lung cancer with bilateral hemispheric metastases.
However, limiting ourselves to two dimensions creates problems in neuropsychiatry. Without distinguishing all three dimensions: left/right, front/back, up/down—students will have trouble making clinical sense of patients who show more complex symptom pictures. For example, it is not easy to make a functional-anatomical interpretation of supranuclear palsy’s symptom picture: memory complaints, executive cognitive loss, extrapyramidal motor dysfunction, oculomotor signs, personality change, and motivational loss (see Case #4 and Conclusions). The 3DA organizes such data into clinical patterns in ways that are helpful anatomically, diagnostically, and therapeutically.
Step 2: What Are the Brain–Behavior Relationships in Three Dimensions?
Presenting brain–behavior relationships in three dimensions speeds up learning. Students may know that hemispheric behavioral organization is complex; what they don’t know is how to keep track of it all, how to see the coherence of brain–behavior relationships, and how to apply these relationships in clinical assessment.
Laterality
Laterality relationships are the most familiar. Therefore, it is generally best to review them first: sensory-motor functions are organized contralaterally in the cerebral hemispheres; language is usually a dominant function of the left hemisphere; visuospatial capacity and attention are dominant capacities of the right hemisphere. These are basics, of course, but they deserve review. Refinements and exceptions can be dealt with later (Table 1). For instance, a more advanced group will appreciate global characterizations of hemispheric function, for example, analytic/synthetic, sequential/holistic, and the relationship of these functional properties to clinical phenomena; for example, aphasia is predominantly a left-hemisphere syndrome, but Han Chinese speakers, perhaps reliant on visuospatial capacities to decipher the complex design of Chinese characters, may be more likely to show aphasia with right-hemisphere lesions.4 Thus, it may not be language that is localized, but the type of information-processing capacity suited to a particular language.
| 3 Questions: | ||
|---|---|---|
| 1. Where is the lesion? | 2. What are the brain–behavior relationships in 3 dimensions? | 3. What is the frontal–subcortical paradox? |
| 3 Dimensions: | ||
| Dimension #1: Laterality | Left | Right |
| Cognitive | Cognitive | |
| Language: dysphasia, dyslexia, dysgraphia | Visuospatial deficits | |
| Other dysfunction of higher cortical functions: apraxia, agnosia | Nonverbal memory deficit | |
| Verbal memory deficit | Nonverbal fluency | |
| Verbal fluency impairment | Left hemi-neglect | |
| Dyscalculia | Behavioral: Dressing apraxia, anosognosia, prosopagnosia | |
| Finger agnosia | ||
| Motor: Contralateral hemiparesis and upper neuron signs | Motor: Contralateral hemiparesis and upper motor neuron signs | |
| Sensory: Contralateral hemisensory loss, including dysgraphesthesia, astereognosis, and extinction with double simultaneous stimulation | Sensory: Contralateral hemisensory loss, including dysgraphesthesia, astereognosis, and extinction with double simultaneous stimulation | |
| Dimension #2: Anteriority | Anterior | Posterior |
| Cognitive | Cognitive | |
| Language: Nonfluent aphasia. | Language: Fluent aphasia | |
| Emotional: Expressive dysprosodia | Emotional: Receptive dysprosodia | |
| Executive cognitive dysfunction: impaired sequencing, planning, working memory; go/no-go deficit; diminished verbal fluency (word-generation) | Visuospatial deficits | |
| Behavioral | Spatial disorientation | |
| Stimulus-bound behavior/environmental dependency | Hemi-neglect | |
| Motivation: apathy, loss of initiative, abulia | Diminished nonverbal fluency (design-generation) | |
| Personality change: disinhibition, loss of social graces, loss of empathy and social judgment | Behavioral: Anosognosia, prosopagnosia, dressing apraxia | |
| Motor: Luria motor-sequencing deficit; gait apraxia; primitive (“frontal release”) signs | ||
| In general: sparing of personality and motivation, although right parietal disease may be associated with personality change, apathy, and emotional indifference. | ||
| Motor: Contralateral hemiparesis (less severe than anterior) | ||
| Sensory: Contralateral hemisensory loss, as above | ||
| Dimension #3: Verticality | Cortical | Subcortical (Basal ganglia) |
| “Frontal” (i.e., anterior) behaviors and cognitive deficits | “Frontal” (i.e., anterior) behaviors and cognitive deficits | |
| Cognitive: Aphasia, agnosia, apraxia | Cognitive: | |
| Executive cognitive impairment | Executive cognitive impairment | |
| Retrieval memory deficit | Retrieval memory deficit | |
| Recall memory deficit (amnesia) | ||
| Emotional: dysprosodia | ||
| Behavioral: Depression, apathy, prosopagnosia, anosognosia, dressing apraxia | Behavioral: Depression, apathy, flat affect | |
| Motor: Contralateral hemiparesis | Motor: Slow speech and gait, dysarthria | |
| Extrapyramidal Motor Signs: rigidity, cogwheeling, dyskinesia | ||
| Sensory: Contralateral cortical sensory loss, as above | ||
| What is the frontal–subcortical paradox? | It refers to the paradox of so-called “frontal lobe” features resulting from subcortical dysfunction. Specifically, it refers to the presence of “anterior” symptoms (above) resulting from disruption of subcortical components of the frontal cortical–basal ganglia–thalamic circuits | |
| 2 DBQs: | DBQ #1: Lateral to Posterior? What features are expected in DAT (or other posterior dysfunction disorders) | Or, ask: Having reviewed laterality brain–behavior relationships, what features are expected with posterior hemispheric dysfunction? |
| DBQ #2: Cortical to subcortical? How do we explain the occurrence of “frontal,” (i.e., anterior hemispheric) features in basal ganglia disease? | ||
| Clinical examples: | Laterality: | |
| 4 Model disorders for 3 dimensions: | Case #1: Tumor, stroke, or head injury with lateralized impairments | |
| Anteriority: | ||
| Case #2: Anterior Hemispheric Dysfunction (e.g., FTD) | ||
| Case #3: Posterior hemispheric dysfunction (e.g., DAT) | ||
| Vertical (Cortical–Subcortical): | ||
| Case #4: Basal ganglia disease (including features of “frontal” dysfunction (i.e., illustrating the frontal-subcortical paradox; e.g., progressive supranuclear palsy) | ||
| Applications and refinements: | 3D approach provides opportunity to introduce and review other key neurobehavioral concepts, including: | |
| °Classification of aphasia, aprosodia, apraxia and agnosia | ||
| Other syndromes amenable to 3D approach: | °Hemispheric specialization: (Verbal/Nonverbal | |
| °Aphasia | Sequential/Holistic) | |
| °Aprosodia | °Cortical networks and localization of function. | |
| °Disorders of attention |
Anteriority
The front/back relationships of hemispheric function are less familiar. A link to the familiar helps, though. Therefore, after introducing the laterality relationships, we remind students of frontal lobe syndromes by posing the question: “What are the behavioral changes we see in people with frontal lobe disease?” This question usually elicits information about personality change, especially disinhibition symptoms. “Inappropriate social behavior,” “aggression,” and “sexual impropriety” are other answers. Students often know that there are problems with executive cognition in frontal lobe disease, although the specifics of executive processes are less familiar. In preparation for the discussion of the frontal–subcortical paradox, it is helpful here to define executive cognition and to point out that executive cognitive functions supplant the older notion of a “frontal lobe syndrome.” Asking about Phineas Gage rounds out the introduction of anterior hemispheric dysfunction because most of us hear in medical school about Gage’s apathy and withdrawal. If “apathy” and “inactivity” have not been volunteered already, they will likely be elicited with the Phineas Gage prompt. Disinhibition, inappropriate behavior, apathy, and these others are features of anterior hemispheric dysfunction. It is helpful to group and label them. I label them in broad strokes as changes in 1) personality, 2) executive cognition, and 3) motivation; this anticipates the anatomical-functional refinements of the three frontal–subcortical circuits whose clinical correlates correspond to this grouping.5
Next are the functions and symptoms associated with posterior hemispheric regions. Bridging to posterior hemispheric functions, I usually ask: “So what would a patient look like if they had cortical disease that spares the prefrontal regions?” At this point, students easily recognize that there will be preservation of personality, motivation, and executive cognitive functions. A few will be able to answer the question: “Can you think of any disorders in which you see exactly this: cognitive loss with preservation of personality?” The answer is dementia of the Alzheimer type (DAT). I introduce DAT here as a model disorder of posterior hemisphere dysfunction. One should note at some point in the discussion that DAT’s typical presentation is predominantly posterior, but may include anterior signs and symptoms, as well. I encourage everyone to recall a patient or a family member with DAT. They usually can. And, when they can, they usually will recall that, if not severely impaired, the person did indeed show preservation of social graces and personality in the face of clear memory impairment.
With a familiar case in mind, we then consider: “Given what we’ve been talking about, what other cognitive changes would you expect to find in DAT?” This question is a dimension-bridging question (DBQ), the first of two; in each instance, a challenge and a prompt for the audience to integrate information across dimensions, as follows: In my experience, this question often is met with uncertainty. We are looking for someone to say language and visuospatial dysfunction. This usually does not happen, though. Language and visuospatial dysfunction are usually thought about in relationship to laterality, especially since they were just introduced as such. Therefore, they are not accessed at this moment because the discussion is characterizing the less-familiar anterior/posterior dimension—unless we paraphrase the DBQ as, “What comes to mind if you reflect on what we were talking about a few minutes ago regarding the left/right dimension?” This question helps students make the connection; the bridge is made: language and visuospatial function, initially thought about as lateralized, are also organized front-to-back—“posteriorized,” one might say. Thereby, students now make the connection. In DAT, we expect personality, motivation, and executive cognition to be relatively spared (early in the disease course), whereas visuospatial and language function—plus memory—are affected early. In other words, visuospatial, language, and memory impairment are the cognitive triad that characterizes the early presentation of DAT.
Classifying visuospatial and language functions as both lateralized and posteriorized, we have now introduced two of the three dimensions. We have also provided a foundation for using DAT and frontal temporal dementia (FTD) as model disorders, DAT as a model disorder of posterior hemispheric dysfunction, and FTD as a model of anterior hemispheric dysfunction. It’s worth making this explicit to students: “Let’s keep FTD and DAT in mind as model disorders of anterior and posterior dysfunction.” This brings in familiar clinical information and cements a bit more solidly the brain–behavior relationships and their 3D associations. This is also a helpful time to note that DAT and FTD are labels for clinical syndromes, closely related to, but distinct from, the diseases (Alzheimer’s disease and a variety of cortical diseases that underlie DAT and FTD, respectively). There are other disorders and pathogenetic processes that can serve these purposes, but DAT and FTD have the virtues of familiarity and utility.
Verticality
The up/down dimension is the cortical–subcortical dimension of brain–behavior relationships. It is easily introduced by relating extrapyramidal motor symptoms to basal ganglia disease and then discussing non-motor symptoms of basal ganglia disease. Thus, staying with the familiar and hoping to keep continuity with the disorders of anterior and posterior cortical dysfunction just offered as model disorders, we simply ask, “What is an example of a disease that is primarily a disease of subcortical structures; that is, defined primarily by basal ganglia pathology?” Parkinson’s disease is easy to work with. Progressive supranuclear palsy (PSP), Huntington’s disease, and Wilson’s disease also serve the purpose. The disease identified by the group will be maintained as the model disorder of subcortical dysfunction. After quickly reviewing distinctive motor features (dyskinesia, rigidity, flexed postured, marche au petit pas, etc.) of, let us say, Parkinson’s disease, we now ask, “And what about non-motor features of Parkinson’s disease? What are some of the behavioral features of Parkinson’s disease?” This is usually a rough spot in the discussion, but, after a little work, participants usually stitch together a pretty accurate profile: these disorders are also associated with deficits in executive cognition, motivation, and personality. We make sure everyone knows that subcortical pathology is often associated with executive cognitive impairment, not just psychomotor slowing. Beyond this, we want especially to make sure everyone sees that this symptom cluster: executive cognitive dysfunction, apathy, personality change—is the cluster we associate with the “frontal lobe syndrome,” that is, the anterior dimension.1 This serves as the jumping-off point for the next DBQ: “How can we make sense of the fact that patients with basal ganglia disease seem to show all the symptoms of frontal lobe disease?” Actually, I like to present the question by generating a little uncertainty: “So what do you think about this? How can we make sense of the fact that patients with basal ganglia disease show all the symptoms of frontal lobe disease? Personality change, apathy, and executive cognitive impairment are the signs of anterior hemispheric dysfunction. They point to the cortex, to the frontal cortex. This is puzzling isn’t it? How can we explain this?”
Step 3: What Is the Frontal–Subcortical Paradox?
Over the past 25 years, delineation of the frontal–subcortical reentrant circuits has become, with little question, the single most important functional-anatomic model for understanding neuropsychiatric dysfunction. Building on the groundbreaking work of Alexander, Delong, and Strick,6 and others,7 clinical neuropsychiatry acquired5 an anatomically-based model for understanding the functions and connectivity of the prefrontal regions of the cerebral cortex. The frontal–subcortical model elegantly parses prefrontal cortex into a small number of regions, each with a specific relationship to a major domain of adaptive capacity—personality change, executive cognition, motivation, oculomotor function, and somatic motor function. Like all models, it has its limits. It oversimplifies. It is incomplete. It is in flux. However, it remains a solid framework for introducing students to the clinical spectrum of hemispheric behavioral dysfunction. And it offers a simple, easily-digested, visual image (Figure 1) for understanding the paradox that patients with basal ganglia disease often have neurobehavioral symptoms that might otherwise be attributed to frontal cortex dysfunction. Therefore, the functional-anatomic connectivity of the frontal–subcortical circuits answers the DBQ we used to introduce the vertical dimension of the 3DA: How do you explain the fact that some patients with basal ganglia disease behave as if they have a frontal lobe syndrome? The reason patients with basal ganglia disease show signs of “frontal lobe” dysfunction is, simply, that the basal ganglia are integral parts of the frontal–striatal–pallidal–thalamic circuits that mediate these capacities. The problem here is not clinical; we do not have to decide whether the lesion is cortical or subcortical. Rather, the problem is that “where is the lesion?,” the first principle of clinical neurology, is not a principle of neurological function. As we learned many years ago: lesions are localized; functions are not. Functions reflect actions and interactions of systems and subsystems, circuits and subcircuits. Functions are anatomically-mediated, but they are not anatomically localized. We want students to understand this. The frontal–subcortical paradox gives us a good opportunity to make the point.
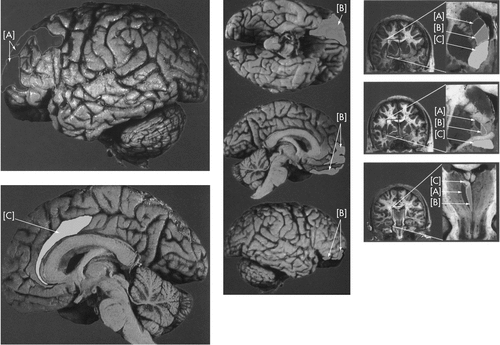
[A]: executive cognitive functioning (dark gray shading); [B]: personality (medium gray shading); [C]: motivation (light gray shading).
How does the 3DA resolve the frontal–subcortical paradox? The 3DA reminds students, or, perhaps more often, introduces them to the functional organization of the cortical–striatal–pallidal–thalamic reentrant circuits. My approach is to present a question: “What is the subcortical connectivity of the cerebral cortex? Choose a point on the cerebral cortex and describe its connections with the basal ganglia and thalamus? An occasional student, usually an M.D.-Ph.D. neuroscientist, will know the answer. The cerebral cortex, virtually every part of it, projects to striatum (i.e., caudate or putamen); striatum projects to globus pallidus; globus pallidus projects to thalamus; and thalamus projects back to the cerebral cortex, roughly the same region of the cerebral cortex from which we began.
This is complicated. So a diagram or two helps. The presentation of Mega and Cummings,5 (Figure 1), because it’s clear and color-coded, makes this particularly easy to visualize and, furthermore, lets us introduce the functional specificity of the prefrontal circuits, three with psychological features (executive cognitive function [A; dark shading], personality [B; light shading], and motivation [C; white]) and two with motor features (general motor and oculomotor). This description foreshadows the use, shortly, of progressive supranuclear palsy as a model disorder in Step 4.
Step 4: Model Disorders of the Three Dimensions
The final step in the 3DA is to demonstrate its clinical use with model disorders. The model disorders offer clinical-pathological paradigms for each of the three dimensions. They also permit students to analyze a clinical case in 3DA terms. Instructors may choose cases according to their background and the experience of their students. The cases I use are: for laterality, a case of stroke; for anteriority, FTD and DAT, illustrating, respectively, anterior and posterior aspects of anteriority; for verticality, progressive supranuclear palsy, to illustrate the subcortical, basal ganglia aspect. Regardless of the conditions chosen, it is particularly important to choose separate cases to illustrate anterior and posterior because clinical features in the anteriority dimension are often intermixed and difficult to distinguish.
Case #1: Laterality
A 62-year-old single business executive with a history of hypertension presents with depression during the first weeks after a hemorrhagic stroke. When he first presented to the emergency room, he was comatose. Brain-imaging demonstrated a left-hemisphere hemorrhagic stroke, centralized in the area of the caudate and putamen. After neurosurgical decompression, the patient was fully conscious, initially appearing to have delirium, but, over the following days, showing more clearly the first signs of a nonfluent aphasia (effortful, sparse speech, with good auditory comprehension and impaired repetition) and depressed mood, along with a dense right hemiparesis. Visuospatial capacity and elementary attention (e.g., immediate recall, digit-span forward) were spared. Tasks of mental control (serial 7s, Trails B) and Luria hand–motor sequencing were markedly impaired. Over 6 months, his mood symptoms improved markedly, but now, at 2-year follow-up, he is again depressed, perhaps because he is frustrated in his forced retirement and lacks social support, since he never had a circle of friends outside of work.
Case #2: Anteriority (Anterior Aspect)
Frontotemporal dementia (FTD): A widower in his early 60s leads the way into the office. He is affable, talking spontaneously, almost pressured at first. Initially, he seems personable, perhaps too friendly, talking too much. He seems indifferent to your welcoming him into the office. He is casually and appropriately dressed, but his shirt is soiled and his hair is in some disarray. He is eating an apple as he enters the room and sits himself down before being invited to do so, setting the half-eaten apple on the table-top. He has no complaint, and does not agree with his son, who is present, that he has become argumentative and depressed. He dismisses his son’s suggestion that he is forgetful, although he can’t recall the agreement he made to “just talk to the doctor, Dad.” His speech is clear; his thought is goal-directed; and, after the first few minutes, his speech is sparse, rather than pressured as it had been initially. His thought content focuses on immediate concerns: Are we almost finished? Where are we going for lunch? His son’s attempts to engage him in conversation are fruitless. He is unconcerned about the son’s concerns and similarly indifferent, perhaps disdainful, as the physician suggests to him that there might be a significant problem if the family went to all these efforts to bring him in for this appointment. Although irritable when challenged, he is unconcerned about what’s happening. When asked about his interests and plans for the future, he offers little—a walk around the block, a TV “soap” in the afternoon, and “a good dump” (i.e., bowel movement), followed later by a few other vulgarities that embarrass his son, who assures you that he would never have talked this way 2 years ago. Gait and motor function are unaffected, except for some difficulty with Luria hand-sequences, the presence of a frontal grasp reflex, and a number of stimulus-bound behaviors, for example, mirroring the examiner’s gestures, repeating some of the examiner’s words, and grabbing for a five-dollar bill when the examiner asks him to name the president depicted on its face.
Case #3: Anteriority (Posterior Aspect)
Dementia of Alzheimer’s type (DAT): A 73-year-old woman, presenting with a complaint of memory loss, enters the office, carefully attired, wearing white gloves and a white velvet hat. She speaks naturally and has a bright, engaging alertness. However, closer listening discloses a slightly stilted speech pattern, a subtle, seeming formality in her choice of words. Occasionally she repeats herself. Her train of thought is organized, but gradually it becomes apparent that she repeats herself periodically. She is somewhat perseverative, apparently not able to generate new ideas. On confrontation naming, she has difficulty coming up with new words and sometimes offers paraphasic responses, for example, a pen is called a “pinkle.” She uses her hands expressively. Her demeanor is dignified but, like her speech, subtly stilted. Her gait is natural and stable. She is polite and considerate. There is no evidence of weakness or slowing when she gestures or walks. She is cheerful, perhaps too good-spirited, given her memory problems. She learns 4 words after only 2 trials, but, 15 minutes later, recalls only 1 of the 4, and does not improve with cueing. She is anxious when her word-finding and memory problems are evident, but she is not depressed. She has difficulty copying a cube and drawing the face of a clock, adding just a few numbers and positioning the hands at the numbers 10 and 11 when asked to show the hands set to 11:10. She shows perseveration in her attempts to copy serial loops and has difficulty in performing three-step Luria hand-sequences.
Case #4: Verticality (Subcortical, Basal Ganglia Aspect)
Progressive supranuclear palsy: A 55-year-old accountant was referred because of depression. He was recently discharged from the hospital where he was admitted after a fall. At that time, history indicated gradually-developing difficulty walking over 6–12 months. His wife was concerned that he was depressed. He acknowledges difficulties completing his work, which he attributes to visual loss. He has become less active socially, largely, he claims, because of his concerns about walking. His wife, however, suggests that he just doesn’t take an interest in seeing friends as he used to. On examination, his gait is slow, flexed, and small-stepped; tone is rigid, but he shows no tremor. Affect is flat. He acknowledges feeling somewhat depressed, although the admitting physician felt that the patient was more apathetic than depressed. Examination of cranial nerves was within normal limits, except for difficulty reading, which was associated with inability to look downward. Evaluation on the Montréal Cognitive Assessment showed a total score of 24, with points lost because of difficulty with the Trails B, clock-drawing, and memory recall task. Recall improved substantially with category and multiple-choice cueing. Neuropsychological assessment confirmed difficulty with mental control and visuospatial perception. On the Wisconsin Card-Sorting task, he was able to complete only one set and showed frequent perseverative errors.
Discussion Points for Examples
Teachers will have their own ideas for how to make use of these cases. Based on the presentation here, one may highlight some of the following. Case #1 illustrates the laterality dimension and permits review of the basic features of hemispheric dominance and asymmetry. More advanced discussion enters into the qualitative differences between the hemispheres (Table 1). Case #1 makes the point that the hemispheres share basic sensorimotor functions, which are organized contralaterally, and that important domains of neuropsychological function—language versus attention and visuospatial capacity—are dominant functions of the left and right hemisphere, respectively. Discussion of other higher cortical functions follows naturally from this. Definitions and classifications of apraxia, agnosia, aphasia, and aprosodia may be introduced as time permits. Less-familiar concepts, for example, Gerstmann’s syndrome and prosopagnosia, may be introduced, depending on the audience. In turning to the anteriority dimension, the model cases demonstrate the fundamental difference between syndromes of anterior and posterior hemispheric dysfunction. DAT and FTD are readily appreciated for this purpose. However, it is equally important to point out that we are demonstrating anatomic-functional relationships that extend to all classes of neurological disease, not just degenerative diseases. It is also helpful to acknowledge the overlap of symptoms between diseases that are preponderantly anterior and posterior, making it clear that clinical diagnosis has limited validity and, as Hickam’s dictum10 tells us, many patients have more than one disease. In either case, the 3DA is a powerful organizing device for characterizing the clinical presentation. This benefit is clearly illustrated as we make use of Cases #2 and #4 to illustrate the intriguing overlap between diseases affecting prefrontal cortex and those affecting subcortical white matter and basal ganglia. Both may produce “frontal syndromes” due to disruption of frontal–subcortical circuits. In this context, we emphasize that lesions may be localized to location, but functions are localized to circuits and subcircuits. The 3DA makes this point in explaining the frontal–subcortical paradox. Students find the frontal–subcortical paradox a tidy way of holding on to the simple and paradoxical fact that patients with subcortical disease, as in Case #4, present so-called “frontal” signs. In using Case #4, it is also helpful to indicate that it is the presence of basal ganglia motor features (rigidity, bradykinesia, movement disorder, etc.) that points more specifically to the presence of basal ganglia disease. Absent motor features, the frontal–subcortical paradox implies that features of prefrontal disease may reflect either cortical or subcortical dysfunction.
Conclusions
Brain–behavior relationships form the practice foundation for neuropsychiatry and behavioral neurology. They are an essential element of content curricula and training programs in neuropsychiatry and behavioral neurology.8,9 The complexity of the brain and its clinical disorders makes it important to have a systematic and useful way to apply them. The three-dimensional approach to neuropsychiatric assessment provides a simple, systematic approach for integrating brain–behavior relationships into this process. It is based on readily-identified, important clinical features of brain dysfunction. With practice, instructors will find that the essence of the 3DA can be effectively communicated in a very short period of time: 10 to 20 minutes is ample for an initial introduction. At the bedside, returning to 3DA principles makes it possible to refine basic points and show students how easily they can bring knowledge into practice.
The 3DA is a process-based approach to teaching neuropsychiatry and behavioral neurology. It is easy to understand and implement. Novice students find that it identifies the basics of behavioral neurology and neuropsychiatry so that scattered bits of clinical and neuroanatomical information quickly funnel into a systematic, easily remembered, and useful approach to dealing with diverse clinical problems. Like general neurology, it is anatomy-based and therefore gives users a disciplined way to assess new cases, regardless of specific disease process.
The 3DA allows modifications according to the interests of the instructor and the sophistication of the student. Although developed as an introduction to the basics of neuropsychiatric assessment, it is refinable to accommodate subtleties of functional-anatomic organization. The basic vocabulary of signs and syndromes can be expanded, for example, to include Gerstmann’s syndrome, subtypes of aphasia, apraxia, aprosodias, etc. Discussion of hemispheric function and organization can be refined to review concepts of hemispheric specialization, for example, sequential and holistic, analytic/synthetic; and complex cases can be elucidated by pointing out anatomically-based symptom clusters, for example, the way symptoms of PSP (Case #4) can be attributed to the dorsolateral (executive cognitive symptoms), orbitofrontal (personality change), mesial (apathy), oculomotor (visual and reading complaints) and supplementary motor (extrapyramidal findings) subcircuits of the frontal–subcortical systems.
In summary (Table 1), the 3DA is a four-step process for teaching neuropsychiatric assessment. The steps are 1) understand localization of function; 2) organize brain–behavior relationships in three dimensions; 3) apply the frontal–subcortical paradox; 4) illustrate the three dimensions with cases that represent model disorders. It uses dimension-bridging questions (DBQs) to prompt knowledge-integration across dimensions. Although it is intended primarily for initial assessment—as a way to orient the practitioner to the salient features of clinical presentation—the initial 3D formulation can be applied to later stages of treatment planning and evaluating changes in clinical status.
Comprehensive curricula1,2 and training programs8,9 in neuropsychiatry and behavioral neurology may readily integrate the 3DA. Also, 3DA principles may be used as springboards to deepen students’ knowledge of the symptoms, syndromes, and diseases that comprise a full content curriculum. In these and other ways, the three-dimensional approach to neuropsychiatric assessment invigorates the content curriculum with practice principles that enhance students’ appreciation of neuropsychiatry’s rich and challenging opportunities.
1 Neurology B, Requirements N: United Council for Neurologic Subspecialties, St. Paul, MN, 2006; available at www.ucns.orgGoogle Scholar
2 : Core curriculum for training in behavioral neurology and neuropsychiatry. J Neuropsychiatry Clin Neurosci 2006; 2006:6–13Crossref, Google Scholar
3 : Lesion location and poststroke depression: systematic review of the methodological limitations in the literature. Stroke 2004; 35:794–802Crossref, Medline, Google Scholar
4 : Crossed aphasia in Chinese: a clinical survey. Brain Lang 1990; 39:347–356Crossref, Medline, Google Scholar
5 : Frontal-subcortical circuits and neuropsychiatric disorders. J Neuropsychiatry Clin Neurosci 1994; 6:358–370Link, Google Scholar
6 : Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 1986; 9:357–381Crossref, Medline, Google Scholar
7 Heimer L, Van Hoesen GW, Trimble M, et al: Anatomy of Neuropsychiatry: The New Anatomy of the Basal Forebrain and Its Implications for Neuropsychiatric Illness. Waltham, MA, Academic Press, 2008Google Scholar
8 : Behavioral neurology and neuropsychiatry fellowship training: the Johns Hopkins model. J Neuropsychiatry Clin Neurosci 2009; 21:335–341Link, Google Scholar
9 : The Perspectives of Psychiatry. Baltimore, MD, Johns Hopkins University Press, 1998Google Scholar
10 : Noble J. David, M.D., reminisces. J Neuroophthalmol 2002; 22:240–246Crossref, Medline, Google Scholar



