Frontotemporal Lobar Degeneration-Modified Clinical Dementia Rating (FTLD-CDR) Scale and Frontotemporal Dementia Rating Scale (FRS) Correlation With Regional Brain Perfusion in a Series of FTLD Patients
Abstract
Severity assessment scales for frontotemporal lobar degeneration (FTLD) have been recently introduced. In the present study, the authors examined whether the FTLD-modified Clinical Dementia Rating (FTLD-CDR) scale and the Frontotemporal Dementia Rating Scale (FRS) correlated with regional brain perfusion in Greek FTLD patients. A total of 47 behavioral variant frontotemporal dementia (bvFTD) patients and 33 primary progressive aphasia (PPA) patients were assessed for demographic data, cognitive reserve (CR), and severity of dementia and underwent brain single-photon emission computed tomography. Both scales were valid in the bvFTD group, predicting frontal lobe perfusion. In the PPA group, both scales were found to predict temporal hypoperfusion only after accounting for CR, which could imply a potential role for reserve in PPA patients.
Frontotemporal lobar degeneration (FTLD) represents a heterogeneous clinical, genetic, and pathological neurodegenerative entity, especially prevalent in young-onset dementia patients.1 It consists of different clinical syndromes (namely, behavioral variant frontotemporal dementia [bvFTD], nonfluent-agrammatic variant of primary progressive aphasia [naPPA], semantic variant PPA [svPPA], and logopenic variant PPA [lvPPA]), with diverse pathological substrates (even Alzheimer’s disease [AD] for most lvPPA cases) and manifests with marked behavioral and personality disorders, language deficits, and social misconduct.2 Furthermore, disease progression varies greatly, with a proportion of patients remaining relatively stable for many years.3 Due to the aforementioned diversities, standard screening tools, as well as neuropsychological tests and severity assessment scales, commonly used in AD fail to capture the unique behavioral and language features of FTLD, causing serious methodological problems in designing clinical therapeutic trials.4
In the past 5 years, there has been important progress in managing these problems, toward a better characterization of FTLD-spectrum disorders. Firstly, new revised diagnostic criteria for bvFTD and PPA subtypes were introduced,5,6 which enable clinicians to recognize different patterns of behavioral and language disturbances and reach to a diagnosis, even in mild cases. Moreover, imaging confirmation of frontal and/or temporal damage (either by means of structural or functional brain imaging) was used in the diagnostic algorithm to increase the level of diagnostic certainty.7 Secondly, the role of cognitive reserve (CR), already established as a mediator of clinical expression of brain pathology in AD,8 has been increasingly studied in bvFTD, as well. Few studies have shown that higher educational- or occupational-level bvFTD patients cope more efficiently with greater brain burden than lower ones,9,10 possibly accounting for the discrepancy between severity of disease and extent of brain pathology. Finally, new standardized tools, such as the Frontal Behavioral Inventory,11 and clinical severity assessment scales have been established to raise specificity for FTLD deficits. In particular, the FTLD-modified Clinical Dementia Rating (FTLD-CDR) scale was introduced4 to expand the standard CDR scale, already used in AD. FTLD-CDR is thought to represent a severity scale specific for FTLD, with evidence from few studies12–14 but only one with brain perfusion data.12 On the other hand, FRS is caregiver-based, incorporates performance in activities of daily living, and could aid in determining disease staging and progression.15
In the present study, we aimed to examine whether FTLD-CDR and FRS scores were correlated with regional cerebral blood flow (rCBF) in the frontotemporal lobes in a large series of clinically defined bvFTD and PPA Greek patients.
Methods
Patients
Eighty patients were enrolled in the study from January 2012 to September 2014, in the neurology department of a tertiary referral center, covering most of Northern-Central Greece. Patients were classified as possible bvFTD or had a clinical diagnosis of naPPA/svPPA according to recently published criteria,5,6 based on clinical and neuropsychological assessment. Neuropsychological evaluation consisted of the Mini-Mental State Examination (MMSE), the Addenbrooke’s Cognitive Examination Revised (ACE-R), the Trail-Making Test A and B, the Story Recall Test, the Rey-Osterrieth complex figure test, Boston Diagnostic Aphasia Examination, and lastly the Frontal Behavioral Inventory for possible neuropsychiatric disturbances. Structural brain imaging (mainly CT or MRI when available), as well as a brain single-photon emission computed tomography (SPECT) perfusion study, was performed for all patients. Exclusion criteria included structural brain lesions, severe cerebrovascular disorders or stroke, hydrocephalus, and major psychiatric comorbidities.
All caregivers and patients were made fully aware of the aims of the study and gave written informed consent. The study protocol was approved by the local university committee, and the principles of the Helsinki Declaration were followed.
Questionnaires
Information on patient demographic characteristics (age, education, and occupation, cognitive reserve level) was gathered from an informed caregiver. Cognitive reserve level was assessed using the Greek version of the Cognitive Reserve Index questionnaire (CRIq), recently adapted for the Greek population.16 The CRIq provides a total score (the Cognitive Reserve Index [CRI]) and three subscores, namely, CRI-Education, CRI-Working Activity, and CRI-Leisure Time, as it gathers information on educational level (formal and informal), occupational attainment, and participation in cognitively demanding leisure time activities, encompassing the amount of time spent on each activity throughout a person’s lifespan.17
Disease severity was estimated using FTLD-CDR, which is the classic six-domain CDR plus two domains (behavior and language), specific for FTLD. A rating of “0” indicates normal behavioral or language status, while ratings of “1,” “2,” and “3” denote mild, modest, and severe deficits, respectively. Total score was calculated after summing individual box scores, by two certified CDR raters (PM, PI) blinded to the SPECT results.
We also administered the FRS to patients’ caregivers; it accounts for seven aspects of the patient’s personality and daily activities: behavior, outing and shopping, household chores and telephone, finances, medications, meal preparation and eating, and, finally, self-care and mobility. Scoring was made on a 3-point scale (“never,” “sometimes,” “all the time”) by recognizing the frequency of a specific disorder.
Brain SPECT Perfusion Study
Patients were administered an intravenous injection of 740 MBq Tc-99m-HMPAO (exametazime), while lying still with their eyes open in a dimly lit and quiet room. After 30 minutes, they were imaged with a single-head gamma camera (Philips, with Pegasys processor). During the study, 120 images were acquired, each of a 20-second duration (total scan time=40 minutes). Images were reconstructed using a Butterworth filter (cut-off frequency=0.35 cycles/cm, order=5) and reorientated in the transverse, sagittal, and coronal levels. Afterward, rectangular (4×4 pixels) regions of interest (ROI) were defined for various cortical areas in each hemisphere (visual, parietal, temporal, and frontal lobe), as well as for the thalami, brainstem, cerebellar hemispheres, and caudate nuclei. The rCBF for each ROI was estimated using a perfusion index, calculated as cortex-to-cerebellum ratio.
Statistical Analysis
Means and standard deviations were calculated for all continuous variables. Differences between groups were examined using t tests for continuous and chi-square tests for categorical variables. Lastly, we conducted a hierarchical multiple linear regression analysis with rCBF scores in both frontal and temporal lobes as the dependent variable, FTLD-CDR or FRS scores as independent variables, and age, sex, education, and duration of illness (defined as time since symptoms onset) as covariates. Especially in the PPA group, moderated regression and slope analysis revealed that CRI scores significantly interacted with severity scales scores in predicting rCBF scores. Therefore, we modified our basic analysis by controlling for CRI status instead of education. This approach enabled us to specifically examine the unique relationship of the FTLD-CDR and FRS with rCBF, accounting for the effect of all other possible predictor variables. All analyses were made using SPSS 22.0, and the alpha level was set a priori at 0.05 (two-tailed). In the analyses of relationships of rCBF with FTLD-CDR or FRS, each test involved multiple comparisons (i.e., four ROIs). Accordingly, the alpha level was Bonferroni corrected a priori to 0.0125.
Results
Eighty patients (53.8% men) fulfilled the inclusion criteria and were enrolled in the study. The mean age of the entire cohort was 67.83 years, with a mean of 2.81 years since symptom onset and 8.94 years of formal education. Forty-seven (58.8%) patients were diagnosed with bvFTD and 33 (41.2%) with PPA (naPPA, N=25, and svPPA, N=8, with the two subgroups not differing in any demographic or basic neuropsychological variables). The mean age in the bvFTD group was 67.66 years, mean time since symptom onset was 3.11 years, and formal education level was 8.11 years (mean age=68.06, 2.39, 10.18 years, respectively, for the PPA group, with the two groups being statistically different in years of education; t=2.392, df=78, p=0.019) (Table 1). The two groups did not differ in terms of general cognitive ability measures (MMSE, ACE-R), but bvFTD patients had higher scores on the Frontal Behavioral Inventory (t=3.195, df=78, p=0.002). Furthermore, MMSE and ACE-R scores did not show any correlation with frontotemporal perfusion, while classic CDR scores correlated with parietal perfusion only.
| Characteristic | All Patients (N=80) | bvFTD Patients (N=47) | PPA Patients (N=33) | p |
|---|---|---|---|---|
| Age (years) | 67.83 (8.3) | 67.66 (8.21) | 68.06 (8.56) | 0.833 |
| Gender (male %) | 53.8 | 53.2 | 54.5 | 0.905 |
| Education (years) | 8.94 (4.01) | 8.06 (3.67) | 10.18 (4.21) | 0.019 |
| Duration of illness (years) | 2.81 (1.77) | 3.11 (2) | 2.39 (1.3) | 0.057 |
| FTLD-CDR | 9.36 (4.96) | 10.3 (4.9) | 8.03 (4.8) | 0.043 |
| FRS | –0.06 (1.52) | –0.45 (1.53) | 0.48 (1.35) | 0.006 |
| CRI | 91.11 (10.6) | 88.11 (8.74) | 95.39 (11.64) | 0.002 |
| CRI-Education | 94.86 (7.11) | 93.34 (5.94) | 97.03 (8.12) | 0.03 |
| CRI-Working activity | 96.24 (14.39) | 93 (11.57) | 100.85 (16.79) | 0.024 |
| CRI-Leisure time | 88.86 (8.57) | 86.74 (7.32) | 91.88 (9.4) | 0.008 |
TABLE 1. Demographic and Clinical Characteristicsa
In terms of CR status, PPA patients had statistically significant higher total CRI, CRI-Education, CRI-Working Activity, and CRI-Leisure Time than bvFTD patients (t=3.198, df=78, p=0.002, t=2.226, df=78, p=0.03, t=2.326, df=78, p=0.024, and t=2.745, df=78, p=0.008, respectively) (Table 1).
The mean FTLD-CDR score was 9.36 for the entire cohort, 10.3 for the bvFTD group, and 8.03 for the PPA group (t=−2.054, df=78, p=0.043 for the difference between groups). Accordingly, the mean FRS score was –0.06 for the entire cohort, –0.45 for the bvFTD group, and 0.48 for the PPA group (t=2.809, df=78, p=0.006 for the difference between groups) (Table 1).
FTLD-CDR scores were strongly and inversely correlated with FRS scores (r=−0.813 p<0.001 for the bvFTD and r=−0.778 p<0.001 for the PPA group), with higher scores in FTLD-CDR correlating with lower scores in FRS, both indicating greater severity of disease.
In the bvFTD group, based on the results from the multiple linear regression analysis after accounting for the effect of age, duration of illness, gender, and education, FTLD-CDR scores were inversely correlated with rCBF in both left and right frontal cortices (F=2.508, df=5, 40, p=0.011, R2=0.239 for the left and F=5.677, df=5, 40, p<0.001, R2=0.415 for the right frontal cortex). Similarly, FRS scores were positively correlated with rCBF in the right frontal cortex (F=6.081, df=5, 40, p<0.001, R2=0.426). In the left frontal cortex, FRS-rCBF correlation did not reach the a priori corrected level of statistical significance (F=2.387, df=5, 40, p=0.014, R2=0.230).
In PPA patients, multiple linear regression analysis, with age, gender, years of formal education, and duration of illness as covariates, failed to demonstrate any correlation between FTLD-CDR or FRS scores and rCBF in any of the brain regions of interest, namely, left and right temporal cortices. However, moderated linear regression analysis revealed a large and significant correlation of CRI scores with rCBF (Figure 1), so we conducted a new multiple regression analysis substituting years of formal education with CRI status (high versus low reserve, with CRI median=96 serving as the cut-off point). In that analysis, FTLD-CDR correlated with bilateral temporal cortex hypoperfusion (F=3.364, df=5, 26, p=0.004, R2=0.393, for the left and F=2.676, df=5, 26, p=0.011, R2=0.331, for the right) and FRS with right temporal cortex hypoperfusion (F=2.821, df=5, 26, p=0.009, R2=0.343). Subsequent slope analysis specified this effect to the high-reserve group of PPA patients (t=−2.259, df=31, p=.03) (Figure 2).
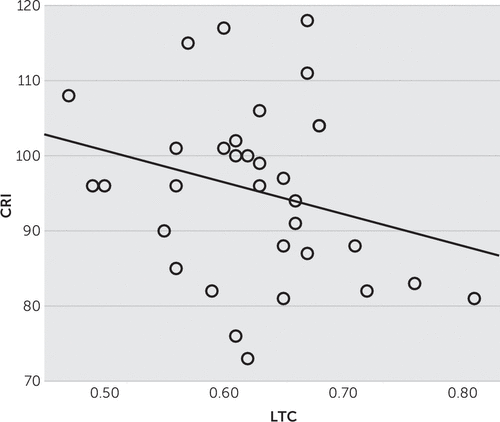
FIGURE 1. Regional Cerebral Blood Flow (rCBF) of the Left Temporal Cortex (LTC) and Cognitive Reserve Index (CRI) Scores in Primary Progressive Aphasia (PPA) Patients Showing the Inverse Correlation Between CRI and LTC

FIGURE 2. Frontotemporal Lobar Degeneration-Modified Clinical Dementia Rating (FTLD-CDR) Scores and Regional Cerebral Blood Flow (rCBF) of the Left Temporal Cortex (LTC) Stratified by Cognitive Reserve Index (CRI) Status (high versus low reserve) Showing the Inverse Correlation Between FTLD-CDR and LTC in High-Reserve Primary Progressive Aphasia (PPA) Patients
Discussion
This is the first brain SPECT perfusion study in a large series of clinically defined FTLD patients in Greece. This is also the first attempt to test FRS against an in vivo proxy of brain damage (construct validity), as well as against another severity assessment scale (convergent validity).
In bvFTD patients, FTLD-CDR scores were inversely correlated with rCBF in the left and right frontal cortices (higher scores correlating with lower perfusion). FRS scores on the other hand, were positively correlated with rCBF in the right frontal cortex (higher scores correlating with higher perfusion). Both scales, therefore, were validated against an in vivo proxy of brain pathology, namely, cerebral blood perfusion in regions commonly affected in bvFTD, after accounting for the possible effect of age, gender, education, and duration of illness. These results are in line with the study of Borroni et al.12 in 77 bvFTD and 20 svPPA patients, where FTLD-CDR scores inversely correlated with brain perfusion in frontal and temporal regions bilaterally. FTLD-CDR was also proven useful in correctly classifying FTLD patients in a recent Spanish study.13
In PPA patients, different at baseline from bvFTD ones (better educated and less severely demented), FTLD-CDR and FRS scores failed to show any correlation with rCBF in any brain region on initial analysis. However, when we took into account the CRI status instead of years of formal education, FTLD-CDR scores inversely correlated with bilateral temporal cortex rCBF and FRS scores with right temporal cortex rCBF.
These results are important in several ways. Firstly, they confirm validity of the FRS in bvFTD patients, both against a proxy of brain pathology and another clinical severity assessment scale. The fact that FRS scores did not show statistically significant correlation with rCBF in the left frontal lobe could be due to the small effect size (Cohen’s f2=0.158), resulting in low power of the study to detect the specific difference (62 bvFTD patients required for a 0.7 study power level). FRS was designed as a caregiver-based instrument, taking into account a lot of aspects of behavioral conduct and performance in activities of daily living and could serve as a tool for better characterization and monitoring progression in FTLD patients. Interestingly, in the PPA group, FRS scores correlated only with right temporal hypoperfusion, which could be due to the fact that FRS deals a lot with the behavioral and daily activities aspects and does not address separately the language disturbances. Secondly, this study provides further evidence of the utility and validity of the FTLD-CDR in bvFTD patients. The only other brain perfusion validation study reported results in the whole group (bvFTD and svPPA) and found correlations with regional blood flow in the temporal cortices as well.12 In our study, the PPA group was more heterogeneous (consisting of naPPA and svPPA), less severely demented, and better educated than the bvFTD group. Additionally, a study by Kim et al.14 in svPPA patients showed that only the language domain of the FTLD-CDR could predict severity of glucose hypometabolism, whereas neither the behavioral domain nor the global score reflected disease progression. Moreover, in our series, CRI status significantly correlated with temporal brain perfusion. These factors could explain the fact that only in further analysis, accounting for CRI status, did scale scores correlate with temporal cortex blood perfusion. Interestingly, this correlation was not evident in the low-CR PPA patients, probably because they exhibited great disease severity even with mild or modest hypoperfusion, or because of a ceiling effect, given the fact that overall the low-CR patients were more severely demented (Figure 2). This also serves as an indirect preliminary evidence of a possible protective role of CR in PPA, with less severe disease in high-reserve patients than low-reserve ones, for the same level of temporal lobe perfusion (Figure 2).
CR hypothesis has been repeatedly tested successfully in AD18 but only recently systematically assessed in FTLD. In particular, we found only a few studies, mainly in bvFTD patients, using either composite neuropsychological measures9 or AD-specific severity scales10 and utilizing various proxies for assessing reserve status.9,10,12 This is the second study using FTLD-specific staging scales to imply that CR (via CRIq, a recently constructed comprehensive instrument) could be a mediator of clinical expression of temporal cortex pathology in PPA patients. In the very few relevant studies, CR was also proven to mitigate the impact of brain lesions in PPA patients.19,20 Moreover, aphasia was less possible in high educational attainment stroke patients21, and occupational attainment more depending on verbal abilities was shown to correlate with right lateralized temporal atrophy, suggesting left temporal reserve.22 In conclusion, the results of our study for the PPA group corroborate existing evidence of the CR theory in language disorders.
There are some limitations in our study. Firstly, the SPECT image analysis performed did not allow for more detailed and specific results in terms of Brodmann’s areas affected. There were neither pathological diagnoses nor genetic testing results (although there were a few cases with a family history of dementia), and finally, conclusions on the specific effect of CR to each PPA subgroup could not be drawn due to the small number of svPPA patients. On the other hand, the large sample, the robustness of the statistic methodology, the direct comparison of FTLD-CDR and FRS, as well as inclusion of CR status, could serve to strengthen the results.
In conclusion, FTLD-CDR and FRS were proven valid in measuring disease severity in Greek FTLD patients. Moreover, CR was shown to possibly mediate clinical expression of disease pathology in PPA patients. Recognition of such a role for CR could result in more appropriate patient selection and interpretation of findings in future clinical trials.
1 : Clinical, genetic and pathological heterogeneity of frontotemporal dementia: a review. J Neurol Neurosurg Psychiatry 2011; 82:476–486Crossref, Medline, Google Scholar
2 : Phenotypic variety in the presentation of frontotemporal lobar degeneration. Int Rev Psychiatry 2013; 25:138–144Crossref, Medline, Google Scholar
3 : Progression in behavioral variant frontotemporal dementia: a longitudinal study. JAMA Neurol 2015; 72:1501–1509.Crossref, Medline, Google Scholar
4 : Development of methodology for conducting clinical trials in frontotemporal lobar degeneration. Brain 2008; 131:2957–2968Crossref, Medline, Google Scholar
5 : Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011; 134:2456–2477Crossref, Medline, Google Scholar
6 : Classification of primary progressive aphasia and its variants. Neurology 2011; 76:1006–1014Crossref, Medline, Google Scholar
7 : Neuroimaging in frontotemporal dementia. Int Rev Psychiatry 2013; 25:221–229Crossref, Medline, Google Scholar
8 : Cognitive reserve. Neuropsychologia 2009; 47:2015–2028Crossref, Medline, Google Scholar
9 : Brain reserve capacity in frontotemporal dementia: a voxel-based 18F-FDG PET study. Eur J Nucl Med Mol Imaging 2007; 34:1082–1087Crossref, Medline, Google Scholar
10 : Relationship between occupation attributes and brain metabolism in frontotemporal dementia. Neuropsychologia 2011; 49:3699–3703Crossref, Medline, Google Scholar
11 : Behavioral disturbances differentiate frontotemporal lobar degeneration subtypes and Alzheimer’s disease: evidence from the Frontal Behavioral Inventory. Int J Geriatr Psychiatry 2013; 28:939–946Crossref, Medline, Google Scholar
12 : The FTLD-modified Clinical Dementia Rating scale is a reliable tool for defining disease severity in frontotemporal lobar degeneration: evidence from a brain SPECT study. Eur J Neurol 2010; 17:703–707Crossref, Medline, Google Scholar
13 : Utility of the Spanish version of the FTLD-modified CDR in the diagnosis and staging in frontotemporal lobar degeneration. J Neurol Sci 2014; 344:63–68Crossref, Medline, Google Scholar
14 : Clinical staging of semantic dementia in an FDG-PET study using FTLD-CDR. Dement Geriatr Cogn Disord 2012; 34:300–306Crossref, Medline, Google Scholar
15 : Clinical staging and disease progression in frontotemporal dementia. Neurology 2010; 74:1591–1597Crossref, Medline, Google Scholar
16 : Adaptation of the Cognitive Reserve Index Questionnaire (CRIq) for the Greek population. Neurol Sci 2016; 37:633–636Crossref, Medline, Google Scholar
17 : Cognitive Reserve Index questionnaire (CRIq): a new instrument for measuring cognitive reserve. Aging Clin Exp Res 2012; 24:218–226Medline, Google Scholar
18 : Brain reserve and dementia: a systematic review. Psychol Med 2006; 36:441–454Crossref, Medline, Google Scholar
19 : Non-fluent progressive aphasia: cerebral metabolic patterns and brain reserve. Brain Res 2007; 1133:178–185Crossref, Medline, Google Scholar
20 : Nature versus nurture in frontotemporal lobar degeneration: the interaction of genetic background and education on brain damage. Dement Geriatr Cogn Disord 2012; 33:372–378Crossref, Medline, Google Scholar
21 : Educational history is an independent predictor of cognitive deficits and long-term survival in postacute patients with mild to moderate ischemic stroke. Stroke 2012; 43:2931–2935Crossref, Medline, Google Scholar
22 : Occupation attributes relate to location of atrophy in frontotemporal lobar degeneration. Neuropsychologia 2010; 48:3634–3641Crossref, Medline, Google Scholar



