Comparison of Anticraving Efficacy of Right and Left Repetitive Transcranial Magnetic Stimulation in Alcohol Dependence: A Randomized Double-Blind Study
Abstract
The objective of this study was to compare the anticraving efficacy of high-frequency repetitive transcranial magnetic stimulation (rTMS) of the right versus left dorsolateral prefrontal cortex (DLPFC) in patients with alcohol dependence. Twenty patients with alcohol dependence syndrome were randomly allocated to receive either right or left rTMS over the right DLPFC (10 sessions at 10 Hz frequency; 20 trains per session; 4.9 seconds per train and intertrain interval 30 seconds) and were assessed on the Alcohol Craving Questionnaire (ACQ-NOW) to measure craving. Two-way repeated-measures analysis of variance for ACQ-NOW total score showed no main effect of group (F[1,18] = 0.0001 but significant main effect of time (F[1,18] = 185.91, p<0.0001, η2 = 0.912). The interaction effect between group and time was not significant. There was significant reduction in craving scores in patients receiving either right or left rTMS with large effect size. However, there was no difference in anticraving efficacy between the two groups.
Alcoholism is one of the most prevalent neuropsychiatric disorders that results from a complex interplay between genetic and environmental factors, leading to deleterious consequences in both physical and psychological domains of the individual along with significant impairment in socio-occupational functioning.1 Alcohol craving presents as an irresistible urge to drink or as intense thoughts about alcohol2 and has significant implication in the development of alcohol dependence and frequent relapses seen in the course of the illness.3 Craving subsumes the intent to use alcohol, anticipation of positive outcome and anticipation of relief from withdrawal symptoms, lack of control over use, and cue-induced autonomic responses.4 Craving is biologically associated with the brain reward center situated in medial forebrain bundle comprising the meso-cortico-limbic dopamine pathway.5,6 However, there have been no studies that have evaluated the cerebral hemispheric lateralization of these constructs of craving. In recent years, several medications have been evaluated for alcohol craving with limited efficacy that warrants the application of novel strategies.
Repetitive transcranial magnetic stimulation (rTMS) is a noninvasive tool that is potentially efficacious in various neuropsychiatric disorders such as depression, mania, and schizophrenia.7 Studies have revealed robust antidepressant properties of left prefrontal high-frequency rTMS8–10; similar findings were observed with right prefrontal low-frequency rTMS,11 although the strength of evidence is low. High-frequency rTMS of right dorsolateral prefrontal cortex (DLPFC) has demonstrated significant reduction in Young Mania Rating Scale (YMRS) score in bipolar mania patients in various studies.12,13 Comparative right versus left DLPFC adjunctive high-frequency rTMS in bipolar manic patients has revealed significant improvement in YMRS score with right prefrontal rTMS, with worsening of mania with left prefrontal rTMS, suggesting that the therapeutic effect of rTMS in mania may show laterality effect opposite to that in depression.14 Several studies have demonstrated the efficacy of high-frequency rTMS of the left DLPFC in reducing negative symptoms of schizophrenia and producing functional improvement.15,16
Few studies have revealed the potential anticraving effects of rTMS in substance dependence. In a randomized sham controlled study,17 eleven nicotine-dependent subjects, were randomly assigned to a course of active or sham-rTMS on consecutive days. Craving, as measured by visual analog scale assessing the desire to smoke, significantly decreased after active compared with sham stimulation. In contrast, the study by Eichhammer et al.18 involving 14 treatment seeking smokers, a single session of high-frequency (20-Hz) rTMS application to the left DLPFC produced reduction in cigarette smoking and craving compared with sham treatment; however, the reduction in craving was not significant. In this study, craving was measured as using a 100-point visual analog scale in which the subjective state desire to smoke was assessed. In a recent outpatient, randomized, double-blind, sham-controlled study,19 48 chronic smokers were randomly assigned to real and sham rTMS stimulation (10 Hz over the left DLPFC, at 100% of MT, 20 trains/day, 50 pulses/train, intertrain interval 15 seconds, for 10 days), each group being subdivided randomly into two subgroups which were presented with either smoking-related or neutral pictures just before the daily TMS intervention. There was significant reduction in cigarette consumption and nicotine dependence, as evaluated objectively by measuring nicotine levels in urine samples and subjectively by participants’ self- reports of craving induced by presentation of smoking cues in form of pictures. In another randomized crossover study,20 involving six right-handed patients with cocaine dependence, two sessions of 10-Hz rTMS at 90% of the individual’s motor threshold, was applied on left or right DLPFC. The right, but not left DLPFC was found to transiently reduce craving (as defined by the desire to consume cocaine on a visual analog scale) by 19% from baseline, which disappeared after 4 hours. In another recent randomized sham-controlled study,6 13 subjects meeting DSM-IV criteria for alcohol dependence, who were abstinent for a minimum duration of 10 days, received active and sham bilateral transcranial direct current stimulation (tDCS) delivered to DLPFC (anodal left/cathodal right and anodal right/cathodal left) for 20 minutes; both anodal left/cathodal right and anodal right/cathodal left significantly decreased alcohol craving as measured on a visual analog scale, compared with sham stimulation, which could not be further increased by visual alcohol cues (viewing a video showing scenes of people drinking in a pleasant way). Our group conducted a prospective, single-blind, sham-controlled study21 involving 45 patients with alcohol dependence syndrome (according to ICD-10 DCR), with Clinical Institute of Withdrawal Assessment in Alcohol Withdrawal (CIWA-Ar) scores ≤10. Patients were allocated to active and sham rTMS in a 2:1 ratio, such that 30 patients received active and 15 patients sham rTMS to the right DLPFC (10 Hz frequency, 4.9 seconds per train, intertrain interval of 30 seconds, and 20 trains per session, total 10 sessions). Alcohol Craving Questionnaire (ACQ-NOW), which is a multidimensional measure of craving, was administered to measure the severity of alcohol craving at baseline, after the last rTMS session and after 1 month of the last rTMS session. Right dorsolateral prefrontal high-frequency rTMS was found to have significant anticraving effects in alcohol dependence, the effect size for treatment with time interaction was large (η2 = 0.401).22
Neuroimaging studies have revealed DLPFC to be a major component of the neural substrate for craving associated with various psychoactive substances including alcohol.6 It has been suggested that the brain substrates for craving can be influenced by cortical rTMS application because of the cortex’s massive interconnections and redundant cortical-subcortical loops.23 The brain reward center situated in medial forebrain bundle is implicated in craving associated with psychoactive substances including alcohol.5 The previous rTMS studies with left DLPFC stimulation, have found reduction in nicotine craving,18,19 and craving induced by the presence of appetitive food.24 However, right DLPFC rTMS stimulation has been found to reduce craving in alcohol dependence21 and cocaine dependence.20 Therefore, the current study was planned to compare the changes in craving parameters with right versus left prefrontal rTMS in alcohol dependence.
Materials and Methods
Study Sample
This was a prospective, hospital-based, single-blind, parallel-group, active-comparator rTMS study conducted over a period of 7 months from August 2009 to February 2010. This study was approved by Institutional ethical committee. Figure 1 shows the CONSORT diagram of flow of participants through the trial. Study sample was collected using purposive sampling method. The sample consisted of 20 (excluding two drop outs) right-handed male patients aged between 18 and 60 years with a diagnosis of alcohol dependence syndrome according to ICD-10 Diagnostic Criteria for Research,2 having CIWA-Ar25 scores of≤10. Written informed consent was obtained from all the participants. Those with comorbid major psychiatric, medical or neurological disorders or with pacemaker or metal in any part of the body were excluded from the study. Patients were randomly allocated to right and left stimulation group in a 1:1 ratio using randomization table. Eventually, ten patients received rTMS over right DLPFC and rest over left.
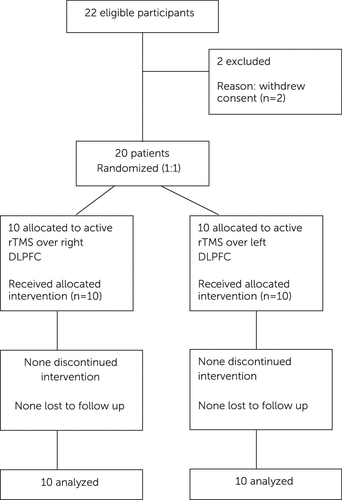
FIGURE 1. CONSORT Diagram Showing the Flow of Participants Through Each Stage of the Triala
Measures
The Handedness Preference Schedule, Hindi version26 was used to determine the handedness of the patients. Severity of Alcohol Dependence Questionnaire Form-C (SADQ-C)27 and ACQ-NOW4 were administered to measure the severity of alcohol dependence and craving, respectively. ACQ-NOW is a 47-item self-administered, multidimensional state measure of acute alcohol craving that has been adapted from the Cocaine Craving Questionnaire (CCQ-NOW).28 It measures five dimensions (subscales) of alcohol craving: factor 1, urges and desires to use alcohol; factor 2, intent to use alcohol; factor 3, anticipation of positive outcome; factor 4, anticipation of relief from withdrawal and negative outcome; and factor 5, lack of control over alcohol use. It has been shown to correlate with other multidimensional measures and visual analogs used to monitor changes in levels of craving from pretreatment through post-treatment.
Procedure
The motor threshold (MT) for the left abductor pollicis brevis (APB) was determined by Neuropack Sigma evoked potential measuring system using a figure-of-eight shaped coil at 1 Hz frequency according to Rossini-Rothwell algorithm.29 According to this, MT was defined as the lowest intensity, which produced five motor-evoked potentials responses of at least 50 µV in 10 trials. Ten daily sessions of rTMS treatments (using Magstim Rapid device; Magstim Company Ltd., Whitland, Wales, UK) were administered over either right or left DLPFC (at 110% of the MT determined) with an air-cooled figure-of-eight coil, angled tangentially to the head. High-frequency (10 Hz) stimulation was administered for 4.9 seconds per train, with intertrain interval of 30 seconds, and a total of 20 trains per session. Each patient received 1000 pulses per day. The rTMS session was started 3 days after the completion of detoxification. ACQ-NOW was administered at baseline, prior to the first rTMS session and immediately following the last rTMS session to measure the changes in craving parameters. During the study period, both the groups received zolpidem 12.5 mg tablets on an as and when required basis at night for insomnia along with vitamin B-complex capsules.
Statistical Analysis
The data obtained was analyzed using the computer software program, Statistical Package for Social Sciences (SPSS version 10.0 for Windows computer program; SPSS Inc., Chicago, IL). The level for alpha was set at p <0.05 (two-tailed) for statistical hypothesis testing with exact probability levels for test statistics shown in the text. Normality of data were examined using histogram and Shapiro-Wilk test. Group differences in clinical characteristics between the right and left group were made using independent sample ‘t’ test for normally distributed data. Repeated-measures analysis of variance (ANOVA) was carried out for the ACQ-NOW total scores, factor scores, and general craving index (GCI) score with two factors: group (right and left) and time (pre-rTMS and post-rTMS). Effect size was reported as eta squared (η2). Pearson’s correlation coefficient (r) was calculated between socio-demographic and clinical variables with change in craving scores.
Results
Sample Characteristics
In our study, 22 patients with the diagnosis of alcohol dependence syndrome fulfilling the inclusion and exclusion criteria were initially recruited, of which two patients dropped out as they withdrew consent because of apprehensions related to the rTMS application. Out of 20 patients, 10 patients each received rTMS over right and left DLPFC, respectively. Sample characteristics are summarized in Table 1. The mean age of patients was 37.10 (SD 10.48) years in the right group and 43.20 (SD 9.74) years in the left group. There was no significant difference in the sociodemographic and clinical variables between the right and left groups. The motor threshold between the two groups was comparable.
| Variables | Right (M±SD) | Left (M±SD) | t (df=18) | p (2-tailed) |
|---|---|---|---|---|
| (N=10) | (N=10) | |||
| Age | 37.10±10.48 | 43.20±9.74 | –1.35 | 0.194 |
| Years of formal education | 9.00±5.85 | 10.20±5.60 | –0.45 | 0.656 |
| Years of alcohol use (duration) | 16.90±8.58 | 17.70±11.14 | –0.18 | 0.859 |
| Age of onset | 20.20±8.32 | 25.50±6.45 | –1.59 | 0.129 |
| Years of dependence | 4.60±2.76 | 5.10±6.04 | –1.39 | 0.182 |
| CIWA-Ar total score | 3.50±1.96 | 2.50±1.27 | 1.36 | 0.192 |
| SADQ total score | 55.00±7.51 | 54.60±7.86 | 0.12 | 0.909 |
| SADQ- A score | 14.40±0.97 | 13.80±1.40 | 1.12 | 0.279 |
| SADQ- B score | 31.00±5.96 | 31.60±6.31 | –0.22 | 0.829 |
| SADQ- C score | 9.60±1.51 | 9.00±1.70 | 0.84 | 0.414 |
| Motor threshold | 49.50±8.64 | 48.50±5.30 | 0.31 | 0.759 |
TABLE 1. Sample Characteristicsa
Change in Craving Scores
The mean ACQ-NOW scores in both the groups are summarized in Table 2. For ACQ-NOW total score, repeated measures ANOVA showed no main effect of group (F[1,18] = 0.0001, p=0.993) but significant main effect of time (F[1,18] = 185.91, p <0.0001, η2 = 0.912). The interaction effect between group and time was not significant (F[1,18] = 0.03, p=0.864). Similarly, for ACQ-NOW factor scores, repeated measures ANOVA showed no main effect of group, but significant main effect of time (p <0.0001). The interaction effect between group and time was not significant. For GCI score, repeated measures ANOVA showed no main effect of group (F[1,18] = 0.004, p=0.948) but significant main effect of time (F[1,18] = 189.57, p <0.0001, η2 = 0.913). The interaction effect between group and time was not significant (F[1,18] = 0.05, p=0.825). In the right rTMS group, five patients (50%) showed response with treatment (50% reduction in ACQ-NOW scores from baseline) compared with 4 patients (40%) in the left rTMS group, and the difference was not significant (Fisher exact significance >0.05).
| Craving Scores | Pre-rTMS | Post-rTMS |
|---|---|---|
| ACQ-T | ||
| Right (M±SD) | 268.10±21.52 | 144.50±36.98 |
| Left (M±SD) | 269.60±30.48 | 142.80±37.16 |
| ACQ–1 | ||
| Right (M±SD) | 52.90±4.86 | 26.90±8.40 |
| Left (M±SD) | 51.30±7.26 | 26.10±7.95 |
| ACQ–2 | ||
| Right (M±SD) | 44.80±6.01 | 19.90±7.43 |
| Left (M±SD) | 49.20±6.74 | 20.60±8.28 |
| ACQ–3 | ||
| Right (M±SD) | 47.90±7.82 | 27.60±7.52 |
| Left (M±SD) | 49.60±9.25 | 27.60±9.47 |
| ACQ–4 | ||
| Right (M±SD) | 57.80±2.44 | 32.90±9.33 |
| Left (M±SD) | 54.70±6.41 | 32.80±10.12 |
| ACQ–5 | ||
| Right (M±SD) | 53.00±6.77 | 30.90±10.12 |
| Left (M±SD) | 52.60±7.06 | 29.30±9.73 |
| GCI | ||
| Right (M±SD) | 8.66±0.70 | 4.70±1.17 |
| Left (M±SD) | 8.70±0.98 | 4.61±1.20 |
TABLE 2. Mean ACQ-NOW scores in right (N=10) and left (N=10) rTMS groupsa
Correlations of Craving Scores With Sociodemographic and Clinical Characteristics
In the right rTMS group, age significantly positively correlated with change in ACQ-NOW factor 5 (r=0.696, p=0.025), with trend toward positive correlation with change in ACQ-NOW total score, factor 1 and 4, and GCI scores. Years of formal education was found to positively correlate with change in ACQ-NOW factor 1 (r=0.768, p=0.010), factor 3 (r=0.765, p=0.010), factor 5 (r=0.645, p=0.044), total score (r=0.722, p=0.018), and GCI (r=0.727, p=0.017), with trend toward positive correlation with factor 2 score. The age of onset of alcohol use was found to correlate negatively with change in ACQ-Total score (r=−0.415, p=0.023), ACQ-factor 1 (r=−0.385, p=0.036), factor 2 (r=−0.401, p=0.028), factor 3 (r=−0.392, p=0.032), factor 5 (r=−0.442, p=0.015), and general craving index (r=−0.415, p=0.023) scores. The years of alcohol dependence similarly was found to positively correlate with change in ACQ-factor 1 score (r=0.399, p=0.029), with evidence of trend toward positive correlation with total, factor 5 and GCI scores. A trend toward positive correlation was found between total duration of alcohol use in years and change in ACQ-factor 5 score. Severity of alcohol dependence (SADQ total score) correlated negatively with change in ACQ-NOW factor 4 (r=−0.794, p=0.006) with a trend toward negative correlation with factors 1, 3, 5, total score, and GCI.
In the left rTMS group, age showed a trend toward positive correlation with change in ACQ-NOW factor 2, whereas no correlation with education or age of onset of alcohol use was found. Duration of alcohol use correlated positively with change in ACQ-NOW factor 2 (r=0.649, p=0.042). Severity of alcohol dependence (SADQ total score) correlated positively with change in ACQ-NOW factor 4 (r=0.666, p=0.035) with a trend toward positive correlation with total score and GCI. This being an exploratory analysis, we conducted several correlations between sociodemographic and clinical characteristics with the craving scores. Considering large number of analyses, there is a possibility that some of the significant correlations are due to chance.
Adverse Effects of rTMS
One patient receiving right rTMS developed nightmare and middle insomnia after the eighth session. Four patients receiving left rTMS reported subjective improvement in sleep following fifth to sixth rTMS sessions. None reported scalp pain or headache following rTMS and none developed seizure.
Discussion
In our study, ACQ-NOW was used in view of its ability to measure multidimensional aspects of craving with high internal consistency and reflect the changes in alcohol craving with rTMS treatment. There was significant reduction in craving scores in patients receiving either right or left rTMS and the effect size was high.22 However, there was no difference in anticraving efficacy between the two groups. Increased activity in the mesolimbic dopaminergic pathway has been implicated in craving associated with alcohol dependence.5 Several dopaminergic antagonists such as clozapine and olanzapine have demonstrated a significant effect in reducing alcohol craving.6 The application of rTMS to the DLPFC could have modulated the altered activity in the mesolimbic pathway through the meso-fronto-limbic connections. Previously, we had found rTMS treatment to right DLPFC had anticraving efficacy.21 High-frequency rTMS application to left and right DLPFC possibly resulted in suppression of the left DLPFC directly or transsynaptically,30 respectively, which reduced alcohol craving.
The mean age of the participants was greater than that of the study by Camprodon et al.20 (age range 19–23 years) but similar to Mishra et al.21 and Boggio et al.6 studies. The mean age of onset of alcohol use in both the groups was greater than that described by Boggio et al.6 (mean 15.0, SD 4.6 years) study. All the patients were abstinent for more than 10 days before the beginning of rTMS session, whereas the abstinence period was 41.0 (SD 51.3) days (a minimum of 10 days) in the Boggio et al.6 study. The rTMS session was started 3 days after the completion of detoxification (duration 7–10 days) in order to prevent the interference of lorazepam in determination of motor threshold. The abstinence period was minimized in our study so as to complete the rTMS sessions within the average duration of stay of the patient in the institute which is approximately 1 month.
Age and education correlated positively with reduction in craving scores in patients receiving right rTMS (moderate effect size), whereas this effect was less marked in left rTMS group. Severity of alcohol dependence had a negative correlation with reduction in craving scores in right rTMS group, whereas a positive correlation was observed between them in those receiving left rTMS (large effect size). It can be inferred that rTMS over left DLPFC is effective in severe alcohol dependence, whereas less severe cases respond to rTMS over right DLPFC. This differential effect might reflect the differences in underlying mechanism through which rTMS acts on both the sides. It can be postulated that rTMS over left DLPFC affects craving circuits directly, whereas stimulation over right affects it indirectly through transcallosal suppression of left DLPFC.30 Further, left-sided rTMS treatment improved subjective sleep in four patients. Such phenomenon was not observed with stimulation over the right side. This finding needs exploration in further studies.
In the present study, rTMS was found to be tolerated well by the patients with benign adverse effect profile as found in the previous studies.20,31 Our study was limited by small sample size. The lack of sham control group is another limitation of our study. The period of abstinence in our study was shorter (10‒13 days) compared with a mean 41 days (minimum of 10 days) in Boggio et al.6 study, which could potentially affect the craving scores. The DLPFC of patients was located using the “5 cm rule,”32 which does not take into consideration the shape and size of a person’s head. This may result in some variations in the exact site of stimulation in the prefrontal cortex. We used the rTMS parameters that were effective in previous study conducted by our group.21 It is possible the lack of difference between left and right rTMS observed in our study could be the result of laterality differences in rTMS effect, similar to that seen in depression (i.e., left high-frequency rTMS versus right low-frequency rTMS).8–11 Further studies are required to optimize TMS parameters such as frequency of stimulation, number of trains, duration of each train, intertrain interval, and number of sessions that will be effective in alcoholism without producing adverse events. The period of rTMS to maintain the gains produced need to be examined with longer follow-up studies. Neurophysiological variables such as quantitative EEG, evoked potentials, frontal activation tasks should be measured along with rTMS in alcohol dependence for more comprehensive assessment of the treatment effect.
1 : Alcohol: effects on neurobehavioral functions and the brain. Neuropsychol Rev 2007; 17:239–257Crossref, Medline, Google Scholar
2
3 : Theories of drug craving, ancient and modern. Addiction 2001; 96:33–46Crossref, Medline, Google Scholar
4 : The Alcohol Craving Questionnaire (ACQ-NOW), in Assessing Alcohol Problems: A Guide for Clinicians and Researchers, 2nd ed. Edited by Allen JP, Wilson VB. NIH Publication No. 03-3745. Bethesda, MD, National Institute on Alcohol Abuse and Alcoholism, 2003, pp 271–281Google Scholar
5 : Brain substrates of craving to alcohol cues in subjects with alcohol use disorder. Alcohol Alcohol 2007; 42:417–422Crossref, Medline, Google Scholar
6 : Prefrontal cortex modulation using transcranial DC stimulation reduces alcohol craving: a double-blind, sham-controlled study. Drug Alcohol Depend 2008; 92:55–60Crossref, Medline, Google Scholar
7 : Transcranial magnetic stimulation: applications in neuropsychiatry. Arch Gen Psychiatry 1999; 56:300–311Crossref, Medline, Google Scholar
8 : Effects of antidepressant pharmacotherapy after repetitive transcranial magnetic stimulation in major depression: an open follow-up study. J Psychiatr Res 2003; 37:145–153Crossref, Medline, Google Scholar
9 : Transcranial magnetic stimulation accelerates the antidepressant effect of amitriptyline in severe depression: a double-blind placebo-controlled study. Biol Psychiatry 2005; 57:162–166Crossref, Medline, Google Scholar
10 : Long-lasting effects of high-frequency repetitive transcranial magnetic stimulation in major depressed patients. Psychiatry Res 2007; 150:181–186Crossref, Medline, Google Scholar
11 : Repetitive transcranial magnetic stimulation (rTMS) in pharmacotherapy-refractory major depression: comparative study of fast, slow and sham rTMS. Psychiatry Res 1999; 88:163–171Crossref, Medline, Google Scholar
12 : Repetitive transcranial magnetic stimulation as an add-on therapy in the treatment of mania: a case series of eight patients. Psychiatry Res 2004; 128:199–202Crossref, Medline, Google Scholar
13 : Efficacy of high-frequency (rapid) suprathreshold repetitive transcranial magnetic stimulation of right prefrontal cortex in bipolar mania: a randomized sham controlled study. J Affect Disord 2009; 117:146–150Crossref, Medline, Google Scholar
14 : Transcranial magnetic stimulation in mania: a controlled study. Am J Psychiatry 1998; 155:1608–1610Crossref, Medline, Google Scholar
15 : Efficacy of adjuvant high-frequency repetitive transcranial magnetic stimulation on negative and positive symptoms of schizophrenia: preliminary results of a double-blind sham-controlled study. J Neuropsychiatry Clin Neurosci 2007; 19:464–467Link, Google Scholar
16 : Treatment of negative symptoms of schizophrenia using repetitive transcranial magnetic stimulation in a double-blind, randomized controlled study. Schizophr Res 2007; 95:151–157Crossref, Medline, Google Scholar
17 : [Transcranial magnetic stimulation for nicotine dependence]. Psychiatr Prax 2003; 30(Suppl 2):S129–S131Crossref, Medline, Google Scholar
18 : High-frequency repetitive transcranial magnetic stimulation decreases cigarette smoking. J Clin Psychiatry 2003; 64:951–953Crossref, Medline, Google Scholar
19 : Repeated high-frequency transcranial magnetic stimulation over the dorsolateral prefrontal cortex reduces cigarette craving and consumption. Addiction 2009; 104:653–660Crossref, Medline, Google Scholar
20 : One session of high-frequency repetitive transcranial magnetic stimulation (rTMS) to the right prefrontal cortex transiently reduces cocaine craving. Drug Alcohol Depend 2007; 86:91–94Crossref, Medline, Google Scholar
21 : Efficacy of repetitive transcranial magnetic stimulation in alcohol dependence: a sham-controlled study. Addiction 2010; 105:49–55Crossref, Medline, Google Scholar
22 : Experimental Design: Procedures for the Behavioral Sciences, 3rd ed. Pacific Grove, CA, Brooks/Cole, 1995Google Scholar
23 : Mechanisms and state of the art of transcranial magnetic stimulation. J ECT 2002; 18:170–181Crossref, Medline, Google Scholar
24 : Effect of left prefrontal repetitive transcranial magnetic stimulation on food craving. Biol Psychiatry 2005; 58:840–842Crossref, Medline, Google Scholar
25 : Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict 1989; 84:1353–1357Crossref, Medline, Google Scholar
26 : Hand preference in India. Int J Psychol 1992; 27:433–442Crossref, Google Scholar
27 : The measurement of alcohol dependence and impaired control in community samples. Addiction 1994; 89:167–174Crossref, Medline, Google Scholar
28 : The development of a cocaine craving questionnaire. Drug Alcohol Depend 1993; 34:19–28Crossref, Medline, Google Scholar
29 : Stimulation of the human motor cortex through the scalp. Exp Physiol 1991; 76:159–200Crossref, Medline, Google Scholar
30 : Prefrontal repetitive transcranial magnetic stimulation (rTMS) changes relative perfusion locally and remotely. Human Psychopharmacol Clin Exp 1999; 14:161–170Crossref, Google Scholar
31 : Report on risk and safety of repetitive transcranial magnetic stimulation (rTMS): suggested guidelines from the International Workshop on Risk and Safety of rTMS (June 1996). Electroencephalogr Clin Neurophysiol 1997; 108:1–16Crossref, Medline, Google Scholar
32 : Mood improvement following daily left prefrontal repetitive transcranial magnetic stimulation in patients with depression: a placebo-controlled crossover trial. Am J Psychiatry 1997; 154:1752–1756Crossref, Medline, Google Scholar



