Effect of High-Frequency Transcranial Magnetic Stimulation on Craving in Substance Use Disorder: A Meta-Analysis
Abstract
Repetitive transcranial magnetic stimulation (rTMS), a noninvasive, neuromodulatory tool, has been used to reduce craving in different substance use disorders. There are some studies that have reported conflicting and inconclusive results; therefore, this meta-analysis was conducted to evaluate the effect of high-frequency rTMS on craving in substance use disorder and to investigate the reasons behind the inconsistency across the studies. The authors searched clinical trials from MEDLINE, Cochrane databases, and International Clinical Trials Registry Platform. The PRISMA guidelines, as well as recommended meta-analysis practices, were followed in the selection process, analysis, and reporting of the findings. The effect estimate used was the standardized mean difference (Hedge's g), and heterogeneity across the considered studies was explored using subgroup analyses. The quality assessment was done using the Cochrane risk of bias tool, and sensitivity analysis was performed to check the influences on effect size by statistical models. After screening and assessment of eligibility, finally 10 studies were included for meta-analysis, which includes six studies on alcohol and four studies on nicotine use disorder. The random-model analysis revealed a pooled effect size of 0.75 (95% CI=0.29 to 1.21, p=0.001), whereas the fixed-model analysis showed a large effect size of 0.87 (95% CI=0.63 to 1.12, p<0.00001). Subgroup analysis for alcohol use disorder showed an effect size of –0.06 (95% CI=–0.89 to 0.77, p=0.88). In the case of nicotine use disorder, random-model analysis revealed an effect size of 1.00 (95% CI=0.48 to 1.55, p=0.0001), whereas fixed-model analysis also showed a large effect size of 0.96 (95% CI=0.71 to 1.22). The present meta-analysis identified a beneficial effect of high-frequency rTMS on craving associated with nicotine use disorder but not alcohol use disorder.
Psychoactive substance use is a major public health problem, which has a debilitating impact on the physical, psychological, and social functioning of an individual and results in substantial health care burden. Alcohol and illicit drug use account for 5.4% of the global annual disease burden, with tobacco use responsible for about 3.7%. Moreover, 12.4% of all deaths worldwide are attributed to alcohol, tobacco, and illegal drug use, and they are the eighth major cause of death globally.1–3 Substance use disorder involves a cluster of behavioral, cognitive, and physiological consequences that develop after repeated substance use.4 Craving has been recognized as a major construct in substance use disorder, which subsumes the intent to use the substance, lack of control over use, anticipation of positive outcome, and/or relief from withdrawal symptoms. Craving has been implicated in causing frequent relapses and maintaining the substance-seeking behavior; hence, it has been the mainstay domain being targeted in the treatment of substance-related disorders.4,5 Despite the availability of various anticraving drugs, the effectiveness of these agents is limited, hence necessitating the application of novel tools that can modulate the target brain circuits and neurochemicals substrates associated with craving.6
Repetitive transcranial magnetic stimulation (rTMS) is a noninvasive tool that has found therapeutic applications in different neurological and psychiatric conditions, by producing cerebral neuromodulation through the modification of cortical excitability, neurotransmitter release, signaling pathway, and gene expression.7–9 Craving related to substance use has been linked with the ascending dopaminergic tracts comprising the meso-cortico-limbic pathway or the “brain reward circuit.” The dorsolateral prefrontal cortex (DLPFC) exerts inhibitory control over the reward circuit through the meso-fronto-limbic connections.10 Stimulating DLPFC by rTMS has been postulated to reduce substance craving possibly by two mechanisms. Firstly, interconnections of the DLPFC with the ventral tegmental area (VTA) increases dopamine excretion from the VTA to the ventral striatum, an area implicated in reward processing. Secondly, stimulation of the DLPFC stimulates glutamate containing cortico-fugal fibers, which end on dopamine containing terminals in the ventral striatum, potentially increasing dopamine excretion and reducing craving.11 In view of the relatively limited and shallow stimulation area targeted by the standard figure-8-shaped TMS coil, recent studies have also used H-coil for deep brain stimulation including the insula, employing similar electromagnetic intensities with limited untoward side effects. As the prefrontal cortex (PFC) and insular cortex are part of the reward system, the stimulation of the DLPFC by figure-8 TMS coil or the stimulation of deeper cortices of medial PFC and insula through H-coil ultimately results in dopaminergic activation in the VTA, thereby reducing craving.
Previous rTMS studies in relation to substance use disorder have revealed rTMS application to significantly reduce craving, particularly in nicotine and cocaine dependence.11–17 However, the few studies of rTMS on craving in alcoholism have yielded inconsistent findings.18–23 In these studies, there has been wide variation in relation to the rTMS intervention protocols, the duration of stimulation, the outcome tools for measuring changes in craving, and, finally, the period of observation.23 In view of this novel and safe therapeutic anticraving intervention and varied but interesting test findings, a meta-analysis of all the studies in relation to rTMS and substance use disorder would be beneficial.
Methods
Development and Registration of Protocol
We developed and followed a standard meta-analysis protocol following PRISMA-P 2015 guidelines24 and registered the protocol in International Prospective Register of Ongoing Systematic Reviews (systematic review registration-PROSPERO 2016: CRD42016032786). This meta-analysis has been conducted and reported conforming to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement.25 The Cochrane handbook26 was used as a methodological reference.
Search Strategy
We searched the MEDLINE and Cochrane databases for randomized controlled trials (RCTs) and controlled clinical trials (CCTs) on transcranial magnetic stimulation (TMS) in patients with substance use disorder published until 2015. Our inquiry was constructed following the PICO-method. Key elements that we used in our search were the “P” (substance use disorder/addicts/addiction/dependence/drug abuse/substance abuse), the “I” (rTMS/ transcranial magnetic stimulation), the “C” (Sham/Placebo), and the “O” (craving). We searched reference lists of published studies, and for unpublished but completed studies we checked the International Clinical Trials Registry Platform (ICTRP) search portal [http://apps.who.int/trialsearch/default.aspx], which is a central database containing the trial registration data sets provided by the different international trial registries including ClinicalTrials.gov.
Study Selection Criteria
Types of studies.
RCTs and CCTs on TMS in patients with substance use disorder published in English-language peer-reviewed journals have been included. All studies included in this meta-analysis had craving reduction as an outcome measure and assessed craving levels in alcohol, nicotine, cocaine, and methamphetamine-dependent patients. The included studies were not restricted by date of publication, craving assessment tool, number of stimulation sessions, site of stimulation, or method of localization of site of stimulation. We have excluded letters to editor, case series, and case reports.
Types of participants.
We have included the studies examining adult human subjects (age: 18–70 years) of both sexes with a diagnosis of substance use disorder fulfilling DSM-5 or ICD-10 criteria. In all the included studies, the following exclusion criteria were followed: use of anticraving medication at admission, any personal or family history of epilepsy, a recent neurosurgical condition, presence of pacemakers or other electronic implants, metal or magnetic objects in brain, unstable medical condition, pregnancy, psychotic episodes, delirium, disorientation, and severe cognitive deterioration.
Types of interventions.
High-frequency rTMS was given to the patients by rTMS machine, which is a high-speed magnetic stimulator connected to a figure-of-eight-formed double 70-mm coil held tangentially to the skull. Resting motor thresholds were performed via visual twitch in the contralateral abductor policis brevis at the beginning of each experiment. In some studies, in order to accurately target the area of the brain, taking into account individual anatomical differences, the precise stimulation site and position of the coil was determined using MRI nonstereotactic guidance.
Types of outcome measures.
Change in craving after rTMS session from baseline (pre rTMS) was measured by different craving assessment tools. In the included studies, the tools used are the Visual Analog Scale (VAS), the short version of Tobacco Craving Questionnaire (sTCQ), the Obsessive Compulsive Drinking Scale (OCDS), the and Alcohol Craving Questionnaire (ACQ-NOW).
Study Selection and Data Collection
Selection of studies.
The selection of relevant studies was done in a stepwise manner. First, all studies were screened based on title and abstract. Then the full text of all studies from this selection was retrieved and read. Inclusion criteria were determined before the literature search was performed. Those studies that met the inclusion criteria were included in the meta-analyses.
Data extraction and management.
Data were abstracted and quality was assessed independently by two investigators (R.M. and B.R.M.) using guidelines published by the Cochrane Collaboration.26 Any disagreement was resolved by discussion between two authors (R.M. and B.R.M.) in consultation with the clinical pharmacologist cum statistical advisor (D.H.). Extracted data included study design, participants, intervention, site of stimulation, outcome measure, and protocol of intervention (frequency of stimulation, number of trains, intertrain interval, motor threshold).
Data Analysis
Meta-analysis was conducted using the Cochrane Program Review Manager Version 5.3.27
Assessment of risk of bias in included studies.
To assess the risk of bias in individual studies, a standardized critical appraisal instrument, the Cochrane Collaboration’s risk of bias tool, was used.26 This tool rates bias of a clinical trial in three categories (low, unclear, and high) on the following domains: random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other bias (if any). Two reviewers (R.M. and B.R.M.) independently evaluated and recorded their judgments and justifications in each domain for each included study.
Measures of treatment effect.
In this meta-analysis, the outcome measure of interest was craving that has been estimated by different craving tools. The standardized mean difference (Hedge's g) has been calculated to estimate the effect size in order to assess the difference in craving levels between active/real and sham/placebo stimulation. Hedge’s g is considered to be a conservative estimate, which is useful for studies with small sample sizes, and the results may be interpreted as reflecting a small (g=0.2–0.5), medium (g=0.5–0.8), or large effect (g >0.8).28 For overall between-group analysis, both random-effect and fixed-effect models were used, irrespective of heterogeneity between individual study samples.
Unit of analysis issues.
“Study” was considered as unit of design instead of the “unit for analyses.” Studies in which two tools were used to assess craving, both parameters were considered separately as two “units of analysis.” We attempted to contact authors on further information on study characteristics when reported data were insufficient for data analysis.
Assessment of heterogeneity.
Keeping in mind the fact that statistical heterogeneity is inevitable due to clinical and methodological diversity in clinical studies, it is important to consider the extent of inconsistency or to quantify inconsistency across the included studies. The chi-squared test was used to assess whether observed differences in results are compatible with chance alone. A low p value (or a large chi-squared statistic relative to its degree of freedom) provides evidence of heterogeneity of intervention effects (variation in effect estimates beyond chance). I2 statistics, which describe the percentage of the variability in effect for an estimate that is due to heterogeneity, will be done for quantifying inconsistency.
Subgroup and sensitivity analysis.
In this meta-analysis, six studies were on alcohol use disorder and another four studies were on nicotine use disorder. Thus, we planned subgroup analysis to assess the effect of high-frequency transcranial stimulation on craving in alcohol and nicotine use disorder individually. Sensitivity analyses were planned to explore the influences on effect size by statistical models (fixed versus random effects).
Assessment of publication bias.
The publication bias across studies was assessed visually using a funnel plot. We also conducted the Egger regression test and Begg and Mazumdar rank correlation test as formal statistical tests for publication bias.
Results
Description of Included Studies
The initial literature search identified 37 potentially relevant studies for assessment. The PRISMA flow diagram illustrates the selection process throughout (Figure 1). At the stage of initial screening, 18 publications were excluded from meta-analysis due to different reasons. Reasons for exclusion were as follows: studies assessing the effect of direct current stimulation, intermittent theta burst stimulation, and low-frequency TMS; functional MRI studies; case reports, letters to the editor, or review articles; and articles in German language. After initial screening, 19 published articles were retrieved for detailed evaluation. In 10 studies, detailed statistical data were not sufficient for calculating standardized mean difference (Hedge's g); therefore, corresponding authors were contacted through e-mail and requested to provide necessary data for the meta-analysis. Six authors responded, whereas three authors did not respond even after reminders; studies from nonresponsive authors were excluded from meta-analysis. One author was unable to be contacted because that author's e-mail address was not provided in the translated article. During this phase, we found that in two studies, there was no sham/placebo control group as a comparator, and in another three studies craving had not been assessed as an outcome parameter. After exclusion of these nine studies, 10 studies were entered into this meta-analysis: six studies on alcohol use disorder and four studies on nicotine use disorder (Tables 1 and 2). Of these studies, the left DLPFC was stimulated in four studies, the right DLPFC was stimulated in three studies, the lateral PFC bilaterally was stimulated in two studies, and the medial PFC was stimulated in one study. The quality assessment of the included studies has been presented through risk of bias summary in Table 3, showing the proportion of studies with each of the judgments (“low risk,” “high risk,” “unclear risk” of bias) for each included study.
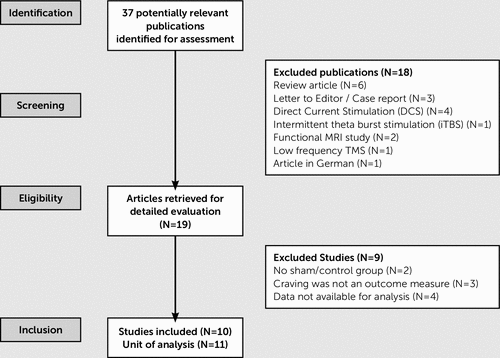
FIGURE 1. Flow Diagram for the Study Selection Process
| Trial and Location | Methods | Participants | Interventions | Outcomes | Number of Participants | Site of Stimulation | Intervention Protocol | Notes/Remarks |
|---|---|---|---|---|---|---|---|---|
| Amiaz et al.11; Israel | Randomized, double-blind, sham-controlled | Patients with nicotine use disorder | High-frequency rTMS | VAS and sTCQ | Sham smoke group: 9/11 subjects finished the 10 days of treatment Real smoke group: 12/13 subjects finished the 10 days of treatment | Left DLPFC | 10 Hz-rTMS, 100% MT, 20 trains/day with an intertrain interval of 15 seconds | Significant reduction in craving |
| Mishra et al.18; India | Randomized, single-blind, sham-controlled | Right-handed male patients with alcohol use disorder | High-frequency rTMS | ACQ-NOW | Sham rTMS: 15 patients; Active rTMS: 30 patients | Right DLPFC | 10 Hz-rTMS, 4.9 seconds per train, intertrain interval 30 seconds, 20 trains per session, total 10 sessions, 110% MT | Significant reduction in craving |
| Höppner et al.21; Germany | Randomized, sham-controlled | Female patients with alcohol use disorder | High-frequency rTMS | OCDS | Sham rTMS: 9 patients Active rTMS: 10 patients | Left DLPFC | 20-Hz, 20 trains of 2.5 seconds, intertrain interval of 42.5 seconds, 1,000 stimuli per day on 10 consecutive working days, 90% MT | No significant changes in craving |
| Herremans et al.22; Belgium | Randomized, single-blind sham-controlled, crossover design | Patients with alcohol use disorder | High-frequency rTMS | OCDS | Sham rTMS: 16 patients; Active rTMS: 15 patients | Right DLPFC | 20-Hz, 40 trains of 1.9 seconds, intertrain interval of 12 seconds, 1560 pulses per session, 110% MT | No significant changes in craving |
| Herremans et al.23; Belgium | Randomized, single-blind sham-controlled, crossover design | Patients with alcohol use disorder | High-frequency rTMS | OCDS | Sham rTMS: 16 patients; Active rTMS: 13 patients | Right DLPFC | 20-Hz, 40 trains of 1.9 seconds, intertrain interval of 12 seconds, 1,560 pulses per session, 110% MT | No significant changes in craving |
| Li et al.12; United States | Randomized, double-blind sham-controlled, crossover design | Right-handed patients with nicotine use disorder | High-frequency rTMS | VAS | 16 (14 completed the study) | Left DLPFC | 10-Hz for 5 seconds, intertrain interval of 10 seconds, 100% MT, each session of 15 minutes with 3,000 pulses | Significant reduction in craving |
| Dinur-Klein et al.33; Israel | Randomized, double-blind sham-controlled | Patients with nicotine use disorder | High- and low-frequency deep rTMS | sTCQ | Sham group: 31 subjects; Low frequency group: 14 subjects; High-frequency group: 32 subjects | Insula and the lateral prefrontal cortex bilaterally | High-frequency sessions consisted of 33 trains of 10 Hz, each lasting 3 seconds, with an intertrain interval of 20 seconds. Total treatment duration was 760 seconds with 990 pulses. Low-frequency sessions consisted of 600 continuous pulses at 1 Hz. | No significant change in craving |
| Pripfl et al.13; Austria | Sham-controlled within-subject design | Patients with nicotine use disorder | High-frequency rTMS | A 5-point rating scale following Cue induced craving (CIC) task | 14 (11 analyzed) | Left DLPFC | 10 Hz, 24 trains, 5 seconds per train, 25 seconds intertrain interval, i.e., 1,200 pulses within 11.6 minutes, 90% MT | Craving ratings were significantly lower after real rTMS stimulation compared with sham stimulation |
| Girardi et al.19; Italy | Open-label add-on deep TMS compared with standard treatment | Patients with alcohol use disorder | High-frequency deep TNS | OCDS | Add-on deep TMS group: 10 subjects; Standard drug treatment group: 10 subjects | Bilateral DLPFC | 20 Hz, 55 trains per session with a 2-second duration each and an intertrain interval of 20 seconds, 120% MT, total of 20 sessions for each patients | Add-on of deep TMS to standard treatment is associated with a significant reduction in craving |
| Ceccanti et al.20; Italy | Randomized, double-blind placebo-controlled | Patients with alcohol use disorder | High-frequency deep rTMS | VAS | Real stimulation: 9 subjects; Sham stimulation: 9 subjects | Medial PFC | 20 Hz, 10 sessions of 30 consecutive trains of 50 stimuli, intertrain interval of 30 seconds, 120% MT | Significant reduction in craving after real rTMS stimulation |
TABLE 1. Characteristics of Trials Included in the Meta-Analysisa
| Trial and Location | Methods | Participants | Intervention | Outcomes | Number of Participants | Site of Stimulation | Intervention Protocol | Reason for Exclusion |
|---|---|---|---|---|---|---|---|---|
| Eichhammer et al.14; Germany | Double-blind crossover trial | Treatment-seeking subjects with nicotine use disorder | High-frequency rTMS | Number of cigarettes smoked during an ad libitum smoking period and effects on craving after a period of acute abstinence | 14 | left DLPFC | 20 trains of rTMS at a rate of 20 Hz for 2.5 seconds over 14 minutes (1,000 pulses/session; intertrain interval: 42.5 seconds). | Published data are inadequate to calculate standardized mean difference. Request mail and subsequent reminders were sent to corresponding author for data with no reply. |
| Camprodon et al.16; United States | Randomized crossover study | Right-handed males with cocaine use disorder | High-frequency rTMS | VAS | 6 | Right and left DLPFC | Each session consisted of 20 trains of a 10-second duration, separated by 1-minute pauses. The frequency of stimulation was 10 Hz, and the intensity was 90% of the individual’s motor threshold. | No placebo/sham comparator group. |
| Staroverov et al.30; Russia | Randomized, placebo-controlled study | Patients with stage II alcohol use disorder | TMT | Hospital Anxiety and Depression Scales, Zung Scale, Spielberger-Khanin Scale, MFI-20 Questionnaire, functional disorders of the autonomic nervous system | Total: 62 | Running magnetic field was directed bitemporally by moving the field from the temporal lobes to the occipital area. | The field scanning frequency was 1–12 Hz with a smooth increase within this range from one procedure to the next during the course of treatment. Courses consisted of 10 daily procedures with exposures lasting 10–20 minutes. | No mention of method of assessment of craving. |
| Study group: 32; Control group: 30 | The intervention protocol has not been discussed in detail. | |||||||
| E-mail address of the corresponding author has not been provided in the translated article (author could not be contacted). | ||||||||
| Staroverov et al.38; Russia | Randomized add-on study | Patients with alcohol use disorder | Transcranial dynamic magnetotherapy | Severity of anxiety and depression, memory, attention, function of autonomic nervous system, psychoemotional status | Total: 54 | Directed bitemporally by moving the field from the temporal lobes to the occipital area. | 1–12 Hz with a smooth increase within this range from one procedure to the next during the course of treatment. Courses consisted of 10 daily procedures with exposures lasting 10–20 minutes. | Craving was not an outcome measure. |
| Study group: 29; Control group: 25 | ||||||||
| Rose et al.15; United States | Single-arm repeated-measures design | Volunteer subjects with nicotine use disorder | High- and low-frequency rTMS | Shiffman-Jarvik Questionnaire, Cigarette Evaluation Questionnaire | 15 | Superior frontal gyrus (SFG), Motor cortex (control condition) | Stimulation was carried out for all three conditions (10-Hz SFG, 1-Hz SFG, 1-Hz MOC) for three periods of 2 minutes and 30 seconds in duration (total period of stimulation 7.5 minutes) at an intensity that was 90% of MT. Thus, the number of pulses was equal for the two 1-Hz conditions, but a greater number of pulses was administered during 10-Hz stimulation. | Published data are inadequate to calculate standardized mean difference. Request mail and subsequent reminders were sent to corresponding author for data with no reply. |
| Prikryl et al39; Czech Republic | Double-blind randomized, placebo-controlled study | Male schizophrenia patients with nicotine use disorder | High-frequency rTMS | Number of cigarettes smoked | Total: 35 | Left DLPFC | Intensity of magnetic stimulation in % of motor threshold (110%), stimulation frequency (10 Hz), number of trains, single train duration (10 seconds), intertrain interval (30 seconds), and total number of stimulation sessions. In each stimulation session, 2,000 TMS pulses were given, with a total of 42,000 pulses per treatment course. | Craving was not an outcome measure. |
| Active stimulation: 18; Sham (placebo): 17 | ||||||||
| Vicario et al.40; Italy | Nonrandomized placebo-controlled | Subjects with nicotine use disorder | Low-frequency TMS | Motor-evoked potential amplitudes and resting motor thresholds recorded from of both tongue and extensor carpi radialis | Total: 20 | Corticobulbar area | TMS frequency during the experimental blocks was less than 0.1 Hz, intensity of 120% of resting motor thresholds. The magnetic stimulation was delivered randomly between 1,000 and 1,300 ms from stimulus onset. | Craving was not an outcome measure. |
| Test group: 11 | ||||||||
| Placebo group: 9 | ||||||||
| Mishra et al.29; India | Randomized, double-blind, active comparator study | Right-handed male patients with a diagnosis of alcohol use disorder | High-frequency rTMS | Severity of Alcohol Dependence Questionnaire Form-C | Total: 20 | Right or left DLPFC | At 110% of the MT determined. | No placebo/sham comparator group. |
| and ACQ-NOW | Right DLPFC: 10, left DLPFC: 10 | High-frequency (10 Hz) stimulation was administered for 4.9 seconds per train, with intertrain interval of 30 seconds, and a total of 20 trains per session. Each patient received 1,000 pulses per day. | ||||||
| Terraneo et al.17; Italy | Between-subject open-label randomized, clinical trial | Treatment-seeking patients with cocaine use disorder | High-frequency rTMS | Cocaine use during the last month (frequency and daily amount), cocaine craving score | Total: 32 | Left DLPFC | 15-Hz frequency, pulse intensity 100% of the resting motor threshold , 60 pulses per train, inter train pause of 15 seconds, 40 stimulation trains, and 2,400 total pulses for a total duration of 13 minutes. | Published data are inadequate to calculate standardized mean difference. Request e-mail and subsequent reminders were sent to corresponding author for data with no reply. |
| Test group: 16; control group (standard treatment): 16 |
TABLE 2. Characteristics of Trials Excluded From the Meta-Analysisa
| Included Studies | Risk of Bias | ||||||
|---|---|---|---|---|---|---|---|
| B1a | B2b | B3c | B4d | B5e | B6f | B7g | |
| Amiaz et al.11 | Low risk | Unclear risk | Low risk | Unclear risk | Low risk | Low risk | Low risk |
| Mishra et al.18 | Low risk | Unclear risk | Low risk | Unclear risk | Low risk | Low risk | Low risk |
| Höppneret al.21 | Low risk | Unclear risk | Low risk | Unclear risk | Low risk | Low risk | Low risk |
| Herremans et al.22 | Low risk | Low risk | Low risk | Unclear risk | Low risk | Low risk | Low risk |
| Herremans et al.23 | Low risk | Unclear risk | Low risk | Unclear risk | Low risk | Low risk | Low risk |
| Li et al.12 | Low risk | Low risk | Low risk | Low risk | Unclear risk | Low risk | Low risk |
| Pripfl et al.13 | Low risk | Low risk | Unclear risk | Unclear risk | Low risk | Low risk | Low risk |
| Dinur-Klein et al.33 | Low risk | Unclear risk | Low risk | Unclear risk | Low risk | Low risk | Low risk |
| Girardi et al.19 | Unclear risk | Unclear risk | High risk | Unclear risk | Low risk | Low risk | Low risk |
| Ceccanti et al.20 | Low risk | Unclear risk | Low risk | Unclear risk | Unclear risk | Low risk | Low risk |
TABLE 3. Risk Summary of Bias: Review of Authors’ Judgments About Each Risk of Bias Item for Each Included Study
Effects of Intervention
To evaluate the effect of high-frequency TMS on craving in substance abuse, the effect sizes (Hedge's g) of included studies were entered in Cochrane Program Review Manager, version 5.3, using both fixed and random-effects models. The test for heterogeneity was not significant (Q=15.08, df=10, p=0.13; I2=34%). In the forest plot, the confidence intervals for the results of individual studies (depicted graphically using horizontal lines) were found to have good overlap, indicating the nonsignificant heterogeneity. The random model analysis revealed a pooled standardized effect size (Hedge’s g) of 0.75 (95% CI: 0.29 to 1.21), indicating an overall medium effect size favoring active stimulation over sham stimulation (z=3.18, p=0.001), whereas the fixed model analysis showed a large effect size of 0.87 (95% CI: 0.63 to 1.12) (Figure 2). In subgroup analyses, the test for heterogeneity was not significant. Subgroup analysis (both fixed and random model) for alcohol use disorder showed a standardized effect size (Hedge’s g) of –0.06 (95% CI: –0.89 to 0.77), indicating no favorable effect of active stimulation over sham stimulation (Figure 3). In the case of nicotine use disorder, random model analysis reveals a standardized effect size (Hedge’s g) of 1.00 (95% CI: 0.48 to 1.55), indicating a statistically significant large effect size favoring active stimulation over sham stimulation (z=3.82, p=0.0001), whereas fixed model analysis also showed a large effect size of 0.96 (95% CI: 0.71 to 1.22) favoring active stimulation (Figure 4). Visually, the funnel plot is almost symmetrical, indicating minimal publication bias across the studies (Figure 5). The Egger regression test (t=0.64, p=0.54) and the Begg and Mazumdar rank correlation test (Kendall’s tau=0.09, p=0.69) were also not significant for publication bias.
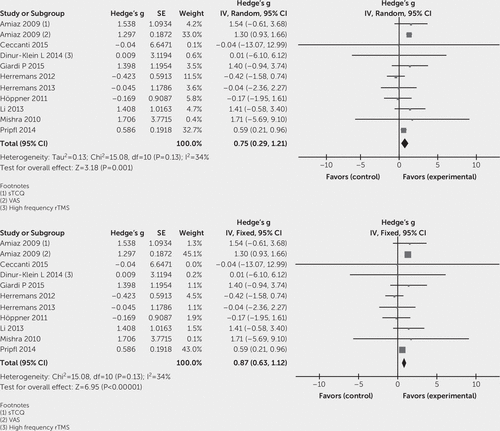
FIGURE 2. Forest Plot for All Included Studies Pooled Together Using Random-Effects and Fixed-Effects Models
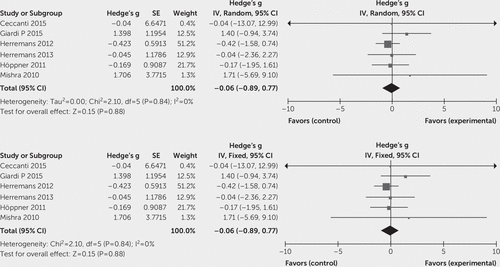
FIGURE 3. Forest Plot for the Subgroup Analysis of Studies on Alcohol Use Disorder Using Random-Effects and Fixed-Effects Models
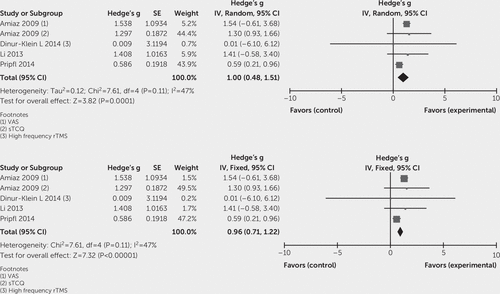
FIGURE 4. Forest Plot for the Subgroup Analysis of Studies on Nicotine Use Disorder Using Random-Effects and Fixed-Effects Models
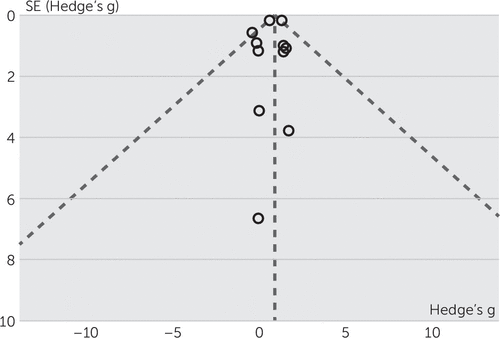
FIGURE 5. Funnel Plot for Publication Bias of the Included Studies
Discussion
The present meta-analysis (both random and fixed model) of 10 studies reveals a significant effect size favoring active rTMS stimulation over sham stimulation in reducing craving in substance dependence. In subgroup analysis, active rTMS stimulation was found to be highly effective in nicotine use disorder but showed no favorable effect in alcohol use disorder. In the presence of heterogeneity, a random-effects meta-analysis weights the studies relatively more equally than a fixed-effect analysis.26 Compared with a fixed-effects model, the random-effects model provides a more conservative estimate of precision and is more appropriate for generalization beyond the included studies.28 But as we were concerned about the influence of small-study effects on the results of our meta-analysis in which there is evidence of between-study heterogeneity (I2>0), we compared the fixed- and random-effects estimates of the intervention effect as recommended by Cochrane Handbook for Systematic Reviews of Interventions.26 The close similarity in our study results suggests that small-study effects have little effect on the intervention effect estimate.
The present meta-analysis was done to evaluate the therapeutic effects of rTMS on craving in substance use disorder. The different study designs, rTMS treatment protocols, site of application, outcome measures, and, finally, period of observation were studied during inclusion in the meta-analysis. It is important to note that nine studies were excluded from analysis. In two studies, there was no sham/placebo control group, but in both these studies a significant decrease in craving levels was observed and therefore important for corroboration of the results of this meta-analysis.16,29 Three studies, for which the authors did not respond to our requests to provide details about their data, were excluded, but these three studies reported a decrease in craving levels compared with sham stimulation.14,17,30 Although these studies were not included for analysis, they are considered as supportive studies of our conclusions.
The previous meta-analysis by Jansen et al.31 focusing on craving involved studies on substance dependence as well as food craving and included the intervention tool rTMS, along with other noninvasive neurostimulation techniques such as transcranial direct current stimulation. For such wide inclusion criteria, the results of the previous meta-analysis could not conclude the effect of rTMS on substance-related craving independently. Hence, we have narrowed down inclusion criteria to include only the studies involving substance use disorder as the study population, rTMS as the intervention tool, and substance-related craving as the outcome measure.
In relation to nicotine use disorder, high-frequency rTMS, either single- or repeated-session, application over the DLPFC has been consistently found to reduce craving and cigarette consumption.11 Chronic nicotine use results in activation of dopaminergic reward pathway, whereas withdrawal is associated with attenuated dopaminergic activity and craving. Application of rTMS to the PFC can induce transient increase in dopamine release in the mesolimbic reward pathway, which “mimics’’ the nicotine intake effect, and thus produces the desired reduction in craving.32,33 High-frequency rTMS can also influence the altered GABA activity in the PFC, which is implicated in cognitive control with addiction.34 It has been speculated that rTMS of the DLPFC might have a beneficial effect on addiction-related altered behavioral functions such as motivation, attentional bias, inhibitory control, and decision making, which might additionally contribute to reduced craving.35
The rTMS studies related to craving in alcohol use disorder again have varied methodology in relation to the protocol for rTMS application and the site of stimulation. Eichhammer et al.14 used a frequency of 20 Hz, but they applied only two sessions that were subthreshold and did not observe any effects on craving. In the study by Herremans et al.22 on recently detoxified alcohol-dependent patients, one single-blind sham-controlled high-frequency rTMS session applied to the right DLPFC did not produce any changes in craving measurement parameter, neither immediately after the stimulation session nor in patients’ natural environment in the next 2 days. In another study by Herremans et al., the primary outcome was to observe for changes in executive function in detoxified alcohol-dependent patients following a single right DLPFC high-frequency rTMS session. In the above mentioned studies, it was consistently concluded that only one rTMS session could be too short to alter alcohol craving in alcohol-dependent patients.23 In the study by Höppner,21 10 sessions of 20-Hz active versus sham rTMS application to the left DLPFC did not reveal any significant differences in relation to changes in craving and mood measurement parameters. The various studies have applied 10 Hz or 20 Hz, both in high-frequency range, but they could have variable modulatory effects on cortical excitability. Another major variation is in relation to the site of stimulation, which can result in different lateralization effect in context to craving substrate. Most high-frequency rTMS studies have involved the right DLPFC stimulation, when researchers have suggested that the dopaminergic substrate implicated in craving are more affected by left DLPFC rTMS than right DLPFC stimulation.10,36,37 Hence, in context to the heterogeneity in rTMS protocols and limited number of treatment sessions, it will be difficult to establish a conclusion regarding the anticraving efficacy of rTMS in alcoholism, with a need for intensive clinical trials in this area.
The present meta-analysis reveals the favorable anticraving efficacy of high-frequency rTMS in nicotine use disorder, but it was found to be not effective in alcohol use disorder. We recommend the need to adopt a uniform treatment protocol and optimize the number of rTMS sessions in relation to treatment of patients with nicotine use disorder. On the other hand, there is a need for further clinical trials with robust rTMS protocols and greater number of treatment sessions to make a final conclusion on the anticraving effects of rTMS in alcohol use disorder.
1 : http://www.who.int/gho/substance_abuse/en/Google Scholar
2 : http://www.who.int/substance_abuse/publications/Media/en/Google Scholar
3 : Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet 2009; 373:2223–2233Crossref, Medline, Google Scholar
4 : http://www.who.int/classifications/icd/en/GRNBOOK.pdfGoogle Scholar
5 : Theoretical frameworks and mechanistic aspects of alcohol addiction: alcohol addiction as a reward deficit disorder. Curr Top Behav Neurosci 2013; 13:3–30Crossref, Medline, Google Scholar
6 : Brain substrates of craving to alcohol cues in subjects with alcohol use disorder. Alcohol Alcohol 2007; 42:417–422Crossref, Medline, Google Scholar
7 : Mechanisms and state of the art of transcranial magnetic stimulation. J ECT 2002; 18:170–181Crossref, Medline, Google Scholar
8 : Transcranial magnetic stimulation as a therapeutic tool in psychiatry: what do we know about the neurobiological mechanisms? J Psychiatr Res 2001; 35:193–215Crossref, Medline, Google Scholar
9 : Transcranial magnetic stimulation: applications in neuropsychiatry. Arch Gen Psychiatry 1999; 56:300–311Crossref, Medline, Google Scholar
10 : Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J Neurosci 2001; 21:RC157Crossref, Medline, Google Scholar
11 : Repeated high-frequency transcranial magnetic stimulation over the dorsolateral prefrontal cortex reduces cigarette craving and consumption. Addiction 2009; 104:653–660Crossref, Medline, Google Scholar
12 : Repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex reduces nicotine cue craving. Biol Psychiatry 2013; 73:714–720Crossref, Medline, Google Scholar
13 : Transcranial magnetic stimulation of the left dorsolateral prefrontal cortex decreases cue-induced nicotine craving and EEG delta power. Brain Stimulat 2014; 7:226–233Crossref, Medline, Google Scholar
14 : High-frequency repetitive transcranial magnetic stimulation decreases cigarette smoking. J Clin Psychiatry 2003; 64:951–953Crossref, Medline, Google Scholar
15 : Repetitive transcranial magnetic stimulation of the superior frontal gyrus modulates craving for cigarettes. Biol Psychiatry 2011; 70:794–799Crossref, Medline, Google Scholar
16 : One session of high frequency repetitive transcranial magnetic stimulation (rTMS) to the right prefrontal cortex transiently reduces cocaine craving. Drug Alcohol Depend 2007; 86:91–94Crossref, Medline, Google Scholar
17 : Transcranial magnetic stimulation of dorsolateral prefrontal cortex reducescocaine use: A pilot study. Eur Neuropsychopharmacol 2016; 26:37–44.Crossref, Medline, Google Scholar
18 : Efficacy of repetitive transcranial magnetic stimulation in alcohol dependence: a sham-controlled study. Addiction 2010; 105:49–55Crossref, Medline, Google Scholar
19 : Add-on deep transcranial magnetic stimulation (dTMS) in patients with dysthymic disorder comorbid with alcohol use disorder: a comparison with standard treatment. World J Biol Psychiatry 2015; 16:66–73Crossref, Medline, Google Scholar
20 : Deep TMS on alcoholics: effects on cortisolemia and dopamine pathway modulation. A pilot study. Can J Physiol Pharmacol 2015; 93:283–290Crossref, Medline, Google Scholar
21 : Repetitive transcranial magnetic stimulation (rTMS) for treatment of alcohol dependence. World J Biol Psychiatry 2011; 12(Suppl 1):57–62Crossref, Medline, Google Scholar
22 : No influence of one right-sided prefrontal HF-rTMS session on alcohol craving in recently detoxified alcohol-dependent patients: results of a naturalistic study. Drug Alcohol Depend 2012; 120:209–213Crossref, Medline, Google Scholar
23 : Reduced intra-individual reaction time variability during a Go-NoGo task in detoxified alcohol-dependent patients after one right-sided dorsolateral prefrontal HF-rTMS session. Alcohol Alcohol 2013; 48:552–557Crossref, Medline, Google Scholar
24 : Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015; 4:1Crossref, Medline, Google Scholar
25 : Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6:e1000097Crossref, Medline, Google Scholar
26 (ed): http://handbook.cochrane.org/Google Scholar
27 Manager R: (RevMan) [Computer program]. Version 5.3. Copenhagen, The Nordic Cochrane Centre, The Cochrane Collaboration, 2014Google Scholar
28 : Introduction to Meta-Analysis. Chichester, West Sussex, John Wiley and Sons, Ltd., 2009Crossref, Google Scholar
29 : Comparison of anticraving efficacy of right and left repetitive transcranial magnetic stimulation in alcohol dependence: a randomized double-blind study. J Neuropsychiatry Clin Neurosci 2015; 27:e54–e59Link, Google Scholar
30 : Transcranial magnetotherapy in the complex treatment of affective disorders in patients with alcoholism. Neurosci Behav Physiol 2009; 39:507–511Crossref, Medline, Google Scholar
31 : Effects of non-invasive neurostimulation on craving: a meta-analysis. Neurosci Biobehav Rev 2013; 37:2472–2480Crossref, Medline, Google Scholar
32 : Nicotine dependence: studies with a laboratory model. Pharmacol Biochem Behav 2001; 70:551–559Crossref, Medline, Google Scholar
33 : Smoking cessation induced by deep repetitive transcranial magnetic stimulation of the prefrontal and insular cortices: a prospective, randomized controlled trial. Biol Psychiatry 2014; 76:742–749Crossref, Medline, Google Scholar
34 : Frontal lobe γ-aminobutyric acid levels during adolescence: associations with impulsivity and response inhibition. Biol Psychiatry 2013; 74:296–304Crossref, Medline, Google Scholar
35 : Neuromodulation of decision-making in the addictive brain. Subst Use Misuse 2010; 45:1766–1786Crossref, Medline, Google Scholar
36 : rTMS of the left dorsolateral prefrontal cortex modulates dopamine release in the ipsilateral anterior cingulate cortex and orbitofrontal cortex. PLoS One 2009; 4:e6725Crossref, Medline, Google Scholar
37 : Repetitive transcranial magnetic stimulation of dorsolateral prefrontal cortex affects performance of the wisconsin card sorting task during provision of feedback. Int J Biomed Imaging 2008; 2008:143238Crossref, Medline, Google Scholar
38 : Efficacy of transcranial magnetotherapy in the complex treatment of alcohol withdrawal syndrome. Neurosci Behav Physiol 2009; 39:891–895Crossref, Medline, Google Scholar
39 : Repetitive transcranial magnetic stimulation reduces cigarette consumption in schizophrenia patients. Prog Neuropsychopharmacol Biol Psychiatry 2014; 49:30–35Crossref, Medline, Google Scholar
40 : Enhanced corticobulbar excitability in chronic smokers during visual exposure to cigarette smoking cues. J Psychiatry Neurosci 2014; 39:232–238Crossref, Medline, Google Scholar



