Higher-Dose Uses of Zolpidem Will Increase the Subsequent Risk of Developing Benign Brain Tumors
Abstract
This study identified 37,810 patients with anxiety or sleep disorder (mean age=53.2 years, SD=16.0 years) who had zolpidem prescribed for at least 2 months from January 1, 2000 through December 31, 2009. Another non-zolpidem cohort was selected by 1:1 matching with the zolpidem cohort on the estimated probability (propensity score) of being treated. The zolpidem cohort had a higher incidence of benign brain tumors compared with the non-zolpidem cohort, particularly for elderly patients. The matched propensity score analysis showed that the highest risk of benign brain tumors occurred in participants with zolpidem exposure ≥520 mg/year (hazard ratio=1.85, 95% confidence interval=1.21–2.82) compared with those not taking zolpidem.
Sleep problems are common, and hypnotic drugs are widely used as treatment in adult medicine.1 Previous studies have revealed that approximately 25% of people are dissatisfied with their sleep quality, and approximately 10% meet the criteria for insomnia syndrome.2,3 Different hypnotic drugs are currently used for initiating sleep, maintaining sleep, avoiding early rising, and improving the efficiency or quality of sleep.4
Zolpidem (e.g., Ambien or Stilnox) is a nonbenzodiazepine hypnotic drug, which can be prescribed for the short-term treatment of insomnia. A single blind trial published in 1991 reported that 10 mg of zolpidem per night is effective and safe for treating sleep disturbances of various origins.5 However, zolpidem users were recently reported to experience a four times or higher risk of death compared with nonusers in the United States.6 We published a population-based study in 2012 and revealed that the use of zolpidem might be associated with an increased risk of subsequent cancer.7 On the basis of these data, we inferred that long-term use of zolpidem might modulate the central nervous system, subsequently resulting in brain tumors. This study used a nationwide population-based database in Taiwan to explore this issue.
Methods
Data Sources
This cohort study used data obtained from the National Health Insurance Research Database (NHIRD). For research purposes, the NHIRD annually releases all medical information from the Taiwan National Health Insurance program to the public, including complete outpatient visits, hospital admissions, prescriptions, disease status, and demographic data for more than 99% of the residents in Taiwan.8 The diagnostic codes provided in the database are in accordance with the ICD-9-CM.
This study obtained a data subset from the NHIRD composed of 1,000,000 randomly sampled beneficiaries who were enrolled in the Taiwan National Health Insurance program from 1996 to 2000, and the medical records collected in the NHIRD from 1996 to 2010 were used for data analysis. The details of the NHIRD have been described, and several Taiwan studies had demonstrated the high accuracy and validity of ICD-9 codes diagnosis in NHIRD.9,10 We confirm that all data were de-identified and analyzed anonymously. In addition, this study was also approved by the Ethics Review Board at China Medical University (CMU-REC-101–012).
Study Participants
By using a prescription variable restriction on the outpatient and inpatient records obtained from the NHIRD, we identified patients with the diagnosis of sleep disorder (ICD-9-CM 370.4 and 780.5, except for sleep apnea syndrome: ICD-9-CM 780.51, 780.53, and 780.57), or anxiety (ICD-9-CM 300.0, 300.2, 300.3, 308.3, and 309.81). Patients who were treated with zolpidem for at least 2 months between January 1, 2000 and December 31, 2009 were the zolpidem cohort. The first treatment date was defined as the index date. Patients were excluded if they had a brain tumor history prior to the index date. Overall, 37,810 patients with a history of zolpidem treatment were included in the case cohort. Using the same exclusion criteria, we randomly selected the nontreated patients from the rest of sleep disorder and/or anxiety patients by 1:1 matching with the treated patient on a propensity score. The propensity score was calculated by a logistic regression to estimate the probability of the treatment assignment given the baseline variables including age, sex, stroke (ICD-9-CM 430–438), dementia (ICD-9-CM 290.0–290.4 and 331.0), epilepsy (ICD-9-CM 345), head injury (ICD-9-CM 850–854 and 959.01), and brain CT or MRI examinations.
Outcome Measurement and Covariates
The study end point was defined by ICD-9-CM codes for benign brain tumors (BBTs; ICD-9-CM 225) or malignant brain tumors (MBTs,; ICD-9-CM 191, 192, 194.3, and 194.4). All enrolled study participants were followed until the diagnosis of a brain tumor, death, withdrawal from the database, or the end of 2010, whichever came first.
We collected information regarding prescriptions, dosages, prescription dates, supply days, and total number of dispensed pills from the database. In addition, this study collected patient zolpidem prescriptions prior to the end points and calculated the average exposure as total zolpidem exposure (milligrams) divided by the period between first exposure and the end points.
Statistical Analysis
Treated and untreated participants were matched on the propensity score. To estimate the propensity score, a logistic regression model was used in which treatment status (taking zolpidem or not) was regressed on the baseline characteristics listed in Table 1. The standardized difference was used to quantify differences in means or prevalence between the two cohorts for continuous or categorical matching variables, respectively. The incidence densities of the two cohorts were calculated stratified by age. To estimate the cumulative incidence of subsequent brain tumors in patients taking zolpidem and non-zolpidem participants, this study conducted survival analysis using the Kaplan-Meier method and based the significance on a log-rank test. Cox proportional hazards models stratifying on the matched pairs were performed to estimate the hazard ratio and 95% confidence intervals (CIs) of developing BBTs and MBTs associated with zolpidem use compared with the non-zolpidem cohort. A two-tailed p<0.05 was considered statistically significant. All statistical analyses were performed using SAS statistical software (version 9.2 for Windows; SAS Institute, Cary, NC).
| Variables | Zolpidem | Standardized difference | |||
|---|---|---|---|---|---|
| No (N=37,810) | Yes (N=37,810) | ||||
| N | Percentage | N | Percentage | ||
| Sex | |||||
| Women | 23,442 | 62.0 | 23,394 | 61.9 | 0.003 |
| Age, years | |||||
| 20–34 | 5443 | 14.4 | 5451 | 14.4 | 0.001 |
| 35–44 | 6679 | 17.7 | 6795 | 18.0 | 0.008 |
| 45–54 | 8791 | 23.3 | 8932 | 23.6 | 0.009 |
| 55–64 | 6721 | 17.8 | 6839 | 18.1 | 0.008 |
| ≥65 | 10,176 | 26.9 | 9793 | 25.9 | 0.023 |
| Mean (SD) | 53.6 | 16.4 | 53.2 | 16.0 | 0.024 |
| Medical history | |||||
| Stroke | 3186 | 8.43 | 3545 | 9.38 | 0.033 |
| Dementia | 1134 | 3.00 | 1236 | 3.27 | 0.015 |
| Epilepsy | 681 | 1.80 | 644 | 1.70 | 0.007 |
| Head injury | 3788 | 10.0 | 3648 | 9.65 | 0.012 |
| Treatment | |||||
| Brain CT or MRI examinations | 2576 | 6.81 | 2845 | 7.52 | 0.028 |
TABLE 1. Baseline Characteristics Between Zolpidem and Non-Zolpidem Cohorts in the Propensity Score–Matched Sample
Results
The baseline demographic and clinical characteristics of the patients in the two cohorts are presented in Table 1. The mean (standard deviation) ages of patients in the zolpidem and non-zolpidem cohorts were 53.2 (16.0) and 53.6 (16.4) years, respectively. Of the 37,810 patients in the zolpidem cohort, some had a medicine history for stroke (9.38%), dementia (3.27%), epilepsy (1.70%), head injury (9.65%), and brain CT or MRI examinations (7.52%).
Overall, during the follow-up period, 147 of the 37,810 patients in the zolpidem cohort experienced a BBT. In addition, the zolpidem cohort (66.7 per 100,000 person-years) had a higher incidence of BBTs compared with the non-zolpidem cohort (50.2 per 100,000 person-years; hazard ratio=1.35, 95% CI=0.97–1.87), but this was not significant. There was a specifically higher incidence of BBTs for elderly participants for both cohort (≥50 years; 76.1 versus 62.5 per 100,000 person-years; Table 2). Regarding the results of the log-rank test and the cumulative incidence curve of BBTs (Figure 1), the risk of BBT was higher in the zolpidem cohort than that in the non-zolpidem cohort (log-rank, p=0.06). Conversely, taking zolpidem or not was not associated with a risk of MBTs (Table 2).
| Age | Zolpidem | ||||||
|---|---|---|---|---|---|---|---|
| No | Yes | Hazard ratio (95% confidence interval) | |||||
| Cases | Person-Year | Ratea | Cases | Person-Year | Ratea | ||
| Benign brain tumors | |||||||
| <50 | 27 | 76430 | 35.3 | 55 | 99600 | 55.2 | 1.19(0.67, 2.13) |
| ≥50 | 58 | 92791 | 62.5 | 92 | 120893 | 76.1 | 1.44(0.97, 2.14) |
| Overall | 85 | 169221 | 50.2 | 147 | 220494 | 66.7 | 1.35(0.97, 1.87) |
| Malignant brain tumors | |||||||
| <50 | 1 | 76504 | 1.31 | 1 | 99781 | 1.00 | 1.00(0.06, 16.0) |
| ≥50 | 2 | 92959 | 2.15 | 3 | 121239 | 2.47 | 1.00(0.14, 7.10) |
| Overall | 3 | 169464 | 1.77 | 4 | 221020 | 1.81 | 1.00(0.20, 4.96) |
TABLE 2. Comparisons of Incidence Density of Benign Brain Tumor and Malignant Brain Tumor Between Zolpidem and Non-Zolpidem Subjects by Age
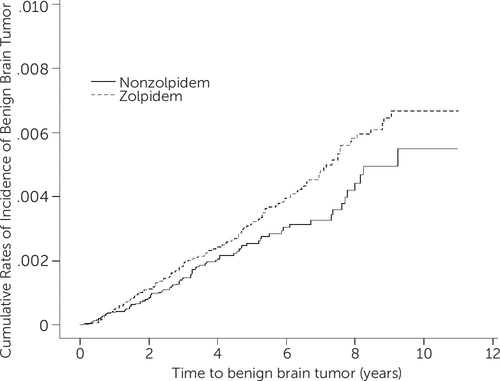
FIGURE 1. Cumulative Incidence of Benign Brain Tumor Compared Between Patients Treated With and Without Zolpidem Using the Kaplan-Meier Method
Moreover, associations between zolpidem dosage and brain tumor risk were analyzed (Table 3). The most significant risk of BBTs occurred in patients with zolpidem exposure ≥520 mg/years (hazard ratio=1.85, 95% CI=1.21–2.82). However, a significant relationship between zolpidem dosage and MBT risk was not observed (hazard ratio=0.50, 95% CI=0.05–5.51 for <520 mg/year; hazard ratio=2.00, 95% CI=0.18–22.1 for ≥520 mg/year).
| Zolpidem Average Exposure (mg/year)a | Number of Events/Number of Patients | Hazard Ratio | 95% Confidence Interval |
|---|---|---|---|
| Benign brain tumors | |||
| None | 85/37,810 | 1 | (Reference) |
| <520 | 47/18,887 | 0.80 | (0.47, 1.37) |
| ≥520 | 100/18,923 | 1.85 | (1.21, 2.82)b |
| Malignant brain tumors | |||
| None | 3/37,810 | 1 | (Reference) |
| <520 | 1/18,887 | 0.50 | (0.05, 5.51) |
| ≥520 | 3/18,923 | 2.00 | (0.18, 22.1) |
TABLE 3. Cox Proportional Hazards Regression Analysis–Measured Hazard Ratio of Benign Brain Tumor and Malignant Brain Tumor by Zolpidem Dosage
Discussion
Zolpidem is a short-acting hypnotic drug that binds at the benzodiazepine binding site on specific GABA receptors and enhances inhibitory neurotransmission.11,12 Common adverse side effects of zolpidem use include nausea or vomiting, amnesia, headaches, hallucinations, short-term memory loss, and sleepwalking.13 The results of this population-based study indicated that zolpidem use also significantly increased the risk of subsequently developing BBT. The risk of developing BBT was higher at older ages (≥50 y) and higher zolpidem dosages (≥520 mg/year) in the zolpidem cohort. Tables 2 and 3 present the findings that should be shared with medical practitioners because these results might influence the long-term prescription of zolpidem, especially in the elderly population. We could not find an increase in the risk of developing MBTs, which probably is because of the small number of patients with MBTs in this study. Additionally, MBTs include metastatic and primary lesions, which could confound the mechanistic relationship of the results.
One possible explanation for the results is that patients with sleep disorders have greater opportunities of undergoing imaging studies; therefore, a greater probability exists for diagnosing brain tumors in these groups. Table 1 showed that patients taking zolpidem had higher probability of undergoing brain CT or MRI examinations than did the non-zolpidem group. Another explanation is that slow-growing BBTs are typically accompanied with dizziness, seizures, sleep disorders, and cognitive problems, which are frequently initially treated with hypnotic drugs before a tumor diagnosis is made.14,15 To rule out this reverse causality, which implies that patients who are already at risk of BBTs might have a greater tendency of using zolpidem than those not at risk of BBTs, we used the Kaplan-Meier method without adjusting for sex, age, and comorbidities. Figure 1 indicates that the risk of BBT increases with the length of time of zolpidem use; thus, reverse causality appeared unlikely.
The most frequently reported BBTs are meningiomas (a tumor of the meninges, comprising 24.0% of all brain tumors), and the most frequently reported MBTs are glioblastomas (a tumor of the neuroepithelial tissue, 22.6%). This indicates that, in most instances, BBTs and MBTs derive from different histologies and have different genetic and molecular expressions.16–18 Zolpidem enhances inhibitory neurotransmission by interacting with the GABA receptor-coupled chloride channel. Apart from their role as an inhibitory neurotransmitter, GABA receptors may regulate various stages of cell proliferation and differentiation in the brain and periphery and thus may be involved in tumor growth by various means.19,20 Furthermore, zolpidem has been reported to promote viral infections, which might suppress immune function and induce the development of BBTs.21 However, the potential mechanisms of hypnotic drugs and brain tumor growth or suppression still remain unclear and controversial. For instance, Gourdeau et al. found that ECO-4601, a benzodiazepine receptor ligand, was shown to bind the peripheral but not the central benzodiazepine receptor and inhibited the growth of CNS tumor cell lines.22 Richardson et al. found benzodiazepine-GABA(A) receptor binding is very low in dysembryoplastic neuroepithelial tumors, a benign brain tumor.23 Recently, Bode et al. reported that knockdown the peripheral type benzodiazepine receptor (now known as translocator protein) and exposure to translocator protein ligands enhances brain tumor proliferation in an animal model.24 Those implied that the change of benzodiapepine binding affinity by zolpidem and other drugs with similar CNS effects might play a role in the neoplastic growth of the brain.
One strength of this study is the nationwide population-based design and representativeness. However, this study has limitations. First, only claims data were available in the NHIRD; other detailed information, such as smoking habits, alcohol consumption, body mass index, socioeconomic status, and family history of cancer, was not provided by the NHIRD. These might be risk factors of brain tumors and could potentially be associated with zolpidem use. However, because the NHIRD contains information regarding nearly the entire population in Taiwan and the Taiwan National Health Insurance reimbursement policy is identical for all patients, it is unlikely that these factors would affect the prescription of zolpidem. Second, the results derived from a cohort study are typically less accurate than those obtained from randomized control trials because a cohort study design is subject to numerous biases related to confounding adjustment. Despite the meticulous study design with adequate control of confounding factors by propensity score matching, a key limitation is any remaining bias regarding unmeasured or unknown confounders. Third, we could follow the codes for zolpidem prescription by administrative billing in the Taiwan National Health Insurance claims but we were unable to contact the patients directly regarding their exact doses of zolpidem use because their identification numbers were anonymous. Finally, prescriptions for these drugs before 1996 were not included in our analysis, which could have caused underestimations of cumulative dosages and might weaken the observed association. However, zolpidem prescription and tumor diagnosis data were exceptionally reliable.
In conclusion, the results of this population-based, retrospective cohort study showed a significant increase in BBT risk associated with zolpidem use. Additional studies including randomized control trials with the same findings are required before any conclusions can be confirmed.
1 : Dawn of a new sleep drug? Wall St J 2011:D1–D4Google Scholar
2 : Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med 2006; 7:123–130Crossref, Medline, Google Scholar
3 : Epidemiology of insomnia in korean adults: prevalence and associated factors. J Clin Neurol 2009; 5:20–23Crossref, Medline, Google Scholar
4 : Characteristics of insomnia in the United States: results of the 1991 National Sleep Foundation Survey. I. Sleep 1999; 22(Suppl 2):S347–S353Medline, Google Scholar
5 : Long-term treatment of insomnia with zolpidem: a multicentre general practitioner study of 107 patients. J Int Med Res 1991; 19:271–279Crossref, Medline, Google Scholar
6 : Hypnotics’ association with mortality or cancer: a matched cohort study. BMJ Open 2012; 2:e000850Crossref, Medline, Google Scholar
7 : Relationship of zolpidem and cancer risk: a Taiwanese population-based cohort study. Mayo Clin Proc 2012; 87:430–436Crossref, Medline, Google Scholar
8 : Taiwan’s National Health Insurance system: high value for the dollar, in Six Countries, Six Reform Models: The Health Reform Experience of Israel, the Netherlands, New Zealand, Singapore, Switzerland and Taiwan. Edited by Okma KGH, Crivelli L. Singapore, World Scientific, 2009, pp 71–204Crossref, Google Scholar
9 : Effectiveness of influenza vaccination in elderly diabetic patients: a retrospective cohort study. Vaccine 2013; 31:718–724Crossref, Medline, Google Scholar
10 : Cancer risk in patients with Graves' disease: a nationwide cohort study. Thyroid 2013; 23:879–884Crossref, Medline, Google Scholar
11 : Zolpidem, a clinical hypnotic that affects electronic transfer, alters synaptic activity through potential GABA receptors in the nervous system without significant free radical generation. Oxid Med Cell Longev 2009; 2:52–57Crossref, Medline, Google Scholar
12 : Zolpidem reduces the blood oxygen level-dependent signal during visual system stimulation. Prog Neuropsychopharmacol Biol Psychiatry 2011; 35:1645–1652Crossref, Medline, Google Scholar
13 : Adverse reactions to zolpidem: case reports and a review of the literature. Prim Care Companion J Clin Psychiatry 2010; 12(6)Medline, Google Scholar
14 : Medical management of patients with brain tumors. Curr Treat Options Neurol 2011; 13:413–426Crossref, Medline, Google Scholar
15 : The relationships between depression and brain tumors. J Neurooncol 2009; 94:153–161Crossref, Medline, Google Scholar
16 : Descriptive epidemiology of primary brain and CNS tumors: results from the Central Brain Tumor Registry of the United States, 1990-1994. Neuro-oncol 1999; 1:14–25Crossref, Medline, Google Scholar
17 : Brain tumors and hormonal factors: review of the epidemiological literature. Cancer Causes Control 2011; 22:697–714Crossref, Medline, Google Scholar
18 : Annual report to the nation on the status of cancer, 1975-2007, featuring tumors of the brain and other nervous system. J Natl Cancer Inst 2011; 103:714–736Crossref, Medline, Google Scholar
19 : Role of gamma-aminobutyric acid in anxiety. Psychopathology 1984; 17(Suppl 1):15–24Crossref, Medline, Google Scholar
20 : Gamma-aminobutyric acid concentrations in benign parotid tumours and unstimulated parotid saliva. J Laryngol Otol 2011; 125:492–496Crossref, Medline, Google Scholar
21 : Evidence that new hypnotics cause cancer. http://escholarship.org/uc/item/12r2f32gGoogle Scholar
22 : Identification, characterization and potent antitumor activity of ECO-4601, a novel peripheral benzodiazepine receptor ligand. Cancer Chemother Pharmacol 2008; 61:911–921Crossref, Medline, Google Scholar
23 : Benzodiazepine-GABA(A) receptor binding is very low in dysembryoplastic neuroepithelial tumor: a PET study. Epilepsia 2001; 42:1327–1334Crossref, Medline, Google Scholar
24 : The 18 kDa translocator protein influences angiogenesis, as well as aggressiveness, adhesion, migration, and proliferation of glioblastoma cells. Pharmacogenet Genomics 2012; 22:538–550Crossref, Medline, Google Scholar



