Antipsychotic Use in a Diverse Population With Dementia: A Retrospective Review of the National Alzheimer’s Coordinating Center Database
Abstract
A cross-sectional analysis examined medication records in the National Alzheimer’s Coordinating Center Database for community-dwelling patients with dementia who visited an Alzheimer’s Disease Center between 2008 and 2014. Hispanic participants had a 1.62-fold greater use of antipsychotic medications, which was largely accounted for by a higher prevalence of neuropsychiatric symptoms and more severe dementia compared with non-Hispanic whites. These results are consistent with reports of later transition to nursing home care among Hispanic participants. Further studies are needed to clarify ethnic differences in how families and physicians address dementia progression and neuropsychiatric symptoms in community-dwelling patients with dementia.
The increased risk for death from antipsychotic medications among elderly patients with dementia has been studied extensively. The U.S. Food and Drug Administration issued a black box warning on atypical antipsychotics in April 2005, and the agency expanded the warning to typical antipsychotics in June 2008.1–4 In addition, overreliance on antipsychotic medications in dementia care has been an area of heavy focus for consumer advocacy groups and the Centers for Medicare & Medicaid Services because of concerns about worsening quality of life, increased health care costs, and increased mortality risk.5 Nevertheless, inappropriate use and overuse of antipsychotic medications for dementia-related neuropsychiatric symptoms (NPSs) in elderly patients have persisted. In May 2012, the Centers for Medicare & Medicaid Services6 launched the Partnership to Improve Dementia Care initiative in an attempt to reduce the overuse of antipsychotic medications in nursing home residents, calling for a 15% reduction in the use of antipsychotics in skilled nursing facilities by the end of 2012.
Emerging research demonstrates that there are ethnic differences in the use of Food and Drug Administration–approved dementia medications. In a Medicare database, use of dementia medications was 30% higher among non-Hispanic whites compared with other racial/ethnic groups.7 However, there has been little or no examination of racial or ethnic differences in antipsychotic medication usage in elderly patients with dementia. Identifying ethnic differences in antipsychotic use could potentially lead to effective intervention programs (e.g., educational or pharmacy/psychiatric consultation programs) aimed at reducing inappropriate antipsychotic use for dementia care. We examined patterns of antipsychotic use in African American, Hispanic, and non-Hispanic white patients with dementia, using existing data from the National Alzheimer’s Coordinating Center (NACC). Our goals were as follows: 1) to characterize the prevalence of antipsychotic use in NACC study participants, overall and by race/ethnicity; 2) to assess whether antipsychotic use was primarily associated with the presence, severity, and type of NPSs, rather than with dementia severity; and 3) to assess whether differences in antipsychotic use across racial/ethnic groups persisted after accounting for NPSs and other demographic and clinical variables.
Methods
Population and Participants
We conducted a retrospective review of prescription medication records for community-dwelling NACC participants diagnosed with dementia. The study sample consists of African American, Hispanic, and non-Hispanic white participants diagnosed with dementia who visited an Alzheimer’s Disease Center between 2008 and 2014 and completed medication forms at the visit. For our cross-sectional analyses, an index visit was determined by the participant’s first record of prescription information starting in 2008. A total of 8,919 participants fit this description (African American, N=983; Hispanic, N=849; and non-Hispanic white, N=7,087). The NACC database consists of data from 34 federally funded Alzheimer’s Disease Centers and is supported by the National Institute on Aging. Details about the NACC consortium and its database have been described previously.8,9 We also considered the subgroup of this population that was diagnosed with either probable or possible Alzheimer’s disease–related dementia (N=7,059). The racial breakdown of this subgroup was as follows: 872 participants were African American, 757 were Hispanic, and 5,430 were non-Hispanic white.
Demographic information about participants included age at visit, gender, educational level, and race/ethnicity. Clinical information included the diagnosis of dementia and whether it was probable or possible Alzheimer’s disease, informant-reported NPSs measured by the Neuropsychiatric Inventory Questionnaire,10,11 and a clinician assessment of dementia severity using the Clinical Dementia Rating (CDR).12–14 The Neuropsychiatric Inventory Questionnaire rates the presence and severity of 12 specific NPSs (delusions, hallucinations, agitation or aggression, depression or dysphoria, anxiety, elation or euphoria, apathy or indifference, disinhibition, irritability or lability, motor disturbance, nighttime behaviors, and appetite and eating) for participants with Alzheimer’s disease and other forms of dementia. For those with affirmative responses, each symptom is further scored by severity (from 1 to 3), with the sum of severity scores used as an overall rating of NPSs. The CDR uses a five-point scale (0, 0.5, 1, 2, and 3) for each of six cognitive domains (memory, orientation, judgment and problem solving, community affairs, home and hobbies, and personal care), based on a physical examination and informant interview. A CDR score of 0 within a particular domain implies no cognitive impairment, whereas a score of 3 is indicative of severe impairment. The CDR sum of boxes ranges from scores of 0 to 18.
Study Outcome
The primary outcome was the use of antipsychotic drugs at the index visit, including miscellaneous antipsychotics, phenothiazine psychotics, thioxanthenes, and first-generation and second-generation antipsychotics, based on the informant-reported medication form from the Uniform Dataset 3.0 (revised in 2008). For a full list of the included medications, please see the NACC Derived Variables documentation.15
Statistical Analysis
Descriptive summaries of the study sample reported continuous variables as means ± standard deviations and categorical as numbers and percentages. Initial univariate logistic regression examined the associations of both the primary and secondary predictors with use of antipsychotic drugs. The key hypothesized predictors were then assessed in a series of three multivariate logistic regression models, including potential demographic confounders. The first model assessed whether the use of antipsychotic medications differed by race/ethnicity, taking into account key demographic variables including gender, age, and education. The second multivariate model added the Neuropsychiatric Inventory Questionnaire sum of severity scores to assess the effect of NPSs on the use of antipsychotics and to determine whether differences across racial/ethnic groups might be accounted for by differences in NPSs. In the third multivariate model, the CDR sum of boxes was added to the model to assess the effect of dementia severity. Secondary analyses repeated these analyses in the subgroup restricted to dementia associated with Alzheimer’s disease.
All reported p values were those of two-sided tests; significance was defined as p<0.05. All analyses were performed using SAS statistical software (version 9.2; SAS Institute Inc., Cary, N.C.) and/or R software (version 3.1.2; Vienna, Austria, R Foundation for Statistical Computing [http://www.R-project.org/]).
Results
Of the 8,919 participants diagnosed with dementia, 930 (10.4%) were reported to be taking one or more antipsychotic medications (panel A of Table 1). Participants were predominantly white, with most having at least a high school education, and there were approximately equal numbers of men and women. Of the 7,059 participants diagnosed with Alzheimer’s disease–related dementia, 625 (8.9%) were taking antipsychotic medication.
| Variable | No Antipsychotic Drug Use (N=7,989) | Antipsychotic Drug Use (N=930) | Percent Taking Antipsychotic Drugs (10.43%) | Univariate Logistic Regression |
|---|---|---|---|---|
| Primary predictor of interest | ||||
| Race/ethnicity | ||||
| White | 6,377 | 710 | 10 | 1.00 |
| Hispanic | 719 | 130 | 15 | 1.62 (1.32–1.98)b |
| African American | 893 | 90 | 9 | 0.9 (0.71–1.13) |
| Key predictors | ||||
| Sociodemographic | ||||
| Age, years | 74±10.5 | 73±11.1 | 0.99 (0.98–1) | |
| <70 | 2,570 | 339 | 12 | 1.23 (1.06–1.43)b |
| 70–75 | 1,522 | 173 | 10 | 1.06 (0.84–1.42) |
| >75 | 3,897 | 418 | 10 | 1.00 |
| Gender | ||||
| M | 3,846 | 473 | 11 | 1.00 |
| F | 4,143 | 457 | 10 | 0.9 (0.78–1.03) |
| Education | 14±3.8 | 14±4.2 | 0.96 (0.95–0.98)b | |
| Less than high school | 952 | 152 | 14 | 1.35 (1.11–1.65)b |
| High school | 3,292 | 388 | 11 | 1.00 |
| College | 1,857 | 186 | 9 | 0.85 (0.71–1.02) |
| Graduate education | 1,888 | 204 | 10 | 0.92 (0.77–1.09) |
| NPS severity | ||||
| Sum of boxes | 5±4.5 | 8±5.9 | 1.12 (1.1–1.13)b | |
| CDR | ||||
| Sum of boxes | 7±4.3 | 12±5.3 | 1.21 (1.19–1.23)b |
Table 1 [A]. Dementia Population: Descriptive Summaries of the Study Sample and Univariate Comparisons of Patients Using Antipsychotics Compared With Those Not Taking Antipsychotic Drugsa
Univariate Analysis
Panel A of Table 1 provides unadjusted odd ratios for association with the use of antipsychotic drugs for study participants with dementia. Unadjusted for age, education, gender, NPSs, and CDR, Hispanic participants in the dementia population were 62% more likely (odds ratio=1.62; 95% confidence interval=1.32–1.98) to be taking antipsychotics compared with non-Hispanic whites. The risk for antipsychotic medication use increased with increasing NPS severity (odds ratio=1.12 for every 1-point increase) and with increasing dementia severity (odds ratio=1.21 for every 1-point increase in the CDR sum of boxes). Older participants were less likely to be taking antipsychotics, with a 1% decrease for every year of age (odds ratio=0.99). Fewer years of formal education increased the odds of antipsychotic use, especially for individuals with less than a high school education (odds ratio=1.35).
Among participants with probable or possible Alzheimer’s disease, Hispanic participants were almost twice as likely to be taking antipsychotics compared with non-Hispanic whites (odds ratio=1.97; 95% confidence interval=1.57–2.46). Of the participants who were taking antipsychotics, 56% endorsed agitation, 33% had disinhibition, 33% reported delusions, and 25% had hallucinations.
Multivariate Analysis
A series of multivariate logistic regression analyses further examined the association of race/ethnicity with antipsychotic use in participants with dementia (panel A of Table 2) and in the subgroup with Alzheimer’s disease (panel B of Table 2), adjusting for age, education, and gender (model I) and sequentially adding NPSs (model II) and CDR (model III).
| Variable | Model I | Model II | Model III |
|---|---|---|---|
| Primary predictor of interest | |||
| Race/ethnicity | |||
| Hispanic compared with white | 1.41 (1.11–1.77)b | 1.21 (0.95–1.54) | 1 (0.77–1.29) |
| African American compared with white | 0.88 (0.69–1.12) | 0.8 (0.61–1.01) | 0.79 (0.61–1.01) |
| Key predictors | |||
| Sociodemographic | |||
| Age | |||
| Effect of 1-year increase | 0.99 (0.98–0.99)b | 1.00 (0.98–1) | 0.99 (0.98–0.99)b |
| Gender | |||
| Women compared with men | 0.86 (0.75–0.99)b | 0.9 (0.78–1.04) | 0.79 (0.68–0.92)b |
| Education | |||
| Less than high school compared with high school | 1.26 (1–1.58)b | 1.17 (0.93–1.48) | 1.10 (0.86–1.4) |
| College compared with high school | 0.83 (0.69–1)b | 0.87 (0.72–1.05) | 0.87 (0.71–1.06) |
| Graduate education compared with high school | 0.89 (0.74–1.06) | 0.93 (0.77–1.12) | 0.95 (0.78–1.16) |
| NPS severity | |||
| Sum of boxes | 1.11 (1.1–1.13)b | 1.08 (1.06–1.09)b | |
| CDR | |||
| Sum of boxes | 1.2 (1.18–1.22)b |
Table 2 [A]. Dementia Population: Nested Logistic Regression Analysis of Antipsychotic Drug Use Among Sociodemographic Factors, Level of Cognitive Impairment as Measured by CDR, and Severity of NPSsa
| Variable | Model I | Model II | Model III |
|---|---|---|---|
| Primary predictor of interest | |||
| Race/ethnicity | |||
| Hispanic compared with white | 1.74 (1.34–2.27)b | 1.46 (1.11–1.92)b | 1.19 (0.88–1.59) |
| African American compared with white | 0.99 (0.75–1.28) | 0.88 (0.66–1.15) | 0.88 (0.66–1.17) |
| Key predictors | |||
| Sociodemographic | |||
| Age | |||
| Effect of 1-year increase | 1.00 (0.99–1.01) | 1.01 (0.99–1.02) | 1.00 (0.99–1) |
| Gender | |||
| Women compared with men | 0.93 (0.78–1.1) | 0.96 (0.81–1.4) | 0.85 (0.71–1.03) |
| Education | |||
| Less than high school compared with high school | 1.20 (0.92–1.56) | 1.11 (0.85–1.46) | 1.03 (0.77–1.37) |
| College compared with high school | 0.86 (0.68–1.08) | 0.92 (0.72–1.16) | 0.91 (0.71–1.17) |
| Graduate education compared with high school | 0.97 (0.77–1.2) | 1.03 (0.81–1.29) | 1.08 (0.85–1.38) |
| NPS severity | |||
| Sum of boxes | 1.12 (1.1–1.13)b | 1.07 (1.06–1.09)b | |
| CDR | |||
| Sum of boxes | 1.23 (1.21–1.26)b |
Table 2 [B]. Probable or Possible Alzheimer’s Disease Population: Nested Logistic Regression Analysis of Antipsychotic Drug Use Among Sociodemographic Factors, Level of Cognitive Impairment as Measured By CDR, and Severity of NPSsa
In both the dementia group and the Alzheimer’s disease subgroup, Hispanics were significantly more likely to be taking antipsychotic medication, even after adjustment for age, education, and gender, although the odds were reduced from the unadjusted models in panels A and B of Table 1. In model II with the NPS severity score added, the odds of taking antipsychotics continued to be elevated for Hispanics and remained significant in the probable or possible Alzheimer’s disease group but were no longer significantly different in the larger dementia group. After adjustment for overall dementia severity (CDR sum of boxes, model III), Hispanics were not significantly more likely to be taking antipsychotics.
| Variable | No Antipsychotic Drug Use (N=6,434) | Antipsychotic Drug Use (N=625) | Percent Taking Antipsychotic Drugs (8.85%) | Univariate Logistic Regression |
|---|---|---|---|---|
| Primary predictor of interest | ||||
| Race/ethnicity | ||||
| White | 4,991 | 439 | 8 | 1.00 |
| Hispanic | 645 | 112 | 15 | 1.97 (1.57–2.46)b |
| African American | 798 | 74 | 8 | 1.05 (0.81–1.36) |
| Key predictors | ||||
| Sociodemographic | ||||
| Age, years | 75±10 | 76±10 | 1.00 (0.99–1.01) | |
| <70 | 1,690 | 150 | 8 | 0.87 (0.71–1.06) |
| 70–75 | 1,204 | 114 | 9 | 0.93 (0.74–1.15) |
| >75 | 3,540 | 361 | 9 | 1.00 |
| Gender | ||||
| M | 2,929 | 285 | 9 | 1.00 |
| F | 3,505 | 340 | 9 | 1.00 (0.85–1.18) |
| Education | 14±3.8 | 14±4.4 | 0.96 (0.94–0.98)b | |
| Less than high school | 837 | 121 | 13 | 1.52 (1.21–1.91)b |
| High school | 2,642 | 251 | 9 | 1.00 |
| College | 1,475 | 119 | 7 | 0.85 (0.67–1.06) |
| Graduate education | 1,480 | 134 | 8 | 0.95 (0.76–1.18) |
| NPS severity | ||||
| Sum of boxes | 4±4.3 | 7±6 | 1.12 (1.1–1.14)b | |
| CDR | ||||
| Sum of boxes | 7±4.2 | 12±5.2 | 1.25 (1.23–1.27)b |
Table 1 [B]. Probable or Possible Alzheimer’s Disease Population: Descriptive Summaries of Study Sample and Univariate Comparisons of Patients Using Antipsychotic Drugs Compared With Those Not Taking Antipsychotic Drugsa
We further examined the effects of NPS severity and dementia severity on racial/ethnic differences in two ways. We looked at the subgroup of participants with higher CDR scores only and saw a significant increase in the odds for taking antipsychotics for Hispanics among those with more severe dementia, even after adjusting for NPS. We also looked at NPSs and found that Hispanic informants generally reported higher levels of NPSs across most levels of CDR, both for participants with dementia and among those with probable or possible Alzheimer’s disease (Figure 1).
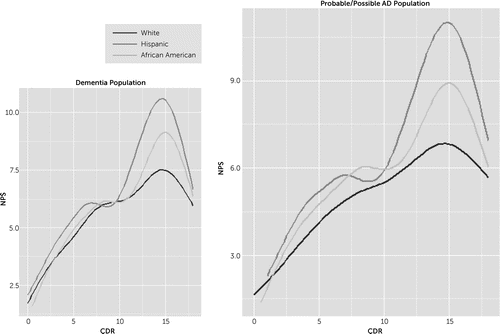
FIGURE 1. Relationship Between the Clinical Dementia Rating and the Caregiver’s Perception of Symptomsa
a Hispanics report higher neuropsychiatric symptom scores across almost all levels of dementia ratings; this is more pronounced as the dementia level increases. AA, African American; CDR, Clinical Dementia Rating; NPS, neuropsychiatric symptom.
Discussion
Use of antipsychotic medications in NACC participants with dementia was relatively common (10% of subjects overall), with higher use in men, in younger participants, and in those with more NPSs or more severe dementia. Use of antipsychotics was 62% higher in Hispanic participants with dementia than in non-Hispanic whites and was almost twofold greater among the subset whose dementia was associated with probable or possible Alzheimer’s disease. This difference was largely accounted for by higher levels of NPSs and dementia severity among Hispanic participants. Among the subjects with the highest levels of CDR, however, Hispanics had higher odds of antipsychotic use even after taking into account the severity of NPSs. There was no difference for antipsychotic use between whites and African Americans.
Hispanic informants also reported greater Neuropsychiatric Inventory Questionnaire severity across most levels of dementia severity, especially at the higher levels (Figure 1), and this subgroup of more impaired subjects seemed to have greater use of antipsychotic medication. This finding suggests that antipsychotic use is primarily driven by NPSs and dementia severity, but Hispanic participants and informants may be coping with greater severity of NPSs that may account for their use of antipsychotics, especially the twofold increase among participants with Alzheimer’s disease. These results are consistent with our study of the Sacramento Area Latino Study on Aging participants with cognitive impairment, which showed that community-dwelling Hispanic participants had higher levels and severity of NPSs compared with other studies (of predominantly white non-Hispanics) using similar recruitment and assessment methods.16 Later work showed that this difference cannot be accounted for by differences in education or source of recruitment.17 One explanation for our findings is that Hispanic families in the community are more likely to be caring for more impaired elderly patients with dementia. One previous study of NACC participants showed lower rates of transition to nursing home care for Hispanic participants with dementia.18 This result is also consistent with studies of nationally representative samples showing that Latino family caregivers report providing more hours of care, on average, to older family members.17,19
Predictors of antipsychotic use have received increasing research attention in various settings, especially skilled nursing facilities, which are more regulated than assisted-living and community settings. Some studies have reported differences across facilities,20,21 in that patients in some nursing facilities consistently have higher antipsychotic use (possibly related to referral patterns). Differences in use have also been reported across countries, possibly related to the availability of dementia-specific units.22 Another study 23 reported a 23% use of antipsychotics in a community sample of adults with dementia in Canada, and the results showed that living in a low-income household was associated with higher antipsychotic use. Findings from the 2002–2004 Aging, Demographics, and Memory Study24 showed that nearly 20% of participants used antipsychotic medication and those who lived with their caregivers were significantly less likely to use antipsychotics. Consistent with our finding that higher NPSs predict antipsychotic use, other studies have found that severe behavioral symptoms25 and hyperactive symptoms (agitation, disinhibition, restlessness, and euphoria) are associated with antipsychotic use.26–28
The strengths of the NACC database for this study include assessing dementia severity and NPSs using the CDR and Neuropsychiatric Inventory Questionnaire in research settings, respectively, as well as using validated instruments and trained research clinicians under standardized conditions. However, there are several limitations. The NACC focuses on a population that has sought consultation and clinical care at federally funded, university-based Alzheimer’s Disease Centers across the United States. In addition, the participants and their caregivers are voluntarily participating in research protocols as part of the Alzheimer’s Disease Center. In general, the Alzheimer’s Disease Centers provide cutting-edge clinical care and research for their catchment areas, and these centers would be expected to have lower antipsychotic use compared with a general community sample. Therefore, the participants may not accurately reflect the general community population. Antipsychotic use in the dementia population is considered “off-label” use and is generally discouraged in favor of nonpharmacological interventions29,30; however, similar to other studies, we were unable to assess the degree to which nonpharmacological interventions are being used in the NACC participants or whether a minority of the antipsychotic use was for approved indications such as for bipolar disorder or schizophrenia. Finally, the findings from this study are cross-sectional in nature and do not address changes in antipsychotic use across time or its relationship with the incidence and progression of dementia and NPSs.
In conclusion, we found that Hispanics, but not African Americans, have increased odds of antipsychotic use in the NACC participants with dementia and probable or possible Alzheimer’s disease compared with non-Hispanic whites and African Americans. The difference is largely accounted for by higher dementia severity and NPSs in Hispanic participants, consistent with other reports that Hispanic patients with dementia experience later transitions to nursing homes and that their families provide more hours of care. Our work also suggests higher use of antipsychotic medications among Hispanic participants with the most severe dementia, regardless of NPS severity. Future studies are needed to examine the onset and progression of troubling NPSs in community-dwelling patients as well as the strategies and decision processes used by families and physicians, especially in the Hispanic community, when considering nonpharmacologic interventions, antipsychotic medications, and placement in nursing homes as dementia progresses.
1 : Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA 2005; 294:1934–1943Crossref, Medline, Google Scholar
2 : ACNP White Paper: update on use of antipsychotic drugs in elderly persons with dementia. Neuropsychopharmacology 2008; 33:957–970Crossref, Medline, Google Scholar
3 : Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med 2005; 353:2335–2341Crossref, Medline, Google Scholar
4 : Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med 2007; 146:775–786Crossref, Medline, Google Scholar
5 National Consumer Voice for Quality Long-Term Care: http://theconsumervoice.org/uploads/files/issues/CVAntipsychoticsIssueBrief.pdfGoogle Scholar
6 US Centers for Medicare & Medicaid Services: http://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/SurveyCertificationGenInfo/National-Partnership-to-Improve-Dementia-Care-in-Nursing-Homes.htmlGoogle Scholar
7 : Racial and ethnic disparities in the treatment of dementia among Medicare beneficiaries. J Gerontol B Psychol Sci Soc Sci 2008; 63:S328–S333Crossref, Medline, Google Scholar
8 :
9 :
10 : Diagnostic accuracy and practice effects in the National Alzheimer’s Coordinating Center Uniform Data Set neuropsychological battery. Alzheimers Dement 2014; 10:675–683Crossref, Medline, Google Scholar
11 :
12 : The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993; 43:2412–2414Crossref, Medline, Google Scholar
13 :
14 : Validation of the new interpretive guidelines for the clinical dementia rating scale sum of boxes score in the National Alzheimer’s Coordinating Center database. Arch Neurol 2010; 67:746–749Medline, Google Scholar
15 National Alzheimer’s Coordinating Center: Description of NACC derived variables to be used in data analysis. www.alz.washington.edu/WEB/dervar.pdfGoogle Scholar
16 : Neuropsychiatric symptoms in Latino elders with dementia or cognitive impairment without dementia and factors that modify their association with caregiver depression. Gerontologist 2003; 43:669–677Crossref, Medline, Google Scholar
17 : Making sense of behavioral disturbances in persons with dementia: Latino family caregiver attributions of neuropsychiatric inventory domains. Alzheimer Dis Assoc Disord 2009; 23:401–405Crossref, Medline, Google Scholar
18 : Measuring Alzheimer disease progression with transition probabilities: estimates from NACC-UDS. Curr Alzheimer Res 2012; 9:1050–1058Crossref, Medline, Google Scholar
19 : Differences in amount of informal care received by non-Hispanic whites and Latinos in a nationally representative sample of older Americans. J Am Geriatr Soc 2005; 53:146–151Crossref, Medline, Google Scholar
20 : After the black box warning: predictors of psychotropic treatment choices for older patients with dementia. Psychiatr Serv 2011; 62:1207–1214Crossref, Medline, Google Scholar
21 : Unexplained variation across US nursing homes in antipsychotic prescribing rates. Arch Intern Med 2010; 170:89–95Crossref, Medline, Google Scholar
22 :
23 : The essential and potentially inappropriate use of antipsychotics across income groups: an analysis of linked administrative data. Can J Psychiatry 2012; 57:488–495Crossref, Medline, Google Scholar
24 : Psychotropic medication burden and factors associated with antipsychotic use: an analysis of a population-based sample of community-dwelling older persons with dementia. J Am Geriatr Soc 2011; 59:2100–2107Crossref, Medline, Google Scholar
25 :
26 : Use of antipsychotic medications in older home-care patients. Report from nine European countries. Aging Clin Exp Res 2008; 20:260–265Crossref, Medline, Google Scholar
27 : Psychotropic drug consumptions at admission and discharge of nursing home residents. J Am Med Dir Assoc 2012; 13:407–412Google Scholar
28 : Prevalence of psychotropic medication use among German and Austrian nursing home residents: a comparison of 3 cohorts. J Am Med Dir Assoc. 2012; 13:187.e7–187.e13Crossref, Medline, Google Scholar
29 : Does pharmacological treatment of neuropsychiatric symptoms in Alzheimer’s disease relieve caregiver burden? Drugs Aging 2012; 29:167–179Crossref, Medline, Google Scholar
30 : Nonpharmacologic management of behavioral symptoms in dementia. JAMA 2012; 308:2020–2029Crossref, Medline, Google Scholar



