Risk Factors for Psychosis Secondary to Temporal Lobe Epilepsy: A Systematic Review
Abstract
The authors critically reviewed all relevant peer-reviewed quantitative research pertaining to the risk factors for psychosis secondary to temporal lobe epilepsy, carrying out an extensive literature search to identify all relevant research studies, and applying specific exclusion criteria; the search yielded 27 original research articles for critical review. These studies were individually assessed for methodological quality. Authors reached consensus on a number of important risk factors for psychosis, including early age at epilepsy onset, history of status epilepticus, hippocampal sclerosis, and left-hemisphere abnormalities. Inconsistencies in defining and diagnosing epileptic psychoses were apparent, as well as the sole use of case–control, cross-sectional, and/or retrospective study designs. There remains a need for research using consistent classification criteria and longitudinal study designs.
The World Health Organization estimates that 50 million people worldwide have epilepsy, making this one of the most common neurological disorders, along with stroke and Alzheimer’s disease.1 Epilepsy is associated with a well-documented range of neurological and physical sequelae, as well as a range of psychological and social stressors and effects.2 It is not surprising, then, that psychiatric comorbidities are frequent in epilepsy, with research demonstrating their presence in 39%−54.1% of all epilepsy patients,3,4 and 10% of all inpatients in one psychiatric hospital had diagnosed epilepsy.5 The crossover between neurological and psychiatric symptoms is apparent.
Psychotic syndromes are frequently found in people with epilepsy, with prevalence rates ranging from 4% to 7% of all people with epilepsy;6,7 this is up to 15 times higher than the 0.4% prevalence in the general population. The rate increases to 7%−11% when temporal lobe epilepsy (TLE) is investigated alone,8 suggesting a greater prevalence of psychosis in those whose seizures originate in the temporal lobes. A large number of studies examining the putative link between psychosis and epilepsy have yielded a broad number of potential risk factors. The broad number of potential risk factors may, however, serve to obscure the key variables associated with TLE and psychosis.
Psychoses of epilepsy are generally classified according to Sachdev9 into three main patterns: ictal, postictal, and interictal psychoses. Ictal psychosis (IP) occurs concurrently with epileptic seizures and is usually a manifestation of nonconvulsive status epilepticus (SE), wherein the brain is in a constant state of seizure. Hallucinations or delusions are often not evident; instead, symptoms may take the form of unusual thoughts and behaviors, such as picking at clothes and oral activity.9 Although the patient is amnesic for this episode, insight remains intact; this can result in misinterpretation of the symptoms by the patient, which can result in further cognitive and behavioral disturbances. This form of psychosis is usually brief, generally lasting only hours. It is believed that IP often remains undiagnosed, with the seizure often imperceptible without an electroencephalogram (EEG); instead, it may appear to be primary psychosis (in cases where epilepsy is undiagnosed) or a state of stupor or delirium. As such, research on this form of psychosis is rare.
Postictal psychosis (PIP) happens when a seizure is followed by psychotic symptoms, often with a period of lucidity of up to 72 hours’ duration in-between.9 This often occurs after seizure clusters or after an increase in seizure frequency.9 PIP typically begins with anxiety, insomnia, and feelings of oppression,10 before presenting through symptoms including delusions, hallucinations, catatonia, and manic or depressive mood. It typically resolves within a few days, but can last up to a month.9 PIP recurs, on average, 2–3 times per year, and may become chronic in a minority of people, developing into IIP in about 10% of people.10
Interictal psychosis (IIP) is not temporally related to seizures, and can occur even when seizures are infrequent or controlled.9 IIP episodes last for several days or weeks, and typically present as affective symptoms, paranoid delusions, auditory hallucinations, and mystical experiences. Affect and cognition are relatively preserved throughout. Just before the onset of IIP, other symptoms may be seen, including insomnia and anxiety,9 and it has been suggested that anxiolytics given at this early stage may prevent the development of a psychotic episode.11
The difficulty in accurately diagnosing psychosis in epilepsy has almost certainly contributed to the mixed results of the relevant research: individuals with epilepsy may be diagnosed with primary psychosis (i.e., unrelated to epilepsy) or secondary psychosis (i.e., arising as a direct result of epilepsy), according to the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5).12 In addition to this, transient, psychotic-like symptoms can also arise as a result of the effects of seizures, antiepileptic drugs and their side effects, and additional life stresses,13 but they may not represent actual psychosis. Therefore, much relies on the ability of the attending neuropsychiatrist to distinguish among such presentations.
The aim of this literature review is to identify, evaluate, and summarize the relevant literature pertaining to risk factors for psychosis (IP, PIP, and IIP) in individuals with TLE. This may yield a clearer picture of the risk for TLE patients for developing psychosis, as well as some understanding of the processes underlying the development of psychotic symptoms.
Methods
We carried out an extensive electronic search in order to identify all literature pertaining to psychosis and TLE published between January 1992 and September 2012. We decided to review only those research studies published in the previous 20 years, as advances in neuro-imaging techniques and revisions to psychiatric diagnostic measures render much of the previous research less consistent with more recent studies. All available electronic databases (PsycINFO, The Cochrane Library, ScienceDirect, PubMed, and UK PubMed Central) were used for this search. The term ‘“temporal lobe epilepsy” was used in every search, along with one of each of the following in turn: psychosis+risk, schizophrenia+risk, and psychiatric comorbid*. This search yielded 1,576 articles. We applied a series of specific exclusion criteria (see Figure 1), resulting in the removal of any studies specifying a primary condition other than epilepsy, those utilizing non-human subjects, investigating the efficacy of medication or surgical techniques, or those in which psychotic symptoms were known to be a direct result of medical or surgical intervention. Finally, all case studies with ≤5 participants, review papers, editorials, and studies not focused specifically on TLE and psychosis were eliminated from the review.
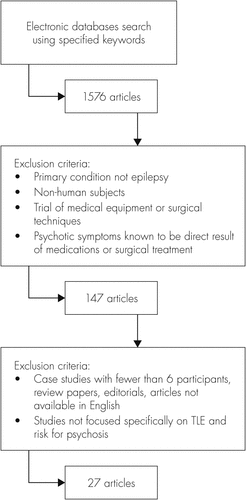
The definition of psychosis used varied among papers; however, all studies that utilized psychiatric assessment and/or standardized clinical assessment measures to identify psychosis were included for review. The bibliographies of the final articles were searched for additional relevant studies. Also, we made contact with eminent researchers in the field to ensure that very recent or in-press articles were not overlooked. This yielded 27 original research articles for review.
The information extracted from these research articles included the study design, sample size, methodologies, risk factors investigated, statistical analyses applied, and results. The quality of each study was assessed by use of the published modification of Downs and Black’s Quality Index, adapted for application to epidemiological studies.14 This index measures quality across reporting (score range: 0–7, external: 0–3, and internal validity: 0–4, and study power: 0–1). The total score range is 0–15, with higher scores indicating higher methodological quality. The Quality Index was also used to categorize studies reporting risk factors into High Quality (total score: 10–15), Moderate Quality (5–9), and Limited Quality (0–4).
For the purposes of this review, “risk factor” will be defined as any factor associated with an increased risk of developing psychosis in patients with TLE. It cannot be assumed that any of the factors identified are causal.
Results
There were 27 research studies for final review (see Table 1). As all studies were case–control or cross-sectional in design, and carried out using existing patients at neurological clinics, no premorbid data were available in any study. As such, causation cannot be determined, and so the factors outlined below are only known to have an association with psychosis in TLE.
| Quality Index Score | Author, Year, Country | Participants | Control Group/ Additional Experimental Group | Study Design | Method of Data Collection | Assessment of Psychosis | Factors Investigated | Statistical Analyses and Results |
|---|---|---|---|---|---|---|---|---|
| 12 | Adachi et al., 2002,25 Japan | 197 individuals with LRE (123 with TLE) and IIP | 456 individuals with LRE (288 with TLE) and NP | Case–control | Medical file review | Neuropsychiatric assessment using ICD–10 criteria | Age at epilepsy onset. | ANCOVA: |
| F=8.19; p=0.004* | ||||||||
| 12 | D’Alessio et al., 2009,17 Argentina | 63 individuals with TLE-P | 60 individuals with TLE-NP | Case–control | Medical file review | Psychiatric assessment using SCID-I and -II | t-tests: | |
| Gender; | p=0.039* | |||||||
| Employment; | p=0.001* | |||||||
| Unilateral HS; | NS | |||||||
| Bilateral HS; | p=0.001* | |||||||
| History of SE; | p=0.017* | |||||||
| Duration of epilepsy >20 yrs; | p=0.02* | |||||||
| Aura; | p=0.054 | |||||||
| Age at epilepsy onset | NS | |||||||
| 12 | Falip et al., 2009,10 Spain | 5 individuals with TLE-PIP | 50 individuals with TLE-NP | Case–control | Review of medical file | Psychiatric assessment | t-tests: | |
| Gender; | NS | |||||||
| History of febrile seizures; | NS | |||||||
| History of SE; | p=0.019* | |||||||
| Seizure frequency; | NS | |||||||
| Duration of epilepsy; | NS | |||||||
| Age at epilepsy onset; | NS | |||||||
| Family history of epilepsy; | NS | |||||||
| Dialeptic or automotor seizures evolving to secondary generalization; | p=0.025* | |||||||
| Lesion side; | χ2 tests: | |||||||
| Etiology; | NS | |||||||
| Nonlateral ictal EEG | NS | |||||||
| p=0.001* | ||||||||
| 12 | Kalinin et al., 2010,24 Russian Federation | 105 individuals with TLE | N/A | Case–control | Clinical and neuropsychological assessments and MRI | Psychiatric assessment using ICD–10 criteria | t-tests: | |
| Handedness; | Paranoia: p NS | |||||||
| Psychoticism: NS | ||||||||
| Laterality focus; | Paranoia: NS | |||||||
| Psychoticism: NS | ||||||||
| Alexithymia | Paranoia: p=0.002* Psychoticism: p=0.006* | |||||||
| 12 | Kanemoto et al., 2001,28 Japan | 132 individuals with epilepsy and IIP | 2,773 individuals with epilepsy and NP | Case–control | Medical file review | Psychiatric assessment using DSM-IV criteria | χ2 tests: | |
| Age at epilepsy onset (<10 years); | χ2=4.87; p <0.05* | |||||||
| Prolonged febrile seizures | χ2=13.73; p <0.01* | |||||||
| 11 | Briellmann et al., 2000,39 Australia | 6 individuals with TLE-PIP | 45 individuals with TLE-NP | Case–control | Collection of temporal lobe tissues | Psychiatric assessment using DSM-IV criteria | Volume loss of the anterior hippocampus; | Mann-Whitney U test: p=0.003*; |
| Mesial dysplasia | Chi square: p=0.006* | |||||||
| 11 | Cunha et al., 2003,37 Portugal | 18 medically-treated individuals with TLE; | N/A | Case–control | Clinical and neuropsychological assessment | Assessment of psychotic symptoms, using SCL–90 (self-administered) | Duration and severity of epilepsy | t-tests: |
| 19 surgically-treated individuals with TLE (examined pre- and postsurgery) | Paranoid Ideation: NS | |||||||
| Psychoticism: NS | ||||||||
| 11 | Kanemoto et al., 1996,27 Japan | 61 individuals with TLE and UHS (31 with PIP); | N/A | Case–control | Medical file review and MRI | Psychiatric assessment, using DSM-IV criteria | χ2 tests: | |
| 50 individuals with TLE with normal MRI (11 with PIP) | UHS; | χ2=9.7; p <0.01* | ||||||
| Age at epilepsy onset (<10 years); | χ2=5.53; p <0.05* | |||||||
| UHS, PIP, and atrophy of temporal neocortex | χ2=6.14; p <0.05* | |||||||
| 11 | Radhakrishnan et al., 2007,43 India | 129 individuals with surgically-treated TLE with CoA build-up; | N/A | Cross-sectional | Psychological and psychiatric assessment; EEG and MRI; collection of temporal lobe tissues | Psychiatric assessment, using ICD–10 criteria | t-tests: | |
| 244 individuals with surgically-treated TLE without CoA build-up | Frequency of IIP in CoA+ and CoA− groups; | p ≤0.001* | ||||||
| Frequency of IIP in Grades 1, 2, or 3 CoA | p=0.006* | |||||||
| 11 | Suckling et al., 2000,41 U.K. | 6 individuals with TLE-P | 26 individuals with TLE -NP | Case–control | Medical file review and collection of temporal lobe tissues | Neuropsychiatric assessment | Fisher’s exact test: | |
| Presence of focal lesions; | p=0.006* | |||||||
| Neuron loss in CA1; | p=0.015* | |||||||
| Neuron loss in CA4; | NS | |||||||
| Neuron loss in dentate gyrus; | NS | |||||||
| Dispersion of dentate granule cells | NS | |||||||
| 10 | De Araújo Filho et al., 2011,35 Brazil | 29 individuals with TLE | 6 individuals with JME | Cross-sectional | Medical file review | Psychiatric assessment, using DSM-IV criteria | Frequencies: 68.9% (20) | |
| 16 with IIP | 4 with IIP | Left-sided MTS; | ||||||
| 13 with PIP | 2 with PIP | Right-sided MTS; | 20.6% (6) | |||||
| Bilateral MTS | 10.3% (3) | |||||||
| 10 | De Oliveira et al., 2010,13 Brazil | 73 individuals with TLE | N/A | Cross-sectional | Clinical questionnaires | Mini-International Neuropsychiatric Interview (MINI) Plus, Version 5.0.0 | Bilateral MTS | Fisher’s exact test: NS |
| 10 | Flügel et al., 2006,26 U.K. | 20 individuals with TLE-IIP | 20 individuals with TLE-NP | Case–control | Neuropsychological assessments; MRI | Neuropsychiatric assessment, using DSM-IV criteria; PANSS | General linear model (multivariate): | |
| Age at onset of epilepsy; | F=10.3; p=0.003* | |||||||
| History of SE; | F=16.1, p=0.00* | |||||||
| Estimate of premorbid IQ; | F=1.4; NS | |||||||
| Current IQ; | F=3.16; NS | |||||||
| Vocabulary; | F=4.4; p=0.04* | |||||||
| Verbal Fluency (animals); | F=8.29; p=0.007* | |||||||
| Verbal Fluency (letters); | F=1.81; NS | |||||||
| Arithmetic; | F=5.09; p=0.03* | |||||||
| Digit Span; | F=3.02; NS | |||||||
| Spatial Span; | F=4.90; p=0.03* F=4.88; p=0.03* | |||||||
| Spatial Working Memory; | ||||||||
| Hippocampal volume | NS | |||||||
| 10 | Gutierrez-Galve et al., 2012,20 U.K. | 22 individuals with TLE-IIP | 23 individuals with TLE-NP; | Case–control | Medical file review and MRI | Neuropsychiatric assessment and PANSS | χ2 test: | |
| 21 healthy individuals | Gender; | χ2=0.58; NS | ||||||
| Handedness; | Fisher’s exact tests: | |||||||
| History of SE; | Fisher’s exact=0.345 | |||||||
| Total brain volume; | Fisher’s exact=0.004* | |||||||
| Age at epilepsy onset; | ANOVAs: | |||||||
| Duration of epilepsy; | F=7.92; p <0.001* | |||||||
| Estimates of Premorbid IQ; | t-tests: | |||||||
| Current IQ; | t = –2.62; p=0.012* | |||||||
| Working Memory Span; | t=1.61; NS | |||||||
| Working Memory Manipulation; | t = –1.06; NS | |||||||
| Frontal cortical thickness; | t = –2.44, p=0.019* | |||||||
| Cortical area; | t = –2.83; p=0.007* | |||||||
| Cortical volume | t=2.84; p=0.007* | |||||||
| ANOVAs: | ||||||||
| F=3.79; p=0.050* | ||||||||
| NS | ||||||||
| NS | ||||||||
| 10 | Kandratavicius et al., 2012,21 Brazil | 14 individuals with TLE-IIP | 16 individuals with TLE and no comorbidity; | Case–control | Collection of temporal lobe tissues | Psychiatric assessment, using DSM-IV criteria | ANOVAs: | |
| 16 individuals with TLE and major depression; | Gender; | NS | ||||||
| 10 normal autopsy samples | Presence of IPI; | NS | ||||||
| Age at epilepsy onset; | F=3.099; p=0.049* | |||||||
| Duration of epilepsy; | NS | |||||||
| Seizure frequency; | NS | |||||||
| Handedness; | NS | |||||||
| HS; | NS | |||||||
| Current IQ; | NS | |||||||
| Education (years); | NS | |||||||
| Seizure type; | Fisher’s exact tests: | |||||||
| Memory in Verbal Tasks; | p=0.02* | |||||||
| Neuronal density in entorhinal cortex; | p=0.003* | |||||||
| Fascia dentata inner molecular layer mossy fiber sprouting | ANOVAs: | |||||||
| F=3.175, p=0.047* | ||||||||
| TLE-IIP and TLE: | ||||||||
| F=4.931; Tukey’s post hoc: p=0.014* | ||||||||
| 10 | Rüsch et al., 2004,38 U.K. | 26 individuals with TLE-P | 24 individuals with TLE-NP; | Case–control | Medical file review and MRI | Neuropsychiatric assessment, using ICD–10 criteria | t-tests: | |
| 20 healthy individuals | Performance IQ; | t=0.203; NS | ||||||
| Verbal IQ; | t=2.307; p=0.02* | |||||||
| Current IQ; | t=1.902; p=0.06 | |||||||
| Cortical gray-matter volumes; | NS | |||||||
| Laterality of HS | NS | |||||||
| 10 | Sundram et al., 2010,30 Ireland | 10 individuals with TLE-P | 10 individuals with TLE-NP | Case–control | Medical file review and MRI | Neuropsychiatric assessment, which was objectively assessed using the OPCRIT | t-tests: | |
| Age at epilepsy onset; | NS | |||||||
| Duration of epilepsy; | NS | |||||||
| Total brain volume; | Mann-Whitney U tests: | |||||||
| Total gray-matter volume; | p=0.07 | |||||||
| Total white-matter volume; | p=0.08 | |||||||
| Gray-matter regional deficits; | NS | |||||||
| White-matter regional deficits | ANCOVAs: | |||||||
| Cluster significance threshold: p=0.002*; | ||||||||
| Cluster significance threshold: p=0.006* | ||||||||
| 10 | Tebartz van Elst et al., 2002,34 U.K. | 26 individuals with TLE-P | 24 individuals with TLE-NP; | Case–control | Medical file review and MRI | Neuropsychiatric assessment, using ICD–10 criteria | ANOVAs: | |
| 20 healthy individuals | Laterality of HS; | NS | ||||||
| EEG abnormalities; | NS | |||||||
| Total brain volumes; | F=11.750; p <0.001* | |||||||
| Hippocampal volumes; | NS | |||||||
| Right amygdala volumes; | F=8.211; p=0.001* | |||||||
| Left amygdala volumes. | F=9.079; p<0.001* | |||||||
| 10 | Umbricht et al., 1995,23 U.S.A. | 8 individuals with TLE-PIP; | 29 individuals with TLE-NP | Case–control | Medical file review | Psychiatric assessment, using DSM-III-R criteria | ANOVAs: | |
| 7 individuals with TLE-IIP | Duration of epilepsy; | NS | ||||||
| Bilateral focus; | Fisher’s exact test: p <0.005* | |||||||
| Seizure clusters; | χ2 tests: χ2=3.75; p=0.05* | |||||||
| Febrile convulsions; | χ2=4.36; p <0.05* | |||||||
| Handedness; | p>0.05 | |||||||
| Age at epilepsy onset; | t-tests: | |||||||
| Time between first seizure and onset of epilepsy; | t=2.67; p <0.05* | |||||||
| Full-scale IQ; | t=2.81; p <0.01* | |||||||
| Verbal IQ. | Mann-Whitney U tests: | |||||||
| z=1.98; p <0.05* | ||||||||
| z=2.11; p <0.05* | ||||||||
| 9 | De Araújo Filho et al., 2008,33 Brazil | 170 individuals with TLE | 100 individuals with JME | Cross-sectional | Medical file review and EEG monitoring | Psychiatric assessment, using SCID-I | Left-sided MTS | χ2 test: p <0.05* |
| 9 | Flügel et al., 2006,18 U.K. | 20 individuals with TLE-IIP | 20 individuals with TLE-NP; | Case–control | Clinical assessments; MRI | Psychiatric assessment, using DSM-IV criteria; PANSS | Mann-Whitney U tests: | |
| 23 healthy individuals | Gender; | Z = –0.64; NS | ||||||
| Estimated Premorbid IQ; | Z = –0.92; NS | |||||||
| Age at epilepsy onset; | Z = –2.91; p=0.004* | |||||||
| Min. and max. seizure frequency; | Z = –0.56; NS | |||||||
| MTR in the left middle and superior temporal gyri | p ≤0.005* | |||||||
| 9 | Flügel et al., 2006,48 U.K. | 20 individuals with TLE-IIP | 20 individuals with TLE-NP | Case–control | Neuropsychological assessments and MRI | Neuropsychiatric assessment; PANSS | General linear model (multivariate): | |
| FA values in frontal left; | F=5.54; p=0.024* | |||||||
| FA values in frontal right; | F=12.18; p=0.001* | |||||||
| FA values in temporal left; | F=5.89; p=0.02* | |||||||
| FA values in temporal right; | F=6.295; p=0.017* | |||||||
| MD values in frontal left; | F=5.203; p=0.029* | |||||||
| MD values in frontal right | F=5.88; p=0.02* | |||||||
| 9 | Fukao et al., 2009,36 Japan | 16 individuals with TLE-P | 41 individuals with TLE-NP | Case–control | Medical file review and magnetoencephalographic measurements | Psychiatric assessment, using DSM-IV criteria | Correlation: | |
| Laterality of focus; | NS | |||||||
| IH-type spike-dipoles; | NS | |||||||
| Left SV-type spike-dipoles; | p=0.002* | |||||||
| Plural types of spike-dipoles; | p=0.001* | |||||||
| Bilateral magnetic spikes; | p=0.046* | |||||||
| MTS | NS | |||||||
| 9 | Gattaz et al., 2011,8 Brazil | 7 individuals with TLE-IIP | 9 individuals with TLE-NP | Case–control | Collection of temporal lobe tissues | Psychiatric assessment, using DSM-IV criteria | Mann-Whitney U tests: | |
| iPLA2 activity; | p=0.016* | |||||||
| cPLA2 activity; | NS | |||||||
| sPLA2 activity; | NS | |||||||
| tPLA2 activity; | NS | |||||||
| Duration of epilepsy; | NS | |||||||
| Frequency of seizures | NS | |||||||
| 9 | Hermann et al., 2000,29 U.S.A. | 54 individuals with TLE | 38 healthy individuals | Case–control | Neuropsychological questionnaires | Assessment of psychotic symptoms, using SCL–90-R | Partial correlation: | |
| Duration of epilepsy; | Paranoid Ideation r=0.46; p=0.001* | |||||||
| Frequency of seizures | Psychoticism: r=0.40; p=0.004* | |||||||
| NS | ||||||||
| 8 | Guarnieri et al., 2005,19 Brazil | 21 individuals with TLE | 23 individuals with TLE-NP | Case–control | SPECT scans | Psychiatric assessment, using DSM-IV criteria | χ2 tests: | |
| 11 with IIP | Gender; | χ2=0.349, NS | ||||||
| 10 with PIP | Marital status; | χ2=1.85; NS | ||||||
| Handedness; | χ2=0.934; NS | |||||||
| Presence of IPI; | χ2=0.02; NS | |||||||
| Resonance laterality; | χ2=0.380; NS | |||||||
| EEG ictal laterality; | χ2=0.509; NS | |||||||
| Education (years); | t-tests: | |||||||
| Age at epilepsy onset; | t=0.102; NS | |||||||
| Duration of epilepsy; | t=0.046; NS | |||||||
| Paid employment; | t=0.480; NS | |||||||
| Seizure frequency; | Fisher’s exact test: | |||||||
| rCBF | F=2.53; NS | |||||||
| Mann-Whitney U test: | ||||||||
| U=214.0; NS | ||||||||
| ANOVA; NS | ||||||||
| 6 | Maier et al., 2000,42 U.K. | 12 individuals with TLE-P | 12 individuals with TLE-NP; | Case–control | Medical file review and MRI | Psychiatric assessment | t-tests (TLE-P and HCs): | |
| 26 individuals with schizophrenia and no epilepsy; | Left NAA; | p <0.001* | ||||||
| 38 healthy individuals | Right NAA; | p <0.001* | ||||||
| Left Cho; | NS | |||||||
| Right Cho; | NS | |||||||
| Left Cr+PCr; | NS | |||||||
| Right Cr+PCr; | NS | |||||||
| Left regional H/A volume; | p=0.0001* | |||||||
| Right regional H/A volume | p=0.004* |
Quality of Studies
The studies were assessed for quality and ranked in order of highest methodological quality (see Table 1). The mean total score on the Quality Index was 10.11 (standard deviation [SD]: 1.39; range: 6–12). The mean subscale scores were 5.78 (SD: 0.64; range: 4–7) for reporting, 1.74 (SD: 0.94; range: 0–3) for external validity, and 2.55 (SD: 0.58; range: 2–4) for internal validity. No study reported a power calculation. These quality scores should be considered alongside the identified risk factors below.
Gender
Earlier studies suggested female gender as a risk factor for psychosis in TLE;15,16 however, the research here identified does not support this finding. Only one study found a statistically significant difference in the number of men and women with TLE and psychosis (TLE-P) in their study, with more men found to exhibit psychosis.17 This result was not replicated in the five other studies investigating this variable.10,18–21
Handedness
Although people with epilepsy are found to demonstrate atypical handedness (left-handedness and ambidexterity) at a significantly higher rate than those without epilepsy,22 handedness was not found to be significantly correlated with the presence of psychotic symptoms in the five studies that compared their groups on this variable.19–21,23,24 It should be noted that many studies continue to use left-handedness or ambidexterity as an exclusion factor in participant recruitment.
Age at Epilepsy Onset
Earlier onset of epilepsy (before age 10) is frequently found to be associated with an increased risk of developing psychosis,18,20,25–28 although psychosis itself is on average found to be first experienced approximately 21 years after the onset of epilepsy.17 Some researchers speculate that this correlation is due to the increased damage to the brain as a result of the longer duration of epilepsy,17,29 with one study finding increasing duration of TLE to be associated with higher scores on all items of the Symptom Checklist−90-R (SCL−90-R).29 However, another study found that earlier age at epilepsy onset continued to be associated with increased risk of psychosis despite duration of epilepsy not differing among their groups.26
Two studies found that earlier epilepsy onset was only associated with IIP:23,27 in one, the TLE-IIP group showed an average onset at age 9 years, whereas the TLE-PIP group showed onset at an average of 19.5 years of age.23 Another study also found earlier onset in IIP, with no association between PIP and age at epilepsy onset.27
Of 12 investigating studies, 4 did not identify earlier age at onset as a risk factor,10,17,19,30 whereas one study found that participants with mesial TLE-IIP were actually diagnosed later than those with mesial TLE and either comorbid depression or no psychiatric comorbidity.21
Seizure History
Febrile convulsions typically occur between the ages of 6 months and 6 years and are associated with a sudden increase in body temperature. These childhood seizures somewhat increase the risk of developing epilepsy and, in rare cases, can result in permanent cerebral impairments, which tend to be unilateral.10 A history of febrile convulsions was found to be inversely related to epileptic psychosis, with fewer febrile convulsions in those with psychosis than in those without (TLE-NP).23 Because febrile convulsions typically cause damage to the hippocampus,23 and some research suggests that delusions may be a result of overactivity of the hippocampal CA1 neurons,31 it was proposed that the cell loss of the CA fields of the hippocampus seen in those with febrile convulsions may actually protect against their overactivity (which can result from interictal epileptiform activity) and subsequent development of delusions.23
On the other hand, one study investigated the relationship between prolonged (lasting 30 minutes or longer) febrile convulsions and IIP and found them to be positively associated.28 This may be partly attributed to the investigation of IIP and not PIP; in the previous study, those with IIP were somewhat more likely to have a history of febrile seizures than were those with PIP.23 In a study comparing people with TLE and PIP to those with TLE alone, no difference was found between the groups on history of febrile seizures.10 Therefore, febrile convulsions may only increase the risk of IIP, and not PIP.
A factor frequently found to be associated with an increased risk for psychosis in patients with TLE is a history of status epilepticus (SE). Every study that investigated this factor found it to be associated with an increased occurrence of psychosis.10,17,20,26 This life-threatening state of persistent seizure (lasting longer than 30 minutes) can quickly result in a significant degree of bilateral cerebral damage, and was found by one study to be associated with immediate occurrence of PIP, as well as with the development of psychotic symptoms several years later in many patients.17 Another study found increased dialeptic and automotor seizures in their TLE-PIP group;10 these are often classified as forms of nonconvulsive SE. Nonconvulsive SE can manifest in psychotic symptoms during the seizure, thus being classified as ictal psychosis.9
Seizure Laterality
Laterality of seizure focus has been associated with epileptic psychoses since Flor-Henry suggested that psychosis was associated with TLE of the dominant hemisphere (as inferred by handedness).32 Eleven studies investigated this variable;10,13,17,19,21,23,24,33–36 five of these found no difference in laterality between TLE-P and TLE-NP groups.13,17,19,24,34 Two studies found that left-sided mesial temporal sclerosis (MTS) was significantly associated with the presence of psychosis, whereas right-sided MTS was not,33,35 and another found a strong correlation between psychosis and left superotemporal-vertical (SV)-type spike-dipole clusters.36 Another study found increased frequency of non-lateralizing ictal EEG in their TLE-PIP group as compared with their control group, showing bitemporal seizures or seizures that quickly spread to the contralateral temporal lobe.10 Similarly, studies have found a higher frequency of secondarily generalized seizures in those with IIP21 and higher frequency of bitemporal seizure foci in a TLE-P group than in the TLE-NP group.23
Seizure Frequency
Seizure frequency has also been suggested as a possible risk factor for psychosis; however, this was not found in the six studies that measured frequency.8,10,18,19,21,29 One study compared SCL−90 scores of medically-treated TLE patients with those of a group who were awaiting surgical treatment because of more frequent and refractory seizures, and found no significant differences in psychotic symptoms.37 Another study found that a TLE-P group experienced more seizure clusters (a series of seizures occurring in a short space of time) than their nonpsychotic counterparts.23
The Limbic System
Temporal lobe epilepsy is sometimes referred to as “limbic epilepsy” by those who prefer to consider epilepsy based on cerebral systems rather than structures.13 The limbic system is situated within the temporal lobes and is involved in such functions as motivation, emotion regulation, and memory. The limbic cortex has the lowest threshold for sustaining epileptiform activity, and abnormalities within the structures of the limbic system—both cortical and subcortical—are frequently found to be present in primary psychosis. This has stimulated research into its role in epileptic psychoses also.38
Anomalies in the limbic cortex were frequently investigated, with 14 studies investigating the hippocampus for various changes or abnormalities.13,17,21,26,27,30,33,34,38–43 Total hippocampal volume was found not to be significantly different between those with and without epileptic psychosis,26,34 with one study further confirming this for the seizure focus side.39 The anterior hippocampus was found to show more deficit in a TLE-NP group than in TLE-PIP.39
A more recent study examined gray- and white-matter volumes separately, finding significant bilateral hippocampal white-matter loss in those with TLE-P as compared with TLE-NP, as well as gray-matter reduction in the left hippocampi of those with TLE-P.30
As previously mentioned, it has been suggested that delusions in primary psychosis may be a result of overactivity of the hippocampal CA1 neurons.31 It is of note, then, that one study found their TLE-NP participants to demonstrate more severe neuron loss in this region of the hippocampus than did their TLE-P group.41 Another study investigated CA1 neuronal loss in TLE patients through the use of measures of corpora amylacea (CoA) build-up,43 which are indicative of the level of neuronal loss. CoA build-up in the CA1 region is usually associated with neurodegenerative diseases and advanced age; however, in this sample, which had an average age of approximately 27 years, they found increased density of CoA deposits to be highly associated with increased frequency of IIP. Those with CoA accumulation were found to experience PIP more frequently also, although the highest density levels were associated with IIP.43 Other researchers have found CoA build-up in CA1 to be associated with the presence of hippocampal sclerosis (HS), with one finding this association to be more likely in slightly older (mean age: 32 years) TLE patients with longer duration of epilepsy.44 Indeed, the study found this increased TLE duration and older age in their group with CoA deposits;43 however, the absence of HS was used as an exclusion criterion in their participant recruitment, thus preventing investigation of this association.43
Given the link between CoA accumulation and HS (or mesial temporal sclerosis [MTS]), it is not surprising that nine studies chose to investigate the presence of HS.13,17,21,27,33–36,38 One of these found bilateral HS to be associated with increased risk of psychosis,17 whereas two found a significant correlation between the presence of psychosis and left-sided HS only.33,35 Another study compared TLE patients with unilateral HS (UHS) with TLE patients with essentially normal MRI and found that those with UHS were significantly more likely to experience psychotic symptoms.27 When this finding was further investigated, it was found that UHS increased the risk for PIP, but not IIP.27 Conversely, two studies, utilizing the same sample of participants, found that HS was more common in a TLE-NP group.34,38 Others failed to find any significant association between HS and psychosis.13,21,36
The presence of N-acetylaspartate (NAA) in the hippocampi of psychotic and nonpsychotic TLE patients, individuals with schizophrenia, and healthy-controls was investigated in one study.42 NAA is an amino acid found within cerebral neurons, which provides a measure of the synaptic activity in those cells; it is therefore used as an indicator of cerebral damage. The researchers found that left-sided NAA was significantly reduced in those with schizophrenia when compared with healthy-controls, and to an even greater extent in those with TLE, with the lowest rates found in TLE-P participants.42 Bilateral NAA reduction was evident in those with TLE as a result of the greater hippocampal cell loss in TLE.42 Despite equal numbers of right- and left-sided seizure foci among participants, increased left-sided NAA reduction was found in the TLE-P group; this did not occur for the TLE-NP group.
Research that found increased activity of phospholipases A2 (PLA2) in the brains of individuals with primary schizophrenia (as well as a subsequent decrease in activity to near-normal levels on receiving antipsychotic medication)40 led to speculation that similar increases may be evident in those with epileptic psychosis. A study of this showed that those with TLE-IIP demonstrated significantly higher activity of the calcium-independent enzyme iPLA2 in the hippocampus than did the TLE-NP control group.8 This enzyme, which catalyses the release of fatty acids from phospholipids, is also found to be elevated in some studies of individuals with schizophrenia,40 thus suggesting some shared processes between primary and secondary psychoses. Animal experiments have found epileptic seizures to increase PLA2 activity;45 this may explain the increased iPLA2 activity in human participants experiencing recurrent seizures. Increased PLA2 activity alters the release of dopamine in the synaptic cleft;8 thus, these findings may provide some support for the dopamine hypothesis of psychosis, suggesting that increased PLA2 leads to the eventual development of psychotic symptoms in some individuals.
The parahippocampal gyrus was examined by only one study reviewed here; significant unilateral white-matter loss, as well as gray-matter loss in the left parahippocampal gyrus, was found in those with TLE-P when compared with TLE-NP participants.30 Within the dentate gyrus, which was also investigated in only one study, the inner-molecular layer of the fascia dentata was found to show decreased levels of a particular type of synaptic reorganization in those with TLE-IIP relative to those with comorbid depression or without comorbidity.21 This synaptic reorganization, mossy fiber sprouting, is believed to occur because of neuronal loss after recurrent seizures, and is held to render the epileptic brain more excitable. It is commonly seen in TLE. It was suggested that such reduction may be due to altered N-methyl-D-aspartate (NMDA) neurotransmission in the hippocampus.21 Animal studies have shown that mossy fiber sprouting is greater when it occurs in reaction to a neuronal injury in a developing brain than in a mature brain.46 This study’s TLE-P participants showed an older age at epilepsy onset than their nonpsychotic counterparts; therefore, these individuals may have displayed less mossy fiber sprouting because their hippocampi were more developed than those of the control group at time of epilepsy onset.
Another study investigated the regional cerebral blood flow (rCBF) of TLE patients with and without psychosis.19 A trend was found for increased rCBF in the right posterior cingulate cortex of the TLE-P group, which was not statistically significant after adjustments were made for multiple comparisons. The posterior cingulate cortex is believed to play a role in such areas as pain perception, episodic memory, and theory of mind, and, indeed, previous research has identified abnormalities in the cingulate cortex in those with primary psychoses.47 The cingulum was also found to show bilateral white-matter deficits and right-hemisphere gray-matter deficits in those with TLE-P when compared with those with TLE-NP.30 Finally, the insula was found to show bilateral gray-matter reductions in those with TLE-P.30
Subcortically, the amygdala was the only structure examined. Only one study examined this, finding bilateral enlargement of the amygdala in the TLE-P group when compared with TLE-NP.34 Another study examined the hippocampus−amygdala complex (H/A) together, finding significant regional bilateral volume reductions in the H/A of TLE-P participants that was not present in TLE-NP patients.42 An area of regional volume reduction in the left H/A was found in both their schizophrenic and TLE-P groups; however, it was not found in the TLE-NP group or in healthy-controls, suggesting its association with psychosis and not epilepsy.42
Temporal and Extratemporal Features
The temporal neocortex of TLE-PIP participants was found to show evidence of increased dysplastic malformations than those with TLE-NP.39 One study compared TLE patients with UHS with TLE patients with normal MRI and found that those with UHS and PIP showed increased atrophy of the temporal neocortex.27 A strong correlation between psychosis and left superotemporal-vertical (SV) type spike-dipole clusters was identified by another study,36suggesting some dysfunction of the left temporal neocortex as a contributor to psychotic symptoms in TLE. However, this study also identified an association between plural types of spike-dipoles and psychosis.
Magnetization transfer imaging (MTI) was applied by one study to identify neural abnormalities through the magnetization transfer ratio (MTR), a measure of macrocellular structural integrity. Looking first at patients with no focal lesions, the researchers found that those with TLE-IIP showed significant areas of MTR reduction in the left middle and superior temporal gyri when compared with the TLE-NP group, who did not differ from healthy controls.18 This difference was not found to be related to age, history of SE, age at epilepsy onset, duration of psychosis or current consumption of antipsychotic medications.
Lower fractional anisotropy (FA) values in the temporal and frontal lobes were found in those with TLE-IIP when compared with those with TLE-NP; the right frontal lobe was found to show this to a greater extent.48 This indicates axonal loss; whereas the finding of increased mean diffusivity (MD) of water molecules in the frontal regions in IIP participants suggests potentially reversible alterations, such as edema. The changes in FA levels were found to be associated with performance on some neuropsychological tests, including verbal fluency and spatial span.48 Higher levels of negative symptoms on the PANSS were found to be associated with greater reductions in the left frontal FA levels.48
One study did not identify any differences in cortical gray matter volume in TLE patients with and without psychosis when compared with healthy controls.38 However, some variance in cortical thickness in those with TLE-IIP was found using surface-based morphometry.20 This study found that those with IIP had reduced cortical thickness of the pars opercularis (in the interior frontal gyrus) when compared with healthy controls; this difference was not found between TLE-NP patients and healthy controls. Cortical thickness was not found to be affected by age of onset or duration of epilepsy or psychosis, or history of SE. Also, the left pars opercularis was found to show reduced cortical area in those with IIP when compared with control groups.20 In the TLE-IIP group, current IQ was found to be associated with the area and volume of the frontal and temporal cortex, as well as volume of the parietal cortex, with higher IQ scores associated with increasing area and volume in those regions.20
Studies of cerebral volume found no significant differences in total cerebral volumes30,34 or total gray- or white-matter volumes30 between TLE-P and TLE-NP participants or healthy controls. However, gray-matter reductions were found in the bilateral inferior, middle, and superior temporal gyri, fusiform gyri, cerebellum, and caudate nuclei, as well as unilaterally in the left inferior parietal lobule.30 White-matter deficits were seen in the bilateral, middle, and inferior temporal gyri, fusiform gyri, corpus callosum, anterior limb of internal capsule, posterior thalamic radiation, and the caudate nuclei.30 Unilateral white-matter deficits were found in the right superior temporal gyrus, left lingual gyrus, and right midbrain.30 This suggests that epileptic psychosis may be a result of more widespread cerebral damage than previously realized.
Cognitive Functioning
Because psychotic phenomena are increasingly found to be associated with impaired cognitive performance,49 this domain has also been investigated in psychoses of epilepsy, with seven of the studies reviewed here addressing it.18,20,21,23,26,34,38 Although estimated premorbid IQ does not tend to differ significantly between TLE-P and TLE-NP groups,18,20,26 some studies have identified lower current IQ in those with comorbid psychotic syndromes than in those without,20,23 whereas others find no significant differences.21,26,38 One study found no difference between groups on episodic memory (with both groups performing below average), but significantly poorer semantic memory and executive abilities were found in those with TLE-IIP.26 The duration of psychosis was found to be significantly correlated with some executive tasks, such as working memory and fluency, with cognitive deterioration appearing to occur progressively alongside psychosis.26
Those with TLE-IIP also demonstrated poorer working memory and working memory manipulation than those with TLE-NP;20 and another study finding their TLE-IIP group to have poorer performance on memory tasks than those with TLE and those with TLE and comorbid depression.21 Verbal IQ was found to be significantly lower in a TLE-P group when compared with TLE-NP (by two studies utilizing the same clinical sample);34,38 another study compared a group with TLE-PIP with a group with TLE-IIP and found the latter group to have lower verbal IQ scores.23 Verbal fluency was also found to be impaired in those with TLE-IIP.26 Only two studies measured differences in level of education, finding no significant difference in the number of years of education held by TLE-P and TLE-NP groups.19,21
Personality Factors
Alexithymia is considered to be a constellation of personality traits that render the individual unable to define, describe, or understand emotions in themselves and others. It is characterized also by a tendency toward highly concrete thinking and poor imaginal processes (lack of fantasies or dreaming). This is often seen in individuals with brain injuries.50 Alexithymia may predispose to the development of psychiatric symptoms, including psychosis.24 Only one of the 27 studies reviewed here identified and investigated this as a potential risk factor, finding that the 24% of their TLE participants who were found to be alexithymic scored higher on all scales of the SCL−90 than nonalexithymic participants.24 This effect was found to be increased when the individual had a right-sided seizure focus, in keeping with the theory that alexithymia results from pathology of the right hemisphere.24
Employment
High rates of unemployment in medical populations are generally held to indicate inability to work because of illness, with one study finding 35.6% of TLE patients classifying themselves as “retired due to illness.”13 One study comparing TLE-P with TLE-NP participants found that those with psychosis were less likely to be employed,17 whereas another study found no significant difference.19 It should be noted that the first study found their participants with psychosis to be significantly more likely to demonstrate epilepsy duration lasting more than 20 years,17 whereas the latter study found no significant differences in epilepsy duration between groups.19 This may suggest that the duration of epilepsy is influential in employment status, with longer-term recurrence of seizures related to higher rates of unemployment. However, a recent study indicates that unemployment rates are significantly higher in the 12 months before first onset of primary psychosis than in the general population;51 therefore, unemployment may also be both predisposing to, and resultant of, psychotic symptoms secondary to TLE.
Discussion
This review has identified a number of factors that are repeatedly found to be associated with secondary psychotic disorders in epilepsy. These have been summarized in Table 2.
| Risk Factor | PIP | IIP | Psychosis Not Ictally-Defined |
|---|---|---|---|
| Early onset of epilepsy (before age 10) | *** | *** | |
| History of prolonged febrile convulsions | *** | ||
| History of status epilepticus | *** | *** | *** |
| Dialeptic and/or automotor seizures | *** | ||
| Left-sided mesial temporal sclerosis | *** | ||
| Bitemporal seizures or seizures spreading quickly to the contralateral temporal lobe | *** | ||
| Secondarily generalized seizures | *** | ||
| Bitemporal seizure foci | *** | ||
| Seizure clusters | *** | ||
| Bilateral hippocampal white-matter loss | *** | ||
| Left hippocampal gray-matter reduction | *** | ||
| Neuron loss in CA1 region of hippocampus | *** | *** | †*** |
| Bilateral hippocampal sclerosis | *** | ||
| Unilateral hippocampal sclerosis | *** | †*** | |
| Reduced synaptic activity in left hippocampus | ** | ||
| Decreased synaptic reorganization in dentate gyrus | *** | ||
| Higher iPLA2 activity in hippocampus | ** | ||
| Regional volume-reduction in hippocampus/amygdala | ** †*** | ||
| Dysplastic malformations or atrophy of temporal neocortex | *** | ||
| Neural abnormalities in left middle and superior temporal gyri | ** | ||
| Axonal loss in temporal & frontal lobes, especially right frontal lobe | ** | ||
| Extratemporal regional gray- and white-matter deficits | *** | ||
| Lower current IQ | *** †*** | *** †*** | |
| Alexithymia | *** |
Onset of epilepsy before age 10 tends to be more likely with psychosis, particularly IIP, with earlier interruptions to neurodevelopmental processes appearing to increase the risk of developing psychosis. Childhood febrile convulsions are also found to have a relationship with the development of psychosis in adulthood, although it is not yet clear what this relationship is. One study found those with a history of febrile convulsions to be less likely to develop psychosis later on, although those with IIP were somewhat more likely to have a history of these seizures than those with PIP.23 Another study supported this, finding IIP to be positively correlated with prolonged febrile convulsions.28
A history of status epilepticus was consistently found to be associated with increased occurrence of psychotic disorders, with one study finding it to be associated both with immediate occurrence of PIP and with the development of psychotic features many years later.17 This seizure form is often nonconvulsive and so may be underdiagnosed; given its association with immediate and delayed psychosis, it may be important to clarify the diagnosis and recording of such seizures.
Hippocampal sclerosis was consistently found to be associated with secondary psychosis, with both unilateral (in particular, the left hemisphere) and bilateral sclerosis correlating with psychotic symptoms. Only two studies (sharing the same sample of participants) found hippocampal sclerosis to be more common in those TLE patients without psychosis.34,38 Further investigation of this area is required; given that hippocampal sclerosis can be identified through MRI, this risk factor may be amenable to relatively routine assessment.
In general, abnormalities in the left hippocampus were found to be somewhat more frequent in those with TLE-P than in any controls. Specifically, reduction of gray matter30,42 and reduction of NAA42 were found to be more common in the left hippocampus in those with secondary psychosis. It is of note that similar findings are reported in studies of primary psychosis. Although Flor-Henry’s theory regarding the involvement of the left hemisphere in epileptic psychosis may be supported here, it is possible that left-hemisphere damage more generally (as opposed to seizure focus) is associated in some way with all psychotic syndromes. However, none of the above studies can determine whether the left-hemisphere alterations occurred before or after onset of psychotic symptoms.
Cell loss in the CA1 region of the hippocampus is also found to be involved in epileptic psychosis. This region, which has highly concentrated dopamine receptors, has been investigated in relation to primary psychosis, with researchers stating that delusions may result from overactivity of the CA1 neurons. The finding of one study reviewed here, that a history of febrile convulsions was associated with reduced risk of psychosis, may be attributed to this idea: because febrile convulsions can result in damage, including CA1 cell loss, to the hippocampus, seizures are less likely to result in cellular hyperactivity, thus possibly protecting against delusions. One study found increased CA1 cell loss in a TLE-NP group relative to TLE-P,41 whereas another measured CoA build-up as an indicator of neuronal loss and found increased deposits in those with TLE-P; however, all participants in this sample also had hippocampal sclerosis.43
Although estimates of premorbid IQ did not differ between the TLE-P and TLE-NP groups, significant differences were found in current IQ, along with verbal IQ and working memory. These were found to be lower in those with TLE-P, with the lowest scores found in people with TLE-IIP.23 One study found this to be associated with the duration of the psychosis,26 suggesting a progressive deterioration of cognitive abilities; therefore, it appears that psychosis either instigates or occurs alongside the decline in cognitive abilities.
Several identified risk factors were found to be associated with interictal psychosis to a greater extent than postictal psychosis. Some researchers have posited that the two disorders exist along a spectrum, with interictal psychosis representing a more progressed condition. Debate continues on this point, with some researchers finding interictal psychosis to be moderately more prevalent in a temporal lobe epilepsy sample than postictal psychosis,33 and others stating that approximately 10% of individuals with postictal psychosis go on to develop interictal psychosis.10 In this review, such factors as earlier onset of epilepsy,27 history of prolonged febrile seizures,28 build-up of CoA in the CA1 region of the hippocampus,43 cerebral volume loss,34,44 decreased full-scale and verbal IQ,23 and reduced memory abilities21 were found to be associated with interictal psychosis to a greater extent than with postictal psychosis. Postictal psychosis, on the other hand, was found to be associated to a greater extent with unilateral hippocampal sclerosis.27 It is apparent that interictal psychosis is associated with more widespread and advanced cerebral damage; however, it is not clear whether this condition represents a more progressed form of postictal psychosis, or whether the two represent separate etiologies. Further investigation is required into these two conditions in order to clarify this.
Limitations of the Current Review
This review has some limitations that we should consider. The search terms applied were necessarily limiting, and although every effort was made to identify all pertinent research, it is possible that some studies were not identified through the methods used. Also, given the advances in imaging technology and neuroscientific methods, only studies from the past 20 years were searched. Therefore, this review is not representative of the earlier research in this area.
Recommendations
This review of the existing literature highlighted three key areas that should be addressed in future research. These are detailed below.
Definition, Diagnosis, and Classification of Psychosis
Some variation is obvious in the definitions of psychosis applied in the 27 studies reviewed here. Although most utilized the criteria for psychotic syndromes outlined by the ICD or the DSM in use at the time of study, a few attempted to reduce the risk for inclusion of seizure-related confusional states through specifying the presence of hallucinations and/or delusions (e.g., Fukao et al.36). As ictal psychoses, at least, do not tend to present in this manner, such a stringent definition of psychosis may have excluded from the study patients with psychosis that presents through thought disorders, affective disturbances, and so on. It is clear that more coherent guidelines for diagnosing epileptic psychoses are required.
The 27 studies also showed some variation as to how psychotic symptoms were diagnosed for the purposes of the research. The majority of the studies utilized a formal psychiatric interview, in which psychotic syndromes were diagnosed according to contemporary DSM or ICD criteria. Some also used standardized measures such as the PANSS52 or MINI-PLUS 5.0.053 to strengthen their assessments. However, two studies29,37 used only the self-administered Symptom Checklist−90 (SCL−90)50,54 or its normed, revised version, SCL−90-R. This is an evaluation of symptom dimensions, and so does not translate to a formal psychiatric diagnosis. However, given that it measures several symptoms on the psychotic dimension, this measure may give some indication of a tendency toward psychosis.
Most researchers also further classified psychoses according to ictal criteria.9 Only a few of the studies failed to separate them thus, with no mention given to the temporal relationship (if any) to seizures. The authors of two such studies justify their failure to separately investigate psychoses according to their relation to ictus by suggesting that, as PIP often progresses to IIP, a joint investigation may be a valid approach.30,36 Although it is possible that PIP and IIP represent two stages of one progressive condition, significant differences are found between the groups. Therefore, their investigation as one entity may limit the validity of research findings.
Study Populations
All participants in each of the 27 studies were existing patients of a neurological service. Although these samples were most likely selected for convenience in terms of identifying individuals with epilepsy and obtaining sufficient medical histories for them, the singular use of an existing medical population may result in findings that are not necessarily generalizable to the entire TLE population. It is possible that individuals receiving ongoing specialized medical attention are undergoing more complex and/or more time-consuming medical treatments than others; it is also possible that these populations may not be representative of all socioeconomic groups, because ongoing medical care can be expensive within some healthcare systems. Also, many of the studies acknowledged that their clinic specialized in treating refractory epilepsies, and so patients at these clinics may not be representative of treatment-responsive TLE patients. Although one study investigated refractoriness and did not find any difference in the frequency of psychiatric disorders between refractory and treatment-responsive TLE,13 this factor should be considered in evaluating the studies.
In order to administer normed assessment measures to their participants, many studies used an IQ <70 as an exclusion factor for participation. Although this allows for use of standardized instruments, it also potentially excludes many participants with psychotic symptoms. As previously seen, cognitive impairments appear to occur progressively over the course of psychosis; IQ was one such impairment. Therefore, excluding participants on the basis of IQ scores may prevent the investigation of a large section of the population with TLE and psychosis.
Study Design
All 27 research studies reviewed were of retrospective case–control or cross-sectional design. These studies could not therefore consider temporal relations among variables, only the incidence or prevalence of medical or psychiatric conditions in specific populations. Indeed, many of the studies took the form of chart and intake assessment reviews, with no active participation by the patients. Although this approach is initially useful in identifying correlations among factors, longitudinal studies are warranted in order to further investigate the specific relationships involved.
Conclusions
This review has identified important risk factors for psychosis secondary to TLE, including onset of epilepsy before age 10 years, history of status epilepticus, hippocampal sclerosis, abnormalities in the left hippocampus, cell loss in the CA1 region, and cognitive impairments. It has also provided recommendations for future research in this area. Through applying more rigorous and consistent diagnostic and research methods, it is expected that our understanding of psychosis in TLE will increase accordingly, thereby allowing for more effective treatment and management of this complex condition.
1
2 : Epilepsy and social identity: the stigma of a chronic neurological disorder. Lancet Neurol 2005; 4:171–178Crossref, Medline, Google Scholar
3 : Psychiatric comorbidity in patients evaluated for chronic epilepsy: a differential role of the right hemisphere? Eur Neurol 2009; 61:350–357Crossref, Medline, Google Scholar
4 : Prevalence of psychiatric comorbidities in temporal lobe epilepsy: the value of structured psychiatric interviews. Epileptic Disord 2010; 12:283–291Medline, Google Scholar
5 : [Frequency of epilepsy in psychiatric inpatients]. Rev Bras Psiquiatr 2005; 27:165–166Crossref, Medline, Google Scholar
6 : Psychosis in epilepsy: frequency and risk factors. J Epilepsy 1995; 8:295–305Crossref, Google Scholar
7 : A multicenter study on the prevalence of psychiatric disorders among new referrals for epilepsy in Japan. Epilepsia 2003; 44:107–114Crossref, Medline, Google Scholar
8 : Increased PLA2 activity in the hippocampus of patients with temporal lobe epilepsy and psychosis. J Psychiatr Res 2011; 45:1617–1620Crossref, Medline, Google Scholar
9 : Schizophrenia-like psychosis and epilepsy: the status of the association. Am J Psychiatry 1998; 155:325–336Crossref, Medline, Google Scholar
10 : Postictal psychosis: a retrospective study in patients with refractory temporal lobe epilepsy. Seizure 2009; 18:145–149Crossref, Medline, Google Scholar
11 : Acute AL symptomatology at disappearance of epileptiform EEG abnormality: paradoxical or “forced” normalization, in Neurobehavioural Problems in Epilepsy: Advances in Neurology, Vol. 55. Edited by Smith D. New York, Raven, 1991, pp 127–142Google Scholar
12
13 : Psychiatric disorders in temporal lobe epilepsy: an overview from a tertiary service in Brazil. Seizure 2010; 19:479–484Crossref, Medline, Google Scholar
14 : Depressive symptoms among mothers of children with epilepsy: a review of prevalence, associated factors, and impact on children. Epilepsia 2009; 50:2344–2354Crossref, Medline, Google Scholar
15 : Factors influencing the occurrence of schizophrenia-like psychosis in patients with temporal lobe epilepsy. Psychol Med 1975; 5:249–254Crossref, Medline, Google Scholar
16 : Psychosis and epilepsy: seizure-type comparisons and high-risk variables. J Clin Psychol 1981; 37:714–721Crossref, Medline, Google Scholar
17 : Psychotic disorders in Argentine patients with refractory temporal lobe epilepsy: a case–control study. Epilepsy Behav 2009; 14:604–609Crossref, Medline, Google Scholar
18 : A magnetization transfer imaging study in patients with temporal lobe epilepsy and interictal psychosis. Biol Psychiatry 2006; 59:560–567Crossref, Medline, Google Scholar
19 : Interictal SPECT in patients with mesial temporal lobe epilepsy and psychosis: a case–control study. Psychiatry Res 2005; 138:75–84Crossref, Medline, Google Scholar
20 : Cortical abnormalities and their cognitive correlates in patients with temporal lobe epilepsy and interictal psychosis. Epilepsia 2012; 53:1077–1087Crossref, Medline, Google Scholar
21 : Differential aberrant sprouting in temporal lobe epilepsy with psychiatric comorbidities. Psychiatry Res 2012; 195:144–150Crossref, Medline, Google Scholar
22 : Incidence of atypical handedness in epilepsy and its association with clinical factors. Epilepsy Behav 2009; 16:330–334Crossref, Medline, Google Scholar
23 : Postictal and chronic psychoses in patients with temporal lobe epilepsy. Am J Psychiatry 1995; 152:224–231Crossref, Medline, Google Scholar
24 : Handedness, alexithymia, and focus laterality as risk factors for psychiatric comorbidity in patients with epilepsy. Epilepsy Behav 2010; 17:389–394Crossref, Medline, Google Scholar
25 : Frequency and age-related variables in interictal psychoses in localization-related epilepsies. Epilepsy Res 2002; 48:25–31Crossref, Medline, Google Scholar
26 : A neuropsychological study of patients with temporal lobe epilepsy and chronic interictal psychosis. Epilepsy Res 2006; 71:117–128Crossref, Medline, Google Scholar
27 : Characteristics of temporal lobe epilepsy with mesial temporal sclerosis, with special reference to psychotic episodes. Neurology 1996; 47:1199–1203Crossref, Medline, Google Scholar
28 : Reexamination of interictal psychoses based on DSM-IV psychosis classification and international epilepsy classification. Epilepsia 2001; 42:98–103Crossref, Medline, Google Scholar
29 : Comorbid psychiatric symptoms in temporal lobe epilepsy: association with chronicity of epilepsy and impact on quality of life. Epilepsy Behav 2000; 1:184–190Crossref, Medline, Google Scholar
30 : Neuroanatomical correlates of psychosis in temporal lobe epilepsy: voxel-based morphometry study. Br J Psychiatry 2010; 197:482–492Crossref, Medline, Google Scholar
31 : Paranoid schizophrenia may be caused by dopamine hyperactivity of CA1 hippocampus. Biol Psychiatry 1992; 31:560–570Crossref, Medline, Google Scholar
32 : Psychosis and temporal lobe epilepsy: a controlled investigation. Epilepsia 1969; 10:363–395Crossref, Medline, Google Scholar
33 : Psychiatric comorbidity in epilepsy: a study comparing patients with mesial temporal sclerosis and juvenile myoclonic epilepsy. Epilepsy Behav 2008; 13:196–201Crossref, Medline, Google Scholar
34 : Amygdala pathology in psychosis of epilepsy: a magnetic resonance imaging study in patients with temporal lobe epilepsy. Brain 2002; 125:140–149Crossref, Medline, Google Scholar
35 : Psychoses of epilepsy: a study comparing the clinical features of patients with focal versus generalized epilepsies. Epilepsy Behav 2011; 20:655–658Crossref, Medline, Google Scholar
36 : Magnetoencephalographic characteristics of psychosis in temporal lobe epilepsy. J Neuropsychiatry Clin Neurosci 2009; 21:455–462Link, Google Scholar
37 : Comparison between the results of the Symptom Checklist–90 in two different populations with temporal lobe epilepsy. Epilepsy Behav 2003; 4:733–739Crossref, Medline, Google Scholar
38 : Absence of cortical gray-matter abnormalities in psychosis of epilepsy: a voxel-based MRI study in patients with temporal lobe epilepsy. J Neuropsychiatry Clin Neurosci 2004; 16:148–155Link, Google Scholar
39 : TLE patients with postictal psychosis: mesial dysplasia and anterior hippocampal preservation. Neurology 2000; 55:1027–1030Crossref, Medline, Google Scholar
40 : Increased plasma phospholipase-A2 activity in schizophrenic patients: reduction after neuroleptic therapy. Biol Psychiatry 1987; 22:421–426Crossref, Medline, Google Scholar
41 : Temporal lobe epilepsy with and without psychosis: exploration of hippocampal pathology including that in subpopulations of neurons defined by their content of immunoreactive calcium-binding proteins. Acta Neuropathol 2000; 99:547–554Crossref, Medline, Google Scholar
42 : Schizophrenia, temporal lobe epilepsy and psychosis: an in-vivo magnetic resonance spectroscopy and imaging study of the hippocampus/amygdala complex. Psychol Med 2000; 30:571–581Crossref, Medline, Google Scholar
43 : Corpora amylacea in mesial temporal lobe epilepsy: clinico-pathological correlations. Epilepsy Res 2007; 74:81–90Crossref, Medline, Google Scholar
44 : The significance of corpora amylacea in mesial temporal lobe epilepsy. Neurol India 2003; 51:277–279Medline, Google Scholar
45 : Secretory phospholipase A2 and phospholipids in neural membranes in an experimental epilepsy model. Acta Neurol Scand 2002; 106:258–262Crossref, Medline, Google Scholar
46 : Fiber architecture of the dentate gyrus following ablation of the entorhinal cortex in rats of different ages: evidence for two forms of axon sprouting in the immature brain. Neuroscience 1981; 6:903–910Crossref, Medline, Google Scholar
47 : Patterns of cerebral blood flow in schizophrenia. Br J Psychiatry 1992; 160:179–186Crossref, Medline, Google Scholar
48 : Diffusion tensor imaging findings and their correlation with neuropsychological deficits in patients with temporal lobe epilepsy and interictal psychosis. Epilepsia 2006; 47:941–944Crossref, Medline, Google Scholar
49 : Should cognitive deficit be a diagnostic criterion for schizophrenia? J Psychiatry Neurosci 2004; 29:102–113Medline, Google Scholar
50 : Alexithymia and emotional empathy following traumatic brain injury. J Clin Exp Neuropsychol 2010; 32:259–267Crossref, Medline, Google Scholar
51 : Simpson Jet al. Unemployment, social isolation, achievement–expectation mismatch and psychosis: findings from the Aesop study. Soc Psychiatry Psychiatr Epidemiol 2008; 43:743–751Crossref, Medline, Google Scholar
52 : The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13:261–276Crossref, Medline, Google Scholar
53 : Mini-International Neuropsychiatric Interview (MINI): validation of a short structured diagnostic psychiatric interview. Rev Brasiliera de Psiquiatria 2000; 22(3)Google Scholar
54 : SCL–90: an outpatient psychiatric rating scale: preliminary report. Psychopharmacol Bull 1973; 9:13–28Medline, Google Scholar



