Depression Symptoms in Chronic Left Hemisphere Stroke Are Related to Dorsolateral Prefrontal Cortex Damage
Abstract
Damage to the brain’s mood regulation systems may contribute to poststroke depression. This study examines relationships between depression symptoms and psychosocial factors and then uses multivariate lesion-symptom mapping to localize depression symptoms in people with chronic left hemisphere stroke. Depression symptoms relate inversely to education and directly to physical disability. Damage in the left dorsolateral prefrontal cortex is associated with greater depression symptoms. These results demonstrate a neurological contribution to depression symptoms in chronic left hemisphere stroke and provide evidence of convergent biological mechanisms for poststroke depression symptoms and major depression with regard to left dorsolateral prefrontal cortex dysfunction.
Depression occurs in 25%−32% of stroke survivors.1 Depression in the subacute phase after stroke independently predicts functional outcomes in the chronic phase, suggesting it negatively impacts recovery.2 Understanding the underlying causes of poststroke depression (PSD) may lead to the development of new treatments that will improve quality of life and functional recovery of stroke survivors.
The etiology of PSD is likely multifactorial, consistent with biopsychosocial models of mental illness,3 but it remains unclear whether the lesion contributes directly to PSD by disrupting the brain’s mood regulation systems.4 Idiopathic major depression is associated with dysfunction in the left dorsolateral prefrontal cortex (DLPFC),5 as is late-life depression,6,7 and depression associated with acute traumatic brain injury.8 Correspondingly, a study of patients with bilateral DLPFC damage from various causes found increased rates of depression compared with patients with lesions elsewhere.9 PSD has previously been associated with left frontal strokes,10,11 particularly with increasing proximity to the left frontal pole.12–14 However, these studies were primarily conducted during the first few months after stroke, during a period of acute adjustment to communication impairments and loss of dominant limb function in many left hemisphere stroke survivors. A meta-analysis examining lateralization of depression at different time points after stroke found that PSD was associated with left hemisphere strokes in the acute phase, but right hemisphere strokes in the chronic phase. This pattern is consistent with differential time courses of neuropsychological syndromes associated with the two hemispheres: resolution of catastrophic reaction and acute adjustment difficulties from aphasia in left hemisphere stroke, and a resolution of anosognosia with persistence of behavioral dysfunction in right hemisphere stroke.4 This meta-analysis, and others,15 have addressed the broad lateralization of depression symptoms to one hemisphere or the other, but have not examined specific localization within the hemispheres. However, some studies have found that within the left hemisphere, the association between anterior strokes and depression diminishes over time.14,16,17 These studies were conducted before the era of modern lesion-symptom mapping18 and used relatively coarse measures of localization (e.g., proximity to the frontal pole). These approaches may not be sensitive to lasting effects of specific damage to structures involved in mood regulation, including the left DLPFC, in the chronic phase after stroke.
Here, we use a new multivariate lesion-symptom mapping method19 to localize depression symptoms within the left hemisphere with greater precision than these earlier studies. Based on the evidence in idiopathic major depression, we hypothesized that strokes of the left DLPFC should disrupt mood regulation systems, resulting in greater depression symptoms compared with other stroke locations, even in the chronic phase of recovery. Although most prior studies on localization of PSD have focused on earlier periods after stroke, testing in the chronic period is consistent with common lesion-symptom association approaches in cognitive neuropsychology, in which the necessity of a given brain structure for the function of interest is demonstrated through a persistent lesion-symptom association in the chronic phase, after adequate time for neuroplastic recovery.20 Because our hypothesis was specifically lateralized to the left DLPFC, we restricted our investigation to survivors of left hemisphere stroke. These approaches are expected to provide a sensitive assessment to determine whether precise localization within the left hemisphere relates to depression symptoms in chronic stroke.
Methods
Study Participants
Left hemisphere stroke survivors were recruited as part of a larger study on language abilities in this population, with the following criteria: stroke at least six months prior to enrollment, native English speaker, able to undergo MRI, comprehension adequate for following study procedures, no history of prior brain injury outside the left hemisphere, no other significant central nervous system disorder, and no history of premorbid psychiatric disorder requiring hospitalization, electroconvulsive therapy, or medication use other than common antidepressants. Presence of aphasia was not an inclusion criterion. One hundred thirteen individuals were screened; 24 subsequently declined participation; 50 were excluded (20 for lesions outside the left hemisphere, seven for other neurological disorders, 12 for MRI contraindications, three nonnative English speakers, seven for failure to complete study procedures, one for stroke less than six months prior to enrollment); 39 participated in the study. Characteristics of the group are shown in Table 1. Twelve patients had moderate-to-severe auditory comprehension deficits, and 27 had mild or no comprehension deficits, based on a Western Aphasia Battery-Revised (WAB-R) Auditory Verbal Comprehension cutoff of 7/10.21
| Characteristic | Mean (SD; Range) | Relationship With SADQ |
|---|---|---|
| Age (years) | 59.9 (9.8; 37.5–77.7) | r=–0.07, p=0.69 |
| Sex (female, male) | 14, 25 | t(37)=0.77, p=0.44 |
| Education (years) | 16.4 (3.1; 12–24) | r=–0.40, p=0.01 |
| Stroke type (ischemic, hemorrhagic) | 33, 6 | t(37)=1.39, p=0.17 |
| Time since stroke (months) | 47.2 (37.1; 7.9–151.2) | r=–0.09; p=0.58b |
| Antidepressant use (yes, no) | 12, 27 | t(37)=0.07, p=0.95 |
| Stroke size (cubic centimeters) | 127.7 (84.8; 3.5–372.0) | r=0.02, p=0.92 |
| WAB-R Aphasia Quotient (/100) | 65.5 (25.2, 13.8–96.1) | r=0.00, p=0.99 |
| WAB-R Auditory-Verbal Comprehension (/10) | 7.7 (1.8; 3.2–9.95) | r=–0.14, p=0.41 |
| WAB-R Spontaneous Speech (/20) | 13.0 (5.8; 1–19) | r=0.01, p=0.94 |
| WAB-R Naming/Word-Finding (/10) | 6.0 (3.0; 0.3–9.9) | r=–0.02, p=0.90 |
| WAB-R Repetition (/10) | 5.9 (2.9; 0.4–9.7) | r=0.09, p=0.60 |
| CLQT Attention Domain score (/215) | 154.5 (48.0; 8–206) | r=–0.07, p=0.67 |
| CLQT Executive Doman score (/40) | 22.1 (6.8; 3–35) | r=–0.27, p=0.09 |
| SAQOL Physical score (1–5) | 3.8 (0.96; 1.9–5) | r=–0.35, p=0.03 |
| SAQOL Communication score (1–5) | 2.9 (0.79; 1.3–5) | r=–0.12, p=0.45 |
| Modified Rankin (0–5) | 2.6 (0.79; 1–4) | ρ=0.03, p=0.84 |
| SADQ score (/30) | 9.51 (4.2; 2–19) |
TABLE 1. Characteristics of Participantsa
Standard Protocol Approvals, Registrations, and Patient Consents
The study was approved by the Georgetown University Medical Center and MedStar Health Research Institute institutional review boards. All participants provided written informed consent.
Assessment
A goal of this study was to include people with aphasia who are frequently excluded from studies of poststroke depression due to comprehension deficits. However, these individuals are typically excluded because there is no well-validated tool for assessing depression in people with comprehension deficits. Here, we used the Stroke Aphasic Depression Questionnaire (SADQ) to assess the severity of depression symptoms.22 The SADQ consists of 21 questions graded on a 0–3 scale with higher values indicating greater depression symptoms. Caregivers completed the questionnaire, although four participants lived independently without caregivers and filled out the SADQ themselves. Because scores based on 10 items of the SADQ have shown somewhat stronger relationships with standard measures of depression, a summary score was calculated for each individual by summing individual question scores on these 10 items.22 There is no standard cutoff score to provide a diagnosis of depression, so SADQ scores were used as continuous measures. See the Discussion section for additional considerations regarding the SADQ. Objective aphasia severity was assessed using the WAB-R, and cognitive deficits were assessed using the Cognitive Linguistic Quick Test (CLQT), a battery designed specifically to test cognition in people with aphasia.23 The modified Rankin scale was used to assess overall disability.24 To assess functional physical and communication disability as perceived by patients and caregivers, we used the physical and communication sections of the Stroke and Aphasia Quality of Life Scale-39 (SAQOL-39).25 Questions are rated on a 1–5 scale, with lower scores indicating greater disability.
MRI Acquisition and Preprocessing
High-resolution three-dimensional T1-weighted MRIs were acquired on a 3.0-T Siemens TIM Trio scanner with the following parameters: TR=1,900 ms; TE=2.56 ms; flip angle=9°; 160 contiguous 1 mm sagittal slices; field of view=250×250 mm; matrix size=246×256, voxel size=1 mm3; slice thickness=1 mm.
Lesions were delineated manually on the T1-weighted images in native space using MRIcron (http://www.mccauslandcenter.sc.edu/mricro/mricron), and checked by two neurologists (S.X. and P.E.T.) blinded to the behavioral data. Lesion masks were warped into the Montreal Neurological Institute (MNI) space using the VBM8 toolbox in SPM8 (http://www.fil.ion.ucl.ac.uk/spm) running under Matlab R2014a. Prior to preprocessing, all images were manually realigned to the anterior commissure to reduce between-subject variability, and lesion tracings were used to mask out damaged tissue to achieve accurate segmentation and spatial normalization. Data were subsequently processed via a procedure of joint spatial normalization and segmentation using the unified segmentation approach.26 The segmented maps were registered to a standard template in MNI space using 12-parameter affine linear and nonlinear warping transformation, and these warps were then applied to the lesion mask (Figure 1).
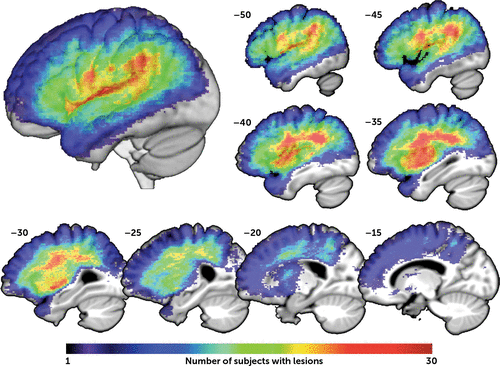
FIGURE 1. Lesion Overlap Mapa
a An overlay of the 39 participants’ lesion masks demonstrates the distribution of left hemisphere areas included in the SVR-LSM analysis. Note that the significance in the SVR-LSM analysis shown in Figure 2 is based on the degree to which patients with and without lesions at a given location differ in their behavior, not how many lesions there are overall at that location, as depicted here. A three-dimensional rendering of the SVR-LSM results is shown in the upper left. Sagittal slices are shown on the right and bottom, at the Montreal Neurological Institute x-coordinates shown. SVR-LSM=support vector regression-based lesion-symptom mapping.
Statistical Analyses
Statistical analyses of behavioral data were performed in SPSS 22 as described below. Time since stroke had a skewed distribution and was log-transformed prior to analyses. SAQOL-39 scores could not be obtained for two participants, so the group mean values were used. All tests were two-tailed.
Multivariate lesion-symptom mapping was carried out using support vector regression-based lesion-symptom mapping (SVR-LSM) running under Matlab R2014a (https://cfn.upenn.edu/~zewang/). SVR-LSM uses a machine learning-based multivariate support vector regression algorithm to find lesion-symptom relationships.19 Only voxels damaged in at least 10% of participants were included in the analysis. The direct total lesion volume control method was applied to control relationships between total lesion size, stroke distribution, and SADQ scores.19 Statistical significance was determined based on 10,000 permutations of the SADQ scores, and the resulting p-map was thresholded at p<0.001, with a cluster threshold of 200 mm3. Because SVR-LSM considers all voxels simultaneously in a single regression model, no correction for multiple comparisons is required.19
Results
As expected, participants overall reported relatively mild depression symptoms, with a median total SADQ score of 9/30 and maximum score of 19/30. There were no significant relationships with age, sex, stroke etiology, time since stroke, total stroke size, or antidepressant use, but there was a protective effect of education on depression symptoms (r=−0.40, p=0.01) (Table 1).
Most of the participants had aphasia and many had cognitive deficits, which could impact depression ratings. We therefore examined relationships between SADQ scores and WAB-R scores, and the CLQT Attention and Executive domain scores. No significant relationships or trends were identified, and examination of scatter plots revealed no apparent nonlinear relationships.
There was an expected significant positive relationship between depression scores and self-reported physical disability (SAQOL physical score r=−0.35, p=0.03), but no relationship with self-reported functional communication disability (SAQOL communication score r=−0.12, p=0.45; Table 1).
Next, the demographic, deficit, and disability measures in Table 1 were entered into a stepwise linear regression with SADQ scores as the dependent variable. Only education survived in the model as predictive of depression symptoms (model, F(1,37)=6.83, p=0.013; standardized beta for education=−0.395, t(37)=−2.61, p=0.013).
SVR-LSM was then used to determine if SADQ scores related to lesion location. To account for the relationship between education and depression symptoms, we performed the SVR-LSM analysis using the residual SADQ score after regressing out education. The SVR-LSM analysis identified a single significant cluster centered in the left DLPFC in which lesions were associated with greater depression symptoms (Figure 2; volume=1,235 mm3; peak MNI coordinates=−33, 21, 26; center=−42, 26, 20). There were no areas in which lesions related to lower SADQ scores.
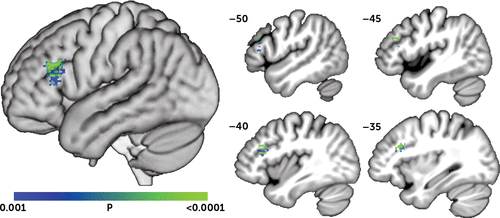
FIGURE 2. Results of SVR-LSM Analysis of Lesion Locations Associated With Depression Symptomsa
a A single cluster centered in the dorsolateral prefrontal cortex was identified in which lesions were associated with greater depression symptoms (p<0.001, cluster volume >200 mm3). A three-dimensional rendering of the SVR-LSM results is shown on the left. Sagittal slices are shown on the right, at the Montreal Neurological Institute x-coordinates shown. SVR-LSM=support vector regression-based lesion-symptom mapping.
Since patients with moderate-severe comprehension deficits would typically be excluded from a study like this one, we next tested the relationship between SADQ scores and DLPFC damage independently in patients with moderate-severe and mild-to-no comprehension deficits. Independent ANOVAs demonstrated that, controlling for education and total lesion volume, SADQ scores related to DLPFC damage (>1/2 of the voxels in the SVR-LSM cluster lesioned) in both groups (moderate-severe comprehension deficit group, F(1,8)=17.09, p=0.003; mild-to-no comprehension deficit group, F(1,23)=9.49, p=0.005).
Discussion
Using a new multivariate lesion-symptom mapping method, we found that lesions in the DLPFC were associated with increased depression symptoms in chronic left hemisphere stroke survivors. These results correspond with prior evidence that left frontal strokes are associated with PSD,10–14,17 and with prior evidence that DLPFC damage from other causes relates to depression symptoms.6,8,9 This study extends these findings by demonstrating a precise localization of the effect within the left DLPFC using an advanced imaging analysis method.
Dysfunction of the left DLPFC has previously been implicated in major depression by studies using a variety of experimental methods.27 The left DLPFC is thought to control negative affect through contextual processing and reappraisal/suppression strategies, and is hypoactive in functional imaging studies of individuals with major depression,5 particularly during emotional judgment tasks.28 Moreover, excitation of the left DLPFC using high-frequency repetitive transcranial magnetic stimulation improves depression and is now cleared by the U.S. Food and Drug Administration for adjunctive treatment of major depression.29,30 Considering the convergence between these findings on major depression and the results of the current study on poststroke depression symptoms, it seems that both may share a common pathophysiological mechanism, at least with regard to the contributions of left DLPFC dysfunction. This convergence also provides support for the use of lesion studies to understand the neuroanatomy of major depression, and lends credence to the notion that some brain stimulation treatments for major depression may also be useful for PSD.31
The question of whether lesion location contributes to PSD has been controversial, with prior studies often providing conflicting results or revealing no association at all.32 This lack of consistency has likely arisen from variation in experimental parameters, including the measurement instruments, the populations examined, and the timing and setting of the testing.3,4,33 In addition, most prior studies have used fairly coarse methods to localize and categorize lesions, with only two recent studies using modern lesion-symptom mapping approaches. One of these recent studies used graph theory analysis of diffusion tensor imaging data to demonstrate that damage within a broad “depression-related network” was associated with increased rates of PSD.34 The other recent notable study used a univariate voxel-wise approach with T1-weighted images, and failed to find a relationship between lesion location and PSD in a cohort of 55 subacute stroke survivors with left, right, or bilateral strokes.35 The authors attribute the negative results to methodological limitations, primarily an overly diverse group of participants who did not have sufficient overlap in lesion locations to provide adequate power for lesion-symptom mapping.
Aside from using a more sensitive multivariate lesion-symptom mapping method than this recent study,19 we also restricted our analysis to left hemisphere stroke survivors only, providing more overlap of lesions in our group and higher power for lesion-symptom mapping. We also examined patients in the chronic phase of recovery, which may have provided sufficient time for normal grief and adjustment responses to resolve, yielding greater specificity for neurologically driven depression symptoms in our measurements. Because aphasia is a common complicating factor in prior studies of PSD, and aphasia occurs in most left hemisphere stroke survivors,36 we also used a different measure of depression symptoms, the SADQ, which was designed for use in stroke survivors with aphasia (however, see the discussion of the limitations of this instrument, below). Moreover, we performed detailed aphasia and cognitive testing to rule out any impact of these deficits on depression symptom ratings prior to examining relationships between depression symptoms and lesion location. Finally, whereas most prior studies investigating localization of PSD have examined lesion location in relation to diagnosis of PSD, we instead examined lesion location in relation to the continuous score on the SADQ, i.e., symptoms of depression, not diagnosis of PSD. This approach acknowledges that depression symptoms after stroke occur on a continuum37 and likely provided greater statistical power in the lesion-symptom mapping analysis, at the cost of reducing our ability to determine whether findings are clinically significant.
In addition to the contribution of left DLPFC damage to depression symptoms, we also confirmed prior evidence that physical disability relates to depression,1 and identified a protective influence of education. This latter result has not been widely identified in other studies of PSD. However, lower education and correspondingly lower socioeconomic status is associated with higher rates of depression in the general population,38 as well as lower likelihoods of seeking mental health services in older adults.39 Level of education in our sample likely serves as a surrogate marker for socioeconomic status and consequently access to support services or social support networks, which help to mitigate PSD symptoms.40
Some limitations of this study are worth noting. First, the SADQ was selected as the measure of depression symptoms to facilitate the inclusion of participants with comprehension deficits. Most prior studies of PSD have excluded these individuals because there are no adequate measures to assess depression in this group. Indeed, the validity of the SADQ has never been clearly established. Furthermore, because the SADQ is administered to caregivers, it lacks questions about the patient’s subjective experience of sadness, which is critical to the diagnosis of depression. One might reasonably question whether the localization observed here truly relates to depression versus other symptoms measured by the SADQ. We are somewhat reassured by our results, however, which are consistent with those of many prior studies on PSD that excluded people with comprehension deficits and used better-validated depression measures. As such, we suggest that despite the significant limitations associated with using the SADQ, our results tentatively fill a gap in the literature by suggesting that the localization of PSD observed in other studies may generalize to stroke survivors with comprehension deficits.
Other limitations include the sample size, which may not have provided sensitivity to detect all possible lesion-symptom relationships. Our analysis is only sensitive to effects in a restricted part of the brain, as shown in Figure 2. The context in which the study was conducted made some standard measures such as the National Institutes of Health Stroke Scale unavailable. Although we excluded individuals with a history of severe psychiatric illness, we did not assess premorbid depression symptoms. The lack of premorbid depression assessment is unlikely to have produced a false-positive lesion-symptom mapping result, but poststroke depression is strongly related to premorbid depression, and its occurrence in this context may relate differently to lesion location. Functional disability was mainly measured using self-report scales, which could be influenced by anosognosia. It should also be noted that overall scores on the SADQ were fairly low in our sample, consistent with prior evidence that chronic left hemisphere stroke in general is not associated with PSD.4,15 Thus, while the current study demonstrates a direct neurological effect of left DLPFC damage on depression symptoms, the clinical importance of the effect to the occurrence or severity of PSD remains unclear.
In summary, we used a new multivariate lesion-symptom mapping method to examine localization of depression symptoms in chronic left hemisphere stroke survivors, and found that lesions of the left DLPFC are related to increased depression symptoms. These results provide evidence of convergent biological mechanisms for poststroke depression symptoms and major depression, and demonstrate a direct neurological contribution to depression symptoms after left hemisphere stroke.
1 : Natural history, predictors and outcomes of depression after stroke: systematic review and meta-analysis. Br J Psychiatry 2013; 202:14–21Crossref, Medline, Google Scholar
2 : Depression is an independent predictor of poor long-term functional outcome post-stroke. Eur J Neurol 2001; 8:315–319Crossref, Medline, Google Scholar
3 : Post stroke depression: epidemiology, pathophysiology, and biological treatment. Biol Psychiatry 2002; 52:253–264Crossref, Medline, Google Scholar
4 : Lesion location and poststroke depression: systematic review of the methodological limitations in the literature. Stroke 2004; 35:794–802Crossref, Medline, Google Scholar
5 : Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of base line activation and neural response data. Am J Psychiatry 2012; 169:693–703Crossref, Medline, Google Scholar
6 : Neuropathological evidence for ischemia in the white matter of the dorsolateral prefrontal cortex in late-life depression. Int J Geriatr Psychiatry 2003; 18:7–13Crossref, Medline, Google Scholar
7 : Reduction of dorsolateral prefrontal cortex gray matter in late-life depression. Psychiatry Res 2011; 193:1–6Crossref, Medline, Google Scholar
8 : Depression in patients with acute traumatic brain injury. Am J Psychiatry 1992; 149:918–923Crossref, Medline, Google Scholar
9 : Distinct regions of prefrontal cortex mediate resistance and vulnerability to depression. J Neurosci 2008; 28:12341–12348Crossref, Medline, Google Scholar
10 : Post-stroke affective or apathetic depression and lesion location: left frontal lobe and bilateral basal ganglia. Eur Arch Psychiatry Clin Neurosci 2007; 257:149–152Crossref, Medline, Google Scholar
11 : Association of frontal subcortical circuits infarcts in poststroke depression: a magnetic resonance imaging study of 591 Chinese patients with ischemic stroke. J Geriatr Psychiatry Neurol 2011; 24:44–49Crossref, Medline, Google Scholar
12 : Mood disorders in stroke patients. Importance of location of lesion. Brain 1984; 107:81–93Crossref, Medline, Google Scholar
13 : Depression in acute and chronic aphasia: symptoms, pathoanatomical-clinical correlations and functional implications. J Neurol Neurosurg Psychiatry 1993; 56:672–678Crossref, Medline, Google Scholar
14 : Two-year longitudinal study of post-stroke mood disorders: dynamic changes in correlates of depression at one and two years. Stroke 1987; 18:579–584Crossref, Medline, Google Scholar
15 : Post-stroke depression and lesion location: a systematic review. J Neurol 2015; 262:81–90Crossref, Medline, Google Scholar
16 : The relationship between poststroke depression and lesion location in long-term follow-up. Biol Psychiatry 1999; 45:187–192Crossref, Medline, Google Scholar
17 : Post-stroke depression: relationships with morphological damage and cognition over time. Ital J Neurol Sci 1995; 16:209–216Crossref, Medline, Google Scholar
18 : Using human brain lesions to infer function: a relic from a past era in the fMRI age? Nat Rev Neurosci 2004; 5:813–819Crossref, Medline, Google Scholar
19 : Multivariate lesion-symptom mapping using support vector regression. Hum Brain Mapp 2014; 35:5861–5876Crossref, Medline, Google Scholar
20 Heilman MKM, Valenstein E (eds): Clinical Neuropsychology. Oxford, United Kingdom, Oxford University Press, 2011Google Scholar
21 Kertesz A: Western Aphasia Battery Test Manual. New York, Grune & Stratton, 1982Google Scholar
22 : The assessment of depression in aphasic stroke patients: the development of the Stroke Aphasic Depression Questionnaire. Clin Rehabil 1998; 12:506–513Crossref, Medline, Google Scholar
23 Helm-Estabrooks N (ed): Cognitive Linguistic Quick Test: Examiner’s Manual San Antonio, Tex, Psychological Corporation, 2001Google Scholar
24 : Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988; 19:604–607Crossref, Medline, Google Scholar
25 : Stroke and Aphasia Quality of Life Scale-39 (SAQOL-39): evaluation of acceptability, reliability, and validity. Stroke 2003; 34:1944–1950Crossref, Medline, Google Scholar
26 : Unified segmentation. Neuroimage 2005; 26:839–851Crossref, Medline, Google Scholar
27 : The functional neuroanatomy of depression: distinct roles for ventromedial and dorsolateral prefrontal cortex. Behav Brain Res 2009; 201:239–243Crossref, Medline, Google Scholar
28 : Imbalance between left and right dorsolateral prefrontal cortex in major depression is linked to negative emotional judgment: an fMRI study in severe major depressive disorder. Biol Psychiatry 2008; 63:369–376Crossref, Medline, Google Scholar
29 : Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry 2007; 62:1208–1216Crossref, Medline, Google Scholar
30 : Rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug-resistant depression. Lancet 1996; 348:233–237Crossref, Medline, Google Scholar
31 : Mood and cognitive effects of transcranial direct current stimulation in post-stroke depression. Neurocase 2011; 17:318–322Crossref, Medline, Google Scholar
32 : Depression after stroke and lesion location: a systematic review. Lancet 2000; 356:122–126Crossref, Medline, Google Scholar
33 : Poststroke depression: prevalence, diagnosis, treatment, and disease progression. Biol Psychiatry 2003; 54:376–387Crossref, Medline, Google Scholar
34 : A significant risk factor for poststroke depression: the depression-related subnetwork. J Psychiatry Neurosci 2015; 40:259–268Crossref, Medline, Google Scholar
35 : Imaging predictors of poststroke depression: methodological factors in voxel-based analysis. BMJ Open 2014; 4:e004948Crossref, Medline, Google Scholar
36 : Poststroke aphasia : epidemiology, pathophysiology and treatment. Drugs Aging 2005; 22:163–182Crossref, Medline, Google Scholar
37 : The Post-Stroke Depression Rating Scale: a test specifically devised to investigate affective disorders of stroke patients. J Clin Exp Neuropsychol 1997; 19:340–356Crossref, Medline, Google Scholar
38 Berkman LF, Breslow L (eds): Health and Ways of Living: The Alameda County Study. New York, Oxford University Press, 1983Google Scholar
39 : Utilization of mental health care services among older adults with depression. J Clin Psychol 2006; 62:299–312Crossref, Medline, Google Scholar
40 : Psychological distress after stroke and aphasia: the first six months. Clin Rehabil 2010; 24:181–190Crossref, Medline, Google Scholar



