Levetiracetam Effect on Adult-Onset Temporal Lobe Epilepsy With Positive Voltage-Gated Potassium Channel Antibody
Abstract
Temporal lobe epilepsy is considered to be the most frequent of all epileptic syndromes. Recently, several retrospective studies suggest that limbic encephalitis (LE) may be a cause for adult onset unexplained seizure disorders in patients. This report describes two cases of adult onset epilepsy with voltage-gated potassium channel antibodies (VGKC-abs)-associated LE that responded well to levetiracetam (LEV). As demonstrated by these two cases and reviewing previous reports, we propose that the therapeutic regimen for VGKC-abs associated seizures still needs to be determined and LEV may be effective in treating this kind of disorders.
Temporal lobe epilepsy (TLE) is considered to be the most frequent of all epileptic syndromes. Previous researches suggested that the majority of TLEs start before the age of 20.1 Several recently retrospective studies suggest that limbic encephalitis (LE) may be a cause for the development of adult onset unexplained seizure disorders in patients.2
LE was initially identified as a paraneoplastic neurologic syndrome usually associating with intracellular onconeural antibodies, such as Hu antibodies, CV2/CRMP5 antibodies, Ma2 antibodies, and amphiphysin antibodies.3 Lately, some types of antibodies against cell membrane antigen, such as voltage-gated potassium channels antibodies (VGKC-abs), N-methyl-D-aspartate receptor (NMDAR) antibodies, and other antibodies, were found in patients who usually do not have cancer.4 Previous studies mainly focused on the features and treatments of LE. Little was known about what kind of antiepileptic drugs (AEDs) was effective in treating this kind of seizure disorders. Here, we describe two cases of adult onset TLE with VGKC-abs-associated LE in China. This is the first reported Chinese case; we propose that levetiracetam (LEV) may be a useful treatment for this kind of TLE.
Case Reports
Case 1
A 57-year-old right-handed woman was admitted to our hospital with a 9-day history of short-term memory loss and psychiatric symptoms without fever. She suffered from recurrent episodes of fear without hallucination when few people around. Moreover, she had two episodes of generalized tonic-clonic seizure (GTCS) 9 days before admission. After admission, the patient appeared to have complex partial seizure (CPS), seven to eight times per day.
Physical examination showed short-term memory impairment and time disorientation. Laboratory tests demonstrated a persistent hyponatremia (117−131mmol/L) and hypochloremia (85.2–94.7mmol/L). Other routine blood tests were normal, such as chest X-ray, CSF examination, and bone marrow puncture. Electroencephalograph (EEG) showed generalized slow activity without epileptic discharges. The Mini-Mental State Examination (MMSE) score was 9/30 (illiterate). Brain MRI demonstrated bilateral increased signal in the medial part of the temporal lobe on T2-weighted and fluid-attenuation inversion recovery (FLAIR) images (Figure 1 a‒c). Positron emission tomography/computed tomography (PET/CT) showed hypermetabolism in bilateral hippocampus (Figure 1 d and e).
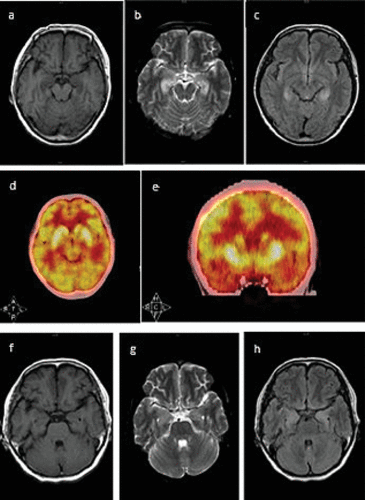
FIGURE 1. Brain MRI and Positron Emission Tomography/Computed Tomography (PET/CT) Images of the Patienta
a [a-c] Brain MRI demonstrated bilateral hyperintense in the medial temporal lobe on T2-weighted and fluid-attenuation inversion recovery (FLAIR) images. [a] T1WI; [b] T2WI; [c] FLAIR image. [d-e]: PET/CT showed hypermetabolism in bilateral hippocampus. [f-h] The brain MRI signal of the medial temporal lobe on T2-weighted and FLAIR images had reduced within 2 months. [f] T1WI; [g] T2WI; [h] FLAIR image.
Suspecting virus encephalitis at first, IV acyclovir (10 mg/kg for 14 days) was started, and seizures were treated with LEV (500 mg bid). Four days later, the frequency of seizure declined obviously to three to four times per day. Two weeks later, the seizure was totally under control, but the psychiatric symptoms did not improve. Fifteen days after admission, we started to give this patient IV methylprednisolone (500 mg/day for 5 days), and her symptom improved dramatically. Then, the patient continued to use AEDs (LEV 500 mg bid) after discharge.
Two months later, the patient returned to our hospital for a follow-up visit. Her serum sodium level had returned to normal (139 mmol/L), and she did not have any psychiatric symptoms or episodes of seizure. MMSE score rose to 13/30 (illiterate). Furthermore, the signal of the medial temporal lobe on T2-weighted and FLAIR images had also reduced within two months (Figure 1 f‒h). Antineuronal antibodies (Hu, Yo, Ri, Ma1/2, CV2/CRMP-5, and amphiphysin) were negative. Serum VGKC-abs level and CSF VGKC-abs level before the prescription of methylprednisolone were 130.54 pmol /L and 161.14 pmol /L, respectively (cutoff value: 100 pmol/L). Antibody titers were measured with ELISA (1:5 dilution, Kingmed Diagnostics, Chengdu, China). We did not test the antibodies 2 months later because the patient refused.
Case 2
A previously healthy 45-year-old woman presented with three episodes of generalized seizure evolving to status epilepticus 3 months prior to admission, each seizure lasting more than 10 minutes. She gradually developed disorientation, and psychiatric symptoms manifested as personality change, unwillingness to communicate with others, anxiety, panic, visual and auditory hallucination, persecutory delusion, and aggressive behavior.
This patient had elevated TPOAb (179.20 IU/L) and TgAb (157.40 IU/L), but other hormones, such as ACTH, LH, GH, FSH, PTC were normal. She also had hyponatremia (130–133 mmol/L) and hypochloremia (95–96 mmol/L). Other laboratory and radiological examinations did not reveal any signs of infection or tumor. Her serum VGKC-abs level and CSF VGKC-abs level before the prescription of methylprednisolone were 112.35 pmol/L and 194.63 pmol/L, respectively. After that, we give the patient IV methylprednisolone (500 mg/day) and oral LEV (500 mg bid) simultaneously. However, 5 days later, the patient was automatically discharged even though her symptoms did not fully resolve. At discharge, IV methylprednisolone was stopped, and LEV was continued to be used in the same dose. When the patient returned to our hospital for a follow-up visit 2 months later, she did not have any episode of seizure and her psychiatric symptoms also improved.
Discussion
Combining the patients’ history, laboratory tests, imaging examinations, and response to treatment, we considered these two patients to be nonparaneoplastic VGKC-abs- associated LE. This is in accordance with newly proposed diagnostic criteria for LE.5,6
VGKC-abs-associated LE constitutes approximately 30% of all autoimmune encephalitis with antibodies to cell membrane antigens.7 VGKCs are widely expressed throughout the entire nervous system and are critical in establishing the resting membrane potential and generation of neuronal action potentials, some subtypes, such as VGKC1.1, VGKC1.2, VGKC1.6, were strongly expressed in the hippocampus molecular layer.8 Recent studies suggest that these antibodies seem to target associated VGKC-complex proteins instead of Kv1 subunits. These targeted proteins include leucine-rich, glioma inactivated 1 (Lgi1), and contactin-associated protein-2 (Caspr2). In patients with positive Lgi1-Abs, the probability of occurrence of seizure is more common than the patients with positive Caspr2-Abs.9 Because the VGKC-abs were detected using ELISA, we did not further detect the Lgi1-Abs/ Caspr2-Abs.
These two cases prompted that VGKC-abs-associated LE may be an important cause for adult onset TLE. The clinical features of epilepsy in previously reported VGKC-abs-associated LE were summarized in Table 1. Case series and case reports from January 2001 to December 2013, which refer to adult onset (>18 years old) VGKC-abs-associated LE, were included in Table 1. Reviews, letters, case series, and case reports concerning other antibodies and children cases were excluded, as well as articles, which only discussed one part of LE (e.g., only MRI findings) or the clinical data are incomplete or unclear. VGKC-abs-associated LE is more common in men above 40 years old. Vincent et al. reviewed 10 patients with positive VGKC-abs associated LE, and seizures were present in nine patients during the acute phase of the disease, including GTCS and/or CPS. Additional features include hallucination, agitation, and behavioral disturbance. Hyponatremia is a common symptom.10 Antibodies to VGKC may also associate with paraneoplastic LE because of thymoma or small cell lung cancer, but more frequently with nonparaneoplastic LE.10
| Reference | Study Location | Study Design | Age (Years) | M:F | VGKC-abs Positive Patients/Patient Number | Epilepsy as Onset Symptom | Antiepileptic Drugs Prescribed | EEG | MRI Findings | Symptoms Recovery Period | MRI recovery period |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Buckley et al.28 Ann Neurol 2001 | UK | Case report | 47–66 | 0:2 | 2/2 | 1/2 | one with VPA; the other with multiple AEDs | Normal | Left hippocampus abnormality | Within 6 months | signal reduced in 2 years |
| Pozo-Rosich et al.29 Ann Neurol. 2003 | UK | Case series | 47–67 | 4:0 | 4/15 | 4/15 | — | — | Bilateral hyperintense in the hippocampus or other areas of the limbic system | — | 3 months |
| Vincent et al.10 Brain. 2004 | UK | Case series | 57(44–79) | 9:1 | 10/10 | 9/10 | PHT,VPA,PB, CLB, LRZ, et al. | Generalized slowing, with focal sharp waves in some cases | 5/10 bilateral medial temporal lobe high signal, 3/10 left-sided hippocampal high signal, 2/10 normal | Did not show, but seizure reduced with the decline of antibodies titers. | < 6 months |
| Thieben et al.24 Neurology. 2004 | USA | Case series | 44–69 | 5:2 | 7/7 | 6/7 | Multiple AEDs (did not show) | All abnormal, 4 had temporal lobe epileptic form activity | Bilateral or unilateral mesial temporal lobe high signal | Within 24 months | one patient fully resolved in 4 month |
| Bien et al.12 Neurology. 2007 | Germany | Case series | 44(24–63) | 16:4 | 4/7 | 9/9 | — | — | 5/9 bilateral | — | — |
| Zuliani et al.30 J Neurol Neurosurg Psychiatry. 2007 | Spain | Case report | middle age | 1:1 | 2/9 with paraneoplastic LE | 2/2 | One used VPA | slowing in the left temporal lobe or generalized slowing and epileptic activity in both temporal lobes | One unilateral | — | — |
| Graus et al.4 Neurology. 2008 | Spain | Case series | 24–44 | 3:1 | 4/7 | 4/7 | — | — | 2/4 bilateral, 2/4 unilateral | — | — |
| Malter et al.11 Ann Neurol. 2010 | Germany | Case control | 55 (44–73) | 6:4 | 10/53 | 6/10 | — | — | 7/10 bilateral mediotemporal encephalitis | 9 months, all seizure free | mean 17 months |
| Irani et al.9 Brain. 2010 | UK | Case control | 63(19–83) | 44:20 | 64/64 | 59/64 | — | — | 40/64 had MRI medial temporal lobe high signal | — | — |
| Lai et al.31 Lancet Neurol. 2010 | USA | Case control | 60(30–80) | 37:20 | 57/57 | 42/57 | — | 26/57 had abnormality of any kind | 43/57 had medial temporal lobe high signal | 12/57 fully recovered with a follow-up of 18 months | — |
| Barajas et al.8 Epilepsia. 2010 | USA | Case report | 64 | 1:0 | 1/1 | 1/1 | LEV, LTG | multiple seizures arising from the left anterior temporal lobe | Left hippocampus and bilateral frontal lobes hyperintense | Within 24 months | 14 months |
| Irani et al.20 Ann Neurol. 2011 | UK | Case series | 64(36–83) | 19:10 | 29/29 | 20/29 | A mean 2.6 AEDs | diffuse slowing (N=9), bilateral frontotemporal slowing (N=6), temporal sharp waves (N=2), normal (N=9) | 10/29 bilateral, 3/29 unilateral medial temporal lobe high signal, 12/29 normal | 14 showed a>50% reduction in seizure frequency over the first month of immunotherapy | — |
| Balint et al.32 J Neurol Sci. 2013 | Germany | Case report | 49 | 0:1 | 1/1 | 1/1 | — | generalized slow activity | Unspecific leptomeningeal signal changes | — | |
| Frisch et al.15 Eur J Neurol. 2013. | Germany | Case control | 57(38–73) | 8:7 | 15/31 | not certain | Average one kind of AEDs | — | 9/15 bilateral, 4/15 left-sided, 1/15 right-sided encephalitic mesiotemporal MRI features | 11/15 reached seizure-free with a follow-up of 25 months | no MRI improvement |
| Irani et al.33 Brain. 2013 | UK | Case series | 68(28–92) | 5:5 | 10/10 | 10/10 | 1–3 AEDs | 3/10 showed diffuse slowing, 6/10 was normal | 2/10 showed abnormalities involving medial temporal lobes, 7/10 had normal MRI | Seizure frequency reduced within 7 –144 days | — |
| Shin et al.23 J Neuroimmunol. 2013 | South Korea | Case series | 60.5(41–78) | 8:6 | 14/14 | 14/14 | — | 8 had epileptiform discharges, 2 had focal rhythmic slowing wave | 9/14 had medial temporal lesions, 5 of them had bilateral lesions | Positive response within a follow-up of 1–24 months, 2/12 relapse | — |
| Saraya et al.34 BMC Neurol. 2013 | Thailand | Case series | 67 | 1:0 | 1/103 | 1/1 | — | — | Meninges and cortex damage | — | — |
TABLE 1. Summary of Clinical Features of Epilepsy in Previously Reported Voltage-Gated Potassium Channel Antibodies (VGKC-abs) Associated Limbic Encephalitisa
The most common abnormalities on brain MRI is increased signal in medial temporal lobe on T2-weighted and FLAIR images, either bilateral or unilateral, but it can also be normal.11, 12 In a systematic review, neuroimaging investigations revealed baseline MRI changes in 70% cases.13 A general tendency of mediotemporal signal regression is within 4–17 months (Table 1), but some patients develop into hippocampal atrophy and hippocampal sclerosis.4,12
The relevance of clinical manifestation and antibodies titers has been confirmed in many researches.14–16 With the antibodies titers declined, the clinical symptom can improve as well as the lesion on brain MRI. The antibodies titers of these two cases were relatively low, may be for the reason that the symptoms of these two cases were relatively mild and the samples were obtained at the early onset of the disease. Furthermore, the levels of VGKC antibodies in the CSF were higher in the CSF compared with serum in both cases, indicating intrathecal synthesis of antibodies, as previous reported in anti-NMDA-receptor encephalitis.17
Recently, there have been frequent descriptions of LE and refractory epilepsy (RE) secondary to antibodies to a broad variety of neuronal antigens.18,19 VGKC-complex antibodies and similar antibodies have been identified in around 10% of unselected patients with unexplained and drug-resistant epilepsy.20 For patient with RE or super RE, it is crucial to establish the underlying cause of seizures.21 Both paraneoplastic and nonparaneoplastic LE may result in RE. Recent researches suggested autoimmune encephalitis may be an essential cause of nonconvulsive status epilepticus, especially anti-NMDAR encephalitis.19 Compared with widely expressed NMDA receptors,17 antibody associated with LE that strongly affects temporal lobe is the VGKC complex antibody.22 Although the incidence of RE associated with anti-VGKC encephalitis remains to be determined, the typical medial temporal regions that anti-VGKC encephalitis affects may be a potential reason for RE.23
The main therapy for LE is immunotherapy. Corticosteroids, intravenous immune globulin (IVIg) and plasma exchange are most frequently used. Other immunosuppressive agents, such as cyclophosphamide and rituximab, can also be utilized.7,11 Although immunotherapy is widely accepted for the treatment of LE, there are many side-effects and other risks.20 Additionally, some patients respond poorly to immunotherapy and fully control of epilepsy needs a long time after immunotherapy and sometimes needs to be treated with AEDs simultaneously.17,18
Previous studies rarely focused on the treatment of seizure. Seizure in this disorder is generally difficult to control and often require treatment with multiple AEDs.24 Only few studies mentioned the AEDs they prescribed. Phenytoin (PHT), phenobarbital (PB), valproic acid (VPA), clobazam (CLB), lorazepam (LRZ), lamotrigine (LTG), carbamazepine (CBZ), topiramate (TPM) and LEV were prescribed in previous reports of patients with positive VGKC-abs (Table 1). But the relationship between the control of seizures and the prescription of AEDs didn’t point out explicitly. Thus, it is unclear which kind of AEDs is more effective for epilepsy induced by VGKC-abs associated LE. In our two cases, after using LEV, the seizure was under control, even before the administration of immunotherapy (Case # 1). In previous case reports, there are a few cases which responded well to AEDs alone.20,25 Barajas et al. also reported a case who was treated with LEV and LTG and the patient reached seizure free within 24 months.8 Until now, LEV has been proved to be safe and effective in treating acute seizure and status epilepticus.26 Swisher et al. suggested that the combination use of PHT, LEV, and pregabalin (PGB) in brain tumor patients with refractory status epilepticus is safe and highly effective.27 As demonstrated by these cases, we proposed that LEV may be a useful AEDs for seizure induced by LE. And this application did not have an obvious psychiatric adverse side effect.
Conclusions
In conclusion, the therapeutic regimen for VGKC-abs-associated seizure still needs to be determined. At what point to start immunotherapy, whether there is a need to administer corticosteroid along with AEDs, and what kind of AEDs should be used are still questions. These two cases indicate that LEV may be effective in treating epilepsy induced by LE. More research should be conducted to confirm this and to provide more evidence for the treatment of epilepsy induced by LE.
1 : Pathology of temporal lobe foci. Adv Neurol 1975; 11:163–185Medline, Google Scholar
2 : Serum antibodies in epilepsy and seizure-associated disorders. Neurology 2005; 65:1730–1736Crossref, Medline, Google Scholar
3 : Antibodies and neuronal autoimmune disorders of the CNS. J Neurol 2010; 257:509–517Crossref, Medline, Google Scholar
4 : Neuronal surface antigen antibodies in limbic encephalitis: clinical-immunologic associations. Neurology 2008; 71:930–936Crossref, Medline, Google Scholar
5 : Limbic encephalitis: a cause of temporal lobe epilepsy with onset in adult life. Epilepsy Behav 2007; 10:529–538Crossref, Medline, Google Scholar
6 : Central nervous system neuronal surface antibody associated syndromes: review and guidelines for recognition. J Neurol Neurosurg Psychiatry 2012; 83:638–645Crossref, Medline, Google Scholar
7 : Limbic encephalitis and variants: classification, diagnosis and treatment. Neurologist 2007; 13:261–271Crossref, Medline, Google Scholar
8 : Adult-onset drug-refractory seizure disorder associated with anti-voltage-gated potassium-channel antibody. Epilepsia 2010; 51:473–477Crossref, Medline, Google Scholar
9 : Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan’s syndrome and acquired neuromyotonia. Brain 2010; 133:2734–2748Crossref, Medline, Google Scholar
10 : Potassium channel antibody-associated encephalopathy: a potentially immunotherapy-responsive form of limbic encephalitis. Brain 2004; 127:701–712Crossref, Medline, Google Scholar
11 : Antibodies to glutamic acid decarboxylase define a form of limbic encephalitis. Ann Neurol 2010; 67:470–478Crossref, Medline, Google Scholar
12 : Limbic encephalitis as a precipitating event in adult-onset temporal lobe epilepsy. Neurology 2007; 69:1236–1244Crossref, Medline, Google Scholar
13 : Treatment of VGKC complex antibody-associated limbic encephalitis: a systematic review. J Neuropsychiatry Clin Neurosci 2013; 25:264–271Link, Google Scholar
14 : Clinical relevance of positive voltage-gated potassium channel (VGKC)-complex antibodies: experience from a tertiary referral centre. J Neurol Neurosurg Psychiatry 2014; 85:625–630Crossref, Medline, Google Scholar
15 : Neuropsychological course of voltage-gated potassium channel and glutamic acid decarboxylase antibody related limbic encephalitis. Eur J Neurol 2013; 20:1297–1304Crossref, Medline, Google Scholar
16 : Voltage-gated potassium channel antibodies associated limbic encephalitis in a patient with invasive thymoma. J Neurol Sci 2006; 250:167–169Crossref, Medline, Google Scholar
17 : Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol 2008; 7:1091–1098Crossref, Medline, Google Scholar
18 : Refractory status epilepticus associated with anti-SSA (anti-Ro) antibodies. Can J Neurol Sci 2012; 39:660–663Crossref, Medline, Google Scholar
19 : Anti-NMDA receptor encephalitis causing prolonged nonconvulsive status epilepticus. Neurology 2010; 75:1480–1482Crossref, Medline, Google Scholar
20 : Faciobrachial dystonic seizures precede Lgi1 antibody limbic encephalitis. Ann Neurol 2011; 69:892–900Crossref, Medline, Google Scholar
21 : Refractory status epilepticus. Curr Opin Crit Care 2012; 18:127–131Crossref, Medline, Google Scholar
22 : Immunological perspectives of temporal lobe seizures. J Neuroimmunol 2013; 263:1–7Crossref, Medline, Google Scholar
23 : VGKC-complex/LGI1-antibody encephalitis: clinical manifestations and response to immunotherapy. J Neuroimmunol 2013; 265:75–81Crossref, Medline, Google Scholar
24 : Potentially reversible autoimmune limbic encephalitis with neuronal potassium channel antibody. Neurology 2004; 62:1177–1182Crossref, Medline, Google Scholar
25 : Tonic seizures: a diagnostic clue of anti-LGI1 encephalitis? Neurology 2011; 76:1355–1357Crossref, Medline, Google Scholar
26 : Levetiracetam use in the critical care setting. Front Neurol 2013; 4:121Crossref, Medline, Google Scholar
27 : Phenytoin, levetiracetam, and pregabalin in the acute management of refractory status epilepticus in patients with brain tumors. Neurocrit Care 2012; 16:109–113Crossref, Medline, Google Scholar
28 : Potassium channel antibodies in two patients with reversible limbic encephalitis. Ann Neurol 2001; 50:73–78Crossref, Medline, Google Scholar
29 : Voltage-gated potassium channel antibodies in limbic encephalitis. Ann Neurol 2003; 54:530–533Crossref, Medline, Google Scholar
30 : Paraneoplastic limbic encephalitis associated with potassium channel antibodies: value of anti-glial nuclear antibodies in identifying the tumour. J Neurol Neurosurg Psychiatry 2007; 78:204–205Crossref, Medline, Google Scholar
31 : Investigation of LGI1 as the antigen in limbic encephalitis previously attributed to potassium channels: a case series. Lancet Neurol 2010; 9:776–785Crossref, Medline, Google Scholar
32 : Caspr2 antibodies in limbic encephalitis with cerebellar ataxia, dyskinesias and myoclonus. J Neurol Sci 2013; 327:73–74Crossref, Medline, Google Scholar
33 : Faciobrachial dystonic seizures: the influence of immunotherapy on seizure control and prevention of cognitive impairment in a broadening phenotype. Brain 2013; 136:3151–3162Crossref, Medline, Google Scholar
34 : Autoimmune causes of encephalitis syndrome in Thailand: prospective study of 103 patients. BMC Neurol 2013; 13:150Crossref, Medline, Google Scholar



