Poststroke Subcortical Aphasia and Neurobehavioral Disturbances Without Motor or Sensory Deficits
To the Editor: Subcortical aphasia may result from lesions of the basal ganglia, anterolateral nuclei of the thalamus, and capsular/pericapsular white matter of the language-dominant hemisphere.1,2 Subcortical aphasia associated with lesions of the posterior limb of the internal capsule (ICp) and adjacent subcortical structures typically involves impaired picture naming (letters better than objects), rapid fluent speech with paraphasias and extended jargon, poor comprehension, and impaired repetition.1,2 Contralateral disturbances of elementary motor and sensory function typically occur; with posterior extension of the capsular lesion, these disturbances may be accompanied by visual field deficits and auditory dysfunction.1,2 Subcortical aphasia associated with lesions of the anterior limb of the internal capsule (ICa) and adjacent structures (often including the genu of the internal capsule [ICg]) typically involves impaired picture naming (objects better than letters), grammatical but slow dysarthric speech output, good semantic comprehension with impaired syntactic comprehension, and repetition impairments primarily for low-probability sentences (with articulatory distortions and word omissions); impaired articulatory agility, buccofacial apraxia, and contralateral hemiparesis also are typically present.1,2 Subcortical aphasia associated with lesions involving the ICa, ICp, and adjacent subcortical structures features more severe language disturbances (i.e., global aphasia) and contralateral motor, sensory, and visual field deficits.1
The neuropsychiatric, motor, and sensory concomitants of subcortical aphasia follow capsular anatomy (Figure 1 [A]). The frontopontine, thalamofrontal, and thalamostriate fibers in the ICa subserve circuits regulating emotion, comportment, motivation, and executive function.3 The ICg comprises fibers of the corticonuclear tract, connecting the motor cortex to contralateral cranial nerve nuclei.3 The ICp comprises fibers of the corticospinal (pyramidal or voluntary motor) tract, as well as sensory fibers originating from the thalamus and, to a lesser extent, from the medial lemniscus; the distal one-third of the ICp includes fibers of the optic and acoustic radiations, as well as temporopontine, parietopontine, and occipitopontine fibers.4 Concomitant neuropsychiatric disturbances (especially executive dysfunction, disordered comportment, diminished motivation, and/or emotional dysregulation) would be expected among persons with subcortical aphasia involving lesions of the ICa and adjacent subcortical structures.3,5–7 By contrast, elementary motor impairments are expected concomitants of subcortical aphasia associated with lesions involving the ICg and/or ICp, and sensory impairments are expected accompaniments of lesions involving the ICp.1,3
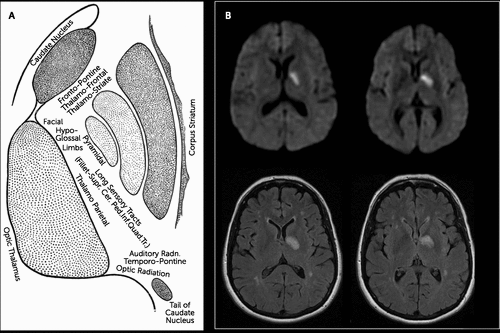
FIGURE 1. White Matter Pathways Within the Internal Capsule [A], and an MRI Scan of the Brain Taken Approximately 3 Days After Stroke [B]a
aThe top and bottom rows in panel B are diffusion-weighted and fluid-attenuated inversion recovery sequences, respectively. Panel A is from the Educational Technology Clearinghouse and the Florida Center for Instructional Technology, College of Education, University of South Florida (etc.usf.edu/clipart/55700/55799/55799_capsule_lg.gif, retrieved June 23, 2014), used with permission.
Contrary to these expectations, we present a case of acute subcortical stroke involving the ICg and ICp as well as adjacent subcortical structures (with caudal extension) associated with subcortical aphasia, apathy, and pathological laughing and no motor or sensory deficits.
Case Report
A 53-year-old, right-handed woman presented 3 days after sudden onset of reduced spontaneous speech, impaired naming and word finding, semantic substitution paraphasias, and diminished self-care. Her National Institutes of Health Stroke Scale score was zero upon admission. However, the examination revealed the following: difficulty with confrontation naming, occasional semantic paraphasias (substitutions), and reduced phrase lengths; brief, uncontrollable, stereotyped, and contextually inappropriate bursts of laughter; and diminished goal-directed thought, emotion, and behavior. The patient had mild hyperreflexia at the right triceps without other motor or sensory abnormalities. An MRI scan of the brain performed 3 days after symptom onset revealed scattered, ill-defined, chronic small vessel ischemic changes in the deep white matter of the cerebral hemispheres and an acute 1.5-cm lacunar stroke principally affecting the ICg and ICp, the medial aspect of the globus pallidus, and the anterior, lateral, and ventral thalamic nuclei as well as the ventral striatum (Figure 1 [B]). The patient’s neurobehavioral abnormalities resolved within the first week after the stroke, but she continued to experience mild residual impairment of naming and word finding (i.e., anomic aphasia). These problems were present during the phone interview 9 months after this event.
Discussion
This patient’s acute stroke was centered over the ICg and the anterior two-thirds of the ICp, involved adjacent subcortical structures, and extended caudally to the ventral striatum, with minimal involvement of the ICa. This lesion would be expected to do the following: produce aphasia predominated by impaired picture naming, rapid fluent speech with paraphasias and extended jargon, poor comprehension, and impaired repetition;1,2 spare frontal thalamostriatal circuits involved in executive function, comportment, and motivation as well as frontal thalamostriatal and frontopontine pathways supporting emotional regulation;3 produce motor deficits via disruption of corticonuclear and corticospinal tracts;1,2,4 and produce sensory deficits via disruption of thalamoparietal, thalamotemporal, and thalamooccipital tracts.1,2 Instead, this lesion produced relatively mild aphasia (with characteristics of both anterior and posterior subcortical aphasia syndromes), prominent neuropsychiatric symptoms, and no overt motor or sensory deficits.
The proximity of the lesion and surrounding edema to the posterior border with the ICa may explain, in part, the patient’s early poststroke neuropsychiatric disturbances; caudal extension of that lesion into the ventral striatum may have also contributed to her dysregulated affect.3,6 At the same time, incomplete involvement of the ICa may account for this patient’s relatively preserved fluency, the transient nature of her symptoms, and her good neuropsychiatric outcome.
The absence of overt and clinically important motor and sensory findings in a patient with a lesion involving the anterior two-thirds of the ICp of sufficient import to produce subcortical aphasia and other neuropsychiatric disturbances is quite unexpected and, to our knowledge, is previously unreported. Rare variant locations of descending and ascending white matter pathways,3,4,8 including a shift of the pyramidal tract within the ICp to a relatively more posterior location, are associated with atypical patterns of motor involvement after capsular stroke.8–10 Given the location of this patient’s capsular lesion, she is suspected of bringing this type of variant capsular anatomy to her stroke. A similarly posterior shift of corticonuclear fibers typically located in the ICg would explain the absence of cranial nerve findings in this patient. This neuroatypical shift in the location of corticonuclear, corticospinal, and sensory fibers within the ICp would concurrently permit shift (or, more accurately, broader distribution) of frontopontine, thalamofrontal, and thalamostriate pathways from the ICa into the ICg and the anterior portion of the ICp. Variant capsular anatomy of this nature offers a parsimonious explanation for this patient’s clinical presentation with poststroke subcortical aphasia and neurobehavioral disturbances without motor or sensory deficits.
1 : Aphasia with predominantly subcortical lesion sites: description of three capsular/putaminal aphasia syndromes. Arch Neurol 1982; 39:2–14Crossref, Medline, Google Scholar
2 : Subcortical aphasia: three different language disorder syndromes? Eur J Neurol 2003; 10:445–448Crossref, Medline, Google Scholar
3 : Disconnection syndromes of basal ganglia, thalamus, and cerebrocerebellar systems. Cortex 2008; 44:1037–1066Crossref, Medline, Google Scholar
4 : A review of corticospinal tract location at corona radiata and posterior limb of the internal capsule in human brain. NeuroRehabilitation 2009; 24:279–283Medline, Google Scholar
5 : Cognitive disconnective syndrome by single strategic strokes in vascular dementia. J Neurol Sci 2012; 322:176–183Crossref, Medline, Google Scholar
6 : Akinetic mutism and mixed transcortical aphasia following left thalamo-mesencephalic infarction. J Neurol Sci 1999; 163:70–73Crossref, Medline, Google Scholar
7 : Loss of motivation for speaking with bilateral lacunes in the anterior limb of the internal capsule. Clin Neurol Neurosurg 1989; 91:325–327Crossref, Medline, Google Scholar
8 : The site of motor corticospinal fibres in the internal capsule of man. A computerised tomographic study of restricted lesions. J Anat 1982; 134:199–208Medline, Google Scholar
9 : Location of human pyramidal tract in the internal capsule: anatomic evidence. Neurology 1975; 25:823–826Crossref, Medline, Google Scholar
10 : Localization of the pyramidal tract in the internal capsule of man. J Neurol Sci 1977; 34:63–70Crossref, Medline, Google Scholar



