Treating Alcohol Use Disorder in U.S. Veterans: The Role of Traumatic Brain Injury
Abstract
Objective:
The authors examined the efficacy of valproate to reduce relapse to heavy drinking among veterans with alcohol use disorder (AUD) and neuropsychiatric comorbidities and whether antecedent traumatic brain injury (TBI) or posttraumatic stress disorder (PTSD) affected treatment response.
Methods:
Participants were male veterans 18–60 years old with an AUD and no other substance use besides nicotine or cannabis. Sixty-two patients were randomly assigned to receive either valproate or naltrexone. Participants were evaluated at baseline and followed weekly for 24 weeks. All participants received standardized psychosocial interventions as well as treatment for coexistent psychiatric conditions.
Results:
During the follow-up period, nine study subjects in the naltrexone group and 14 in the valproate group relapsed to heavy drinking, but the difference did not reach statistical significance. Participants with a history of moderate to severe TBI were more likely to relapse to heavy drinking compared with those with no TBI (hazard ratio=4.834, 95% CI=1.103–21.194, p=0.033). PTSD status did not significantly affect outcome.
Conclusions:
Intensive outpatient programs are efficacious alternatives to treat AUD in veterans, although the role of pharmacological treatment is not completely elucidated. Glutamatergic agents appear to be less effective than opiate antagonists to prevent relapse to heavy drinking and to increase cumulative abstinence. Future studies should examine novel pharmacological and nonpharmacological options.
The 1-year and lifetime prevalence of alcohol use disorders (AUD) has been estimated to be 13.9% and 29.1%, respectively, in the adult U.S. civilian population (1). In veterans, the lifetime prevalence of AUDs was estimated to be 32%, whereas estimates of their past-year prevalence vary between 5% and 21% (2). In addition to this high prevalence, veterans are vulnerable to develop complex forms of addictive disorders characterized by the presence of coexistent psychiatric conditions, particularly posttraumatic stress disorder (PTSD) and mood disturbance (3). Furthermore, the high frequency of traumatic brain injury (TBI) among veterans conveys an additional risk of developing refractory forms of AUD, probably related to the disruption of prefrontal circuits mediating reward expectation, impulse control, and emotional regulation (4).
A history of TBI is frequently present among clients of substance abuse treatment programs (5). A recent survey of 7,784 patients enrolled in state-funded treatment programs in Kentucky showed that 31.7% reported a lifetime history of one or more TBIs with loss of consciousness. In this sample, patients with recurrent TBI had more severe psychiatric problems, more cognitive deficits, and greater impulsivity (6). Consistent with this report, 36 of 50 seriously ill patients (72%) admitted to a specialized dual diagnosis program were shown to have a history of TBI (7) and had worse treatment outcomes than patients without TBI (7).
The relationship between TBI and alcohol misuse is bidirectional. It is well known that alcohol misuse is a risk factor for TBI, as evidenced by the fact that alcohol intoxication is involved in approximately 50% of TBI cases in civilian settings (8). On the other hand, previous epidemiological studies reported that TBI patients without a history of misuse have a greater risk of developing AUD than control subjects during the first 3 years following trauma (9, 10).
TBI is characterized by axonal damage and neuronal loss in vulnerable regions of the prefrontal cortex (PFC), hippocampus, thalamus, striatum, amygdala, and forebrain nuclei (11, 12). AUD may also produce structural and metabolic changes in these structures (13, 14). It is plausible that TBI and AUD may act synergistically to disrupt the neural circuits that mediate stress responses (15, 16), the prefrontal modulation of emotional processing (17), and critical aspects of addictive behavior such as stimulus salience attribution, reward expectation, and response inhibition (18, 19).
Pharmacological options to treat AUD include naltrexone (20), acamprosate, and topiramate (21). Of these drugs, naltrexone is the only one that has been rigorously tested in the veteran population. Krystal et al. (22) conducted a multicenter, randomized controlled trial of naltrexone efficacy to treat alcohol dependence in conjunction with standardized psychosocial treatment among 627 veterans. They did not find differences between the active drug and placebo in any of their outcome measures (22). Secondary analysis of their data, however, suggested that naltrexone can reduce heavy drinking among alcohol-dependent patients receiving antidepressants (23).
Anticonvulsants have a role in the treatment of addictive disorders, probably through their effects on glutamatergic and GABAergic neurotransmission (24). For example, Beresford et al. (25), reported retrospective data obtained through review of medical charts from 18 patients with a history of TBI, affective lability, and alcohol dependence treated with mood stabilizing medication for 6 weeks. They observed beneficial effects of anticonvulsants on emotional lability and alcohol misuse (25). Previous trials support valproate’s efficacy to treat alcohol withdrawal (26, 27) and to prevent relapse after completion of detoxification (27, 28). In addition, valproate has been shown to be efficacious to reduce alcohol misuse among patients with coexistent psychiatric disorders (29).
The primary goal of this study was to examine AUD treatment outcomes in a veteran population with neuropsychiatric comorbidities. We hypothesized that veterans receiving valproate would be less likely to relapse into heavy drinking than veterans receiving naltrexone. In addition, the study examined if the antecedent of TBI or the presence of PTSD affected treatment response.
Methods
Experimental Design
This was a double-blind active-controlled parallel group randomized controlled trial. Patients with AUD were evaluated at the time of enrollment in outpatient treatment and followed weekly for 24 weeks. All patients received standardized psychosocial interventions as well as usual treatment for coexistent psychiatric conditions. In addition, they were randomly assigned to receive either valproate or naltrexone. The study protocol is detailed in the online supplement. The protocol was approved by the institutional review boards at the University of Iowa and Baylor College of Medicine. Informed consent was obtained from every participant.
Study Participants
Participants were male veterans 18–60 years old, with an AUD and no other substance use besides nicotine or cannabis. All study subjects underwent successful detoxification before starting rehabilitation. Veterans were enrolled in the outpatient substance abuse treatment programs at two sites: the Iowa City VA Medical Center and the Michael E. DeBakey VA Medical Center in Houston. Veterans with psychotic disorders, significant medical comorbidities (e.g., study subjects with moderate to severe heart failure, end-stage renal disease, or evidence of moderate to advanced liver disease), and multiple AUD treatment failures (i.e., three inpatient residential treatments during the 2 years before enrollment) were excluded from the study. The rationale of the last criterion was to prevent excessive attrition related to the recruitment of extremely refractory patients. Further details regarding inclusion and exclusion criteria are provided in the online supplement.
Randomization
Patients who met inclusion criteria were randomly assigned using a permuted blocks randomization scheme. Treatment randomization was stratified by psychosocial treatment frequency, because this might be a major predictor of recovery. Thirty-one participants were randomly assigned to receive valproate, and 31 were assigned to receive an active control (naltrexone) (Figure 1).
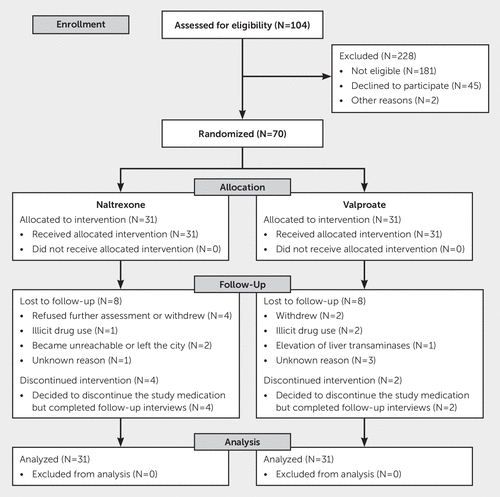
FIGURE 1. CONSORT flow diagram
Patients were excluded if they did not meet the drinking criteria, showed evidence of substance abuse different from nicotine or cannabis, currently required therapy with valproate or naltrexone, had severe complicating medical illness, or had other/multiple reasons for exclusion (Figure 1).
Primary and Secondary Outcomes
The primary outcome variable was the time to relapse to heavy drinking, defined by having five or more drinks in a sitting, and was assessed using the timeline follow-back method (30). A structured questionnaire reviewed the amount of alcohol that the patient had consumed on each of the days of the previous week. Additional outcomes included time to the first drink as well as the percentage of drinking weeks during the follow-up period. The evaluation of drinking outcomes was done by experts from the Iowa Consortium for Substance Abuse Research and Evaluation. Information sources were the participants’ report and VA medical records. Evaluators were not involved in any aspect of treatment and were blind to the randomized intervention status.
TBI Assessment
To ascertain TBI, we used the definition provided by the VA/Department of Defense clinical practice guideline (https://www.ncbi.nlm.nih.gov/books/NBK189784). TBI was further categorized as mild, moderate, or severe based on the presence and duration of loss of consciousness, the length of posttraumatic amnesia, and the duration of altered mental status associated with the event.
Pertinent information was obtained from veterans’ self-report and review of medical records, including neuroimaging reports. Detailed information was generally obtained from cases of moderate to severe TBI. However, this was not true for most veterans suffering mild TBI; we had to rely in their self-report of the event. If a patient experienced multiple TBI events of different severity, the higher severity level was assigned.
Neuropsychiatric Assessment
Diagnosis of comorbid psychiatric conditions was made using the Mini-International Neuropsychiatric Interview (31); the severity of depressive, anxiety, and PTSD symptoms was measured by the Hamilton Depression Rating Scale (32), the Hamilton Anxiety Rating Scale (33), and the PTSD Checklist (34). Severity of AUD was assessed through the Alcohol Use Disorders Identification Test (35). Estimation of the likelihood that symptoms and functional status of an individual resulted from lifetime TBI exposure was done using the Ohio State University TBI Inventory (36).
Psychosocial Intervention
Treatment was based on the Matrix Intensive Outpatient Drug and Alcohol Treatment Model (Substance Abuse and Mental Health Services Administration [SAMHSA] level 2) (37). This model is an integrated therapeutic approach incorporating evidence-based treatment modalities, including cognitive behavioral therapy, motivational enhancement therapy, 12-step facilitation, group therapy and social support, and individual supportive psychotherapy and education (for further details, see the online supplement).
Within this theoretical background, participants were offered the possibility of having conventional outpatient treatment (SAMHSA level 1) instead of the intensive program. This flexible approach was adopted to include those veterans with AUD who sought rehabilitation treatment but had limited time to devote to an intensive outpatient program.
Valproate and Naltrexone Dosing
Sodium valproate extended release tablets were initiated at a dosage of 250 mg per day, taken approximately 30 minutes after a meal. Dosage was increased up to a dose of 1,000 mg per day. We used a fixed dose of valproate to minimize side effects and increase tolerability within a population with mild liver abnormalities. Therapeutic levels of valproate have not been established for the treatment of AUDs. However, in this study, determination of valproate levels contributed to the assessment of compliance with the medication regimen. Naltrexone was given once per day in a dose of 25 mg for the first 4 days and 50 mg thereafter, up to completion of the protocol. All naltrexone and valproate pills were identical in appearance. Compliance was assessed each week concurrently with the determination of alcohol consumption.
Adverse Events
An adverse event was defined as any undesirable medical event with new onset or significant exacerbation during the study, regardless if it was considered to be related to the study medication. Adverse events were graded on a three-point ordinal scale: mild, moderate, and severe. Further details on adverse events and criteria for early termination of the study are presented in the online supplement.
Statistical Analysis
All randomly assigned participants were included for analysis, following the intention-to-treat principle. Time to relapse to heavy drinking, the primary outcome, was compared between the two study groups using the planned two-sample log-rank test. The Kaplan-Meier curve was produced to estimate the probability of remaining relapse free when follow-up time elapsed. Unadjusted hazard ratio was estimated from a Cox proportional hazards model that controlled for study group (naltrexone versus valproate) only. For sensitivity analysis, we obtained adjusted p value and hazard ratio for the group effect in a Cox proportional hazards model adjusted for site (Houston versus Iowa) as a covariate and psychosocial treatment frequency (high versus low) as strata, using the exact partial likelihood.
For secondary outcomes, time to first drinking was compared between the two study groups using the log-rank test. The percentage of drinking weeks was analyzed using the generalized estimating equation with logit link and exchangeable working correlation, where the outcome was binary and indicated whether the participant had any drinking during each follow-up week.
As a secondary analysis, we tested a multivariate Cox proportional hazards model for the primary outcome, with study group, site, TBI severity, and PTSD status adjusted as covariates and treatment frequency included as strata. Baseline covariates that were unbalanced between the two randomization groups were also controlled for. The effects of TBI and PTSD were clinically important, as they represent frequent comorbidities in the veteran population whose functional neuroanatomy overlaps with the circuits involved in addiction. p values were based on the likelihood ratio test. Secondary analyses were not adjusted for multiple comparisons, and results should be interpreted as exploratory. The Cox proportional hazards assumption was tested and verified for all covariates. All analyses were conducted in SAS (version 9.4).
Results
Study Group
We screened 290 male veterans enrolled for AUD rehabilitation treatment at the Substance Use Disorders services of the Iowa City VA Medical Center or the Michael E. DeBakey VA Medical Center in Houston. A total of 62 patients were eligible and were randomly assigned to pharmacological treatment between February 2012 and June 2015 (Figure 1).
Of the study subjects enrolled in Texas, 18 received naltrexone and 19 received valproate. Of the study subjects enrolled in Iowa, 13 received naltrexone and 12 received valproate. When randomly assigned, 46 (74%) participants were also receiving intensive outpatient rehabilitation treatment and 16 (26%) were followed by substance abuse counselors on a weekly basis (i.e., high- and low-frequency rehabilitation treatment, respectively).
Baseline Characteristics
The baseline characteristics of our study group are summarized in Table 1. Baseline characteristics were well balanced between the two groups, except that the percentage of participants that lived by themselves was significantly greater in the valproate group. The percentage of participants with no TBI, mild TBI, or moderate to severe TBI was 40.3%, 38.7% and 21.0%, respectively; 25.8% of the sample had PTSD. The distribution of PTSD was similar among those with and without TBI (24.3% versus 28%, odds ratio [OR] 0.827, 95% CI=0.261–2.615, p=0.746).
| Total (N=62) | Valproate group (N=31) | Naltrexone group (N=31) | |||||
|---|---|---|---|---|---|---|---|
| Characteristic | Mean or N | SD or % | Mean or N | SD or % | Mean or N | SD or % | pb |
| Age (years) (mean ± SD) | 47.4 | 9.2 | 46.7 | 9.6 | 48.1 | 8.8 | 0.662 |
| Race/ethnicityc | 0.349 | ||||||
| White | 33 | 54.1 | 17 | 54.8 | 16 | 53.3 | |
| African American | 23 | 37.7 | 13 | 41.9 | 10 | 33.3 | |
| Other | 5 | 8.2 | 1 | 3.2 | 4 | 13.3 | |
| Hispanic/Latino | 5 | 8.2 | 3 | 9.7 | 2 | 6.7 | 1.000 |
| Marital status | 0.302 | ||||||
| Single | 24 | 38.7 | 12 | 38.7 | 12 | 38.7 | |
| Married | 17 | 27.4 | 6 | 19.4 | 11 | 35.5 | |
| Divorced | 19 | 30.6 | 11 | 35.5 | 8 | 25.8 | |
| Widowed | 2 | 3.2 | 2 | 6.5 | 0 | 0 | |
| Employment | 0.800 | ||||||
| Full-time | 17 | 27.4 | 10 | 32.3 | 7 | 22.6 | |
| Part-time | 5 | 8.1 | 3 | 9.7 | 2 | 6.5 | |
| Volunteer work | 1 | 1.6 | 1 | 3.2 | 0 | 0 | |
| Full-time student | 3 | 4.8 | 1 | 3.2 | 2 | 6.5 | |
| Retired | 1 | 1.6 | 0 | 0 | 1 | 3.2 | |
| Unemployed | 24 | 38.7 | 10 | 32.3 | 14 | 45.2 | |
| Disabled | 11 | 17.7 | 6 | 19.4 | 5 | 16.1 | |
| Living environment | 0.007 | ||||||
| Living independently | 25 | 40.3 | 19 | 61.3 | 6 | 19.4 | |
| Living with nuclear family | 14 | 22.6 | 5 | 16.1 | 9 | 29 | |
| Living with parents or close relatives | 8 | 12.9 | 1 | 3.2 | 7 | 22.6 | |
| Living in a residential care facility or group home | 11 | 17.7 | 4 | 12.9 | 7 | 22.6 | |
| Homeless or living in a shelter | 4 | 6.5 | 2 | 6.5 | 2 | 6.5 | |
| Maximum education level | 0.595 | ||||||
| Below or partial high school | 3 | 4.8 | 1 | 3.2 | 2 | 6.5 | |
| High school graduate | 18 | 29 | 7 | 22.6 | 11 | 35.5 | |
| Partial college (≥1 year completed) | 33 | 53.2 | 18 | 58.1 | 15 | 48.4 | |
| College education and above | 8 | 12.9 | 5 | 16.1 | 3 | 9.7 | |
| Study site | 0.796 | ||||||
| Iowa City | 25 | 40.3 | 12 | 38.7 | 13 | 41.9 | |
| Houston | 37 | 59.7 | 19 | 61.3 | 18 | 58.1 | |
| HAM-D (mean ± SD)c | 10.1 | 5.8 | 10.1 | 4.2 | 10.1 | 7.1 | 0.413 |
| HAM-A (mean ± SD)d | 10.5 | 4.3 | 10.6 | 3.8 | 10.4 | 4.8 | 0.659 |
| YMS (mean ± SD)e | 2.7 | 3.9 | 2.5 | 2.6 | 2.9 | 4.9 | 0.470 |
| AUDIT (mean ± SD)f | 24.4 | 7.7 | 23.9 | 6.6 | 25 | 8.9 | 0.617 |
| TBI severity | 0.739 | ||||||
| No TBI | 25 | 40.3 | 11 | 35.5 | 14 | 45.2 | |
| Mild | 24 | 38.7 | 13 | 41.9 | 11 | 35.5 | |
| Moderate to severe | 13 | 21.0 | 7 | 22.6 | 6 | 19.4 | |
| Number of TBIs (mean ± SD) | 1.4 | 1.5 | 1.6 | 1.6 | 1.2 | 1.4 | 0.268 |
| Age at first TBI (years) (mean ± SD)g | 19.6 | 12.1 | 17.7 | 10.8 | 21.8 | 13.3 | 0.314 |
| Current PTSD | 16 | 25.8 | 9 | 29 | 7 | 22.6 | 0.562 |
| PCL total (mean ± SD)h | 37.7 | 15.8 | 40 | 16.3 | 35.5 | 15.4 | 0.308 |
TABLE 1. Baseline characteristics of the study participantsa
Primary Outcome
During the follow-up period, nine study subjects in the naltrexone group and 14 in the valproate group relapsed to heavy drinking. Although the Kaplan-Meier survival curves (Figure 2) showed a separation in relapse-free probability between the two groups, the difference was not statistically significant (log-rank χ2=2.99, df=1, p=0.084). The unadjusted hazard ratio for naltrexone versus valproate was 0.486 (95% CI=0.209–1.130). The estimated probability of remaining relapse free after 24 weeks of follow-up was 0.657 (95% CI=0.436–0.809) in the naltrexone group and 0.441 (95% CI=0.236–0.628) in the valproate group.
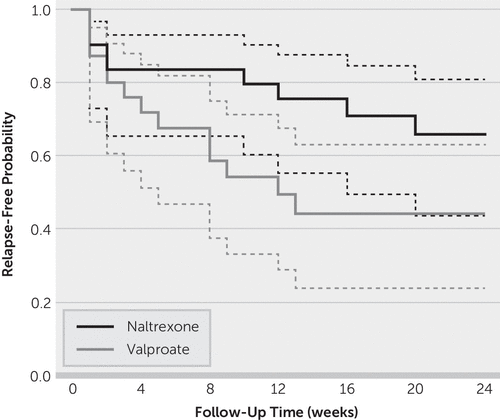
FIGURE 2. Survival analysisa
a The solid lines correspond to the Kaplan-Meier estimates, and dashed lines represent the corresponding 95% confidence interval.
In sensitivity analysis, the adjusted hazard ratio for naltrexone versus valproate in the stratified Cox model was 0.436 (95% CI=0.180–1.056, likelihood ratio χ2=3.55, df=1, p=0.060).
Secondary Outcomes
Comparable results were obtained when comparing time to first drinking (unadjusted hazard ratio=0.710, 95% CI=0.373–1.350, log-rank χ2=1.38, df=1, p=0.241) and the percentage of drinking weeks (Wald χ2=1.24, df=1, p=0.265) between the two study groups, where the unadjusted odds of drinking in the naltrexone group were 0.600 (95% CI=0.245–1.474) times that in the valproate group. Conclusions remained the same when we controlled for rehabilitation treatment frequency and site.
Multivariate Analysis of the Primary Outcome
According to the multivariate Cox proportional hazards model, participants with a history of moderate to severe TBI were more likely to relapse into heavy drinking when compared with those with no TBI (hazard ratio=4.834, 95% CI=1.103–21.194, p=0.033). This was not the case for veterans with a history of mild TBI (hazard ratio=1.669, 95% CI=0.518, 5.380, p=0.385). PTSD status, on the other hand, did not significantly affect outcome (hazard ratio=1.759, 95% CI=0.605–5.116, p=0.300).
The model-based survival curve by randomization group and the frequency of psychosocial treatment are shown in Figure 3. Participants who were enrolled in the intensive outpatient treatment program were more likely to avoid relapse into heavy drinking compared with those participants who had one weekly session with their counselor, independent of which pharmacological treatment they received.
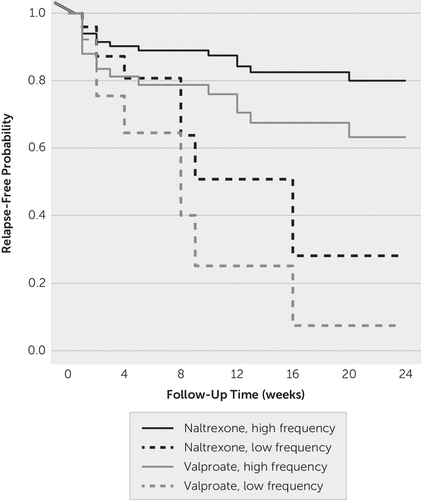
FIGURE 3. Effect of psychosocial treatment frequencya
a The data were estimated from the multivariable Cox proportional hazards model stratified by the frequency of rehabilitation treatment. All other model covariates were set at the overall mean.
Compliance With Study Medication and Concurrent Medication Use
Of 30 participants in the naltrexone group, six (20%) were ever noncompliant with their study medication during the follow-up. Of 31 participants in the valproate group, four (12.9%) were ever noncompliant. There were no significant differences between the valproate and the naltrexone groups in the level of noncompliance with the study medication (OR=1.688, 95% CI=0.425–6.704, Fisher’s exact p=0.508). Similarly, no significant between-group difference was detected in concurrent medication use (Table 2). Overall, the percentages of participants who used concurrent medications were 69.4% for antidepressants, 8.1% for antianxiety agents, 9.7% for antipsychotics, 8.1% for gabapentin, and 8.1% for prazosin.
| Total | Naltrexone group | Valproate group | Odds ratiob | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Medicationa | N | % | N | % | N | % | Estimate | 95% CI | pc |
| Antidepressantsd | 43 | 69.4 | 21 | 67.7 | 22 | 71.0 | 0.86 | 0.29–2.53 | 0.783 |
| Antianxiety agentse | 5 | 8.1 | 2 | 6.5 | 3 | 9.7 | 0.64 | 0.10–4.15 | >0.9 |
| Antipsychoticsf | 6 | 9.7 | 3 | 9.7 | 3 | 9.7 | 1.00 | 0.19–5.39 | >0.9 |
| Gabapenting | 5 | 8.1 | 2 | 6.5 | 3 | 9.7 | 0.64 | 0.10–4.15 | >0.9 |
| Prazosinh | 5 | 8.1 | 3 | 9.7 | 2 | 6.5 | 1.55 | 0.24–10.01 | >0.9 |
TABLE 2. Concurrent medication by randomization group and type
In our group of participants treated with valproate, the mean total valproate level was 42.7 µg/ml, the SD was 19.4 µg/ml, and the range varied from 9 to 72.7 µg/ml.
Adverse Events
There were no serious adverse events for any participant in the trial. There were no significant differences between the valproate or naltrexone groups in the frequency of adverse events. However, valproate had more sedating effects than naltrexone. The percentage of study subjects who had at least one of the adverse events listed during the follow-up is presented in Table 3.
| Odds ratioa | |||||
|---|---|---|---|---|---|
| Event | Naltrexone group (%) | Valproate group (%) | Estimate | 95% CI | pb |
| Abdominal pain | 16.1 | 9.7 | 1.79 | 0.39–8.27 | 0.707 |
| Anemia | 3.2 | 0.0 | – | – | >0.9 |
| Bleeding | 3.2 | 3.2 | 1.00 | 0.06–16.74 | >0.9 |
| Chest pain | 6.5 | 3.2 | 2.07 | 0.18–24.07 | >0.9 |
| Confusion | 12.9 | 12.9 | 1.00 | 0.23–4.42 | >0.9 |
| Diarrhea | 29.0 | 32.3 | 0.86 | 0.29–2.53 | 0.783 |
| Dizziness | 25.8 | 22.6 | 1.19 | 0.37–3.82 | 0.767 |
| Drowsiness | 22.6 | 48.4 | 0.31 | 0.10–0.93 | 0.034 |
| Hair loss | 6.5 | 0.0 | – | – | 0.492 |
| Hypomania | 3.2 | 6.5 | 0.48 | 0.04–5.62 | >0.9 |
| Insomnia | 16.1 | 9.7 | 1.79 | 0.39–8.27 | 0.707 |
| Lightheadedness | 25.8 | 12.9 | 2.35 | 0.63–8.81 | 0.199 |
| Nasal bleed | 3.2 | 3.2 | 1.00 | 0.06–16.74 | >0.9 |
| Nasal inflammation | 9.7 | 3.2 | 3.21 | 0.32–32.74 | 0.612 |
| Nausea | 29.0 | 9.7 | 3.82 | 0.92–15.81 | 0.054 |
| Slowness of movements | 3.2 | 6.5 | 0.48 | 0.04–5.62 | >0.9 |
| Sweating | 32.3 | 16.1 | 2.48 | 0.73–8.37 | 0.138 |
| Tremor | 12.9 | 6.5 | 2.15 | 0.36–12.69 | 0.671 |
| Worsening depression | 6.5 | 9.7 | 0.64 | 0.10–4.15 | >0.9 |
| Other | 64.5 | 64.5 | 1.00 | 0.35–2.83 | >0.9 |
TABLE 3. Percentage of participants with adverse events by type and randomization group
Discussion
The primary aim of this study was to test valproate’s efficacy to reduce relapse to heavy drinking in a group of veterans with AUD and neuropsychiatric comorbidities, including TBI and PTSD. Survival analysis suggested no significant difference between the two arms. As expected, participants who were enrolled in an intensive outpatient program were less likely to relapse into heavy drinking than participants receiving a less intensive outpatient option, independent of which pharmacological treatment they received.
Regarding the effect of coexistent neuropsychiatric conditions, veterans with a history of moderate to severe TBI were more likely to relapse into heavy drinking when compared with veterans without TBI exposure. This increased vulnerability was not observed among veterans with a history of mild TBI. On the other hand, a current diagnosis of PTSD or the severity of anxiety, depressive, and PTSD symptoms did not appear to affect treatment outcome. Finally, besides mild sedation, there were no significant differences between the valproate and naltrexone groups in the frequency of adverse events.
The principal limitation of the present study is related to the relatively small sample size and, consequently, the limited power to identify subgroup differences. Though the estimated relapse-free probability in the naltrexone group was larger than that in the valproate group (Figure 2), the difference was statistically nonsignificant. In fact, with the estimated event rate and effect size in the current study, a sample size of 164 is needed to detect the between-group difference with 80% power. The study design did not include a placebo arm because both investigators and reviewers of the grant application considered it would be unethical to give participants a placebo. The lack of a placebo control restricts the interpretation of the results. In addition, reliance on veterans’ self-report increases the possibility of retrospective bias. Finally, considering valproate’s teratogenic effects and the fact that most veterans enrolled in AUD programs are male, women were excluded from the present study. This limits the generalizability of the findings.
Given these limitations, what are the implications of these findings? In first place, they support the idea that, in the veteran population, an intensive outpatient program is more efficacious that other less intensive psychosocial treatment options. Furthermore, it has been suggested that an intensive outpatient program might be as effective as inpatient residential treatment (38).
Our findings suggest that opiate antagonists may be more effective than valproate to prevent relapse into problematic alcohol use among U.S. veterans. However, the present study is underpowered to establish this conclusively. It can be argued that the study subjects enrolled in our study were closer to the phenotype of reward drinkers driven by positive reinforcement rather than relief drinkers among whom negative reinforcement is a determinant factor (39). However, most of these veterans were in advanced stages of the addiction cycle, when negative reinforcement is more prominent, and many had coexistent stress-related psychopathology.
Alternatively, valproate may not be an efficacious modulator of glutamatergic transmission, and other agents of this kind may be more appropriate to treat addictive disorders in this group of patients. However, a large and rigorous randomized controlled trial reported that acamprosate (a medication that influences glutamate synaptic homeostasis) did not prevent relapse into heavy drinking, whereas naltrexone did (20).
Chronic AUD is characterized by cravings, executive dysfunction, and compulsive behavior (40). The pathophysiology of cravings and compulsive drinking involves disruption of glutamatergic circuits connecting the PFC, the striatum, and the extended amygdala (41). Increased excitatory input leads to neuroplastic changes in the amygdala, the nucleus accumbens, and dorsal striatum mediated by the activation of N-Methyl-D-aspartate and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic-acid receptors and associated with cravings and drug-seeking behavior (42–44). Pharmacological modification of glutamatergic circuits may result in a reduction of these behavioral abnormalities. Specifically, valproate contributes to normalizing glutamate neurotransmission through multiple mechanisms, including blockade of sodium and voltage gated potassium channels, increased GABAergic transmission, and the up-regulation of glutamate transporters in astrocytes (45, 46). However, cravings are also associated with an increase in the phasic activity of dopaminergic neurons ascending from the ventral tegmental area to striatal and limbic targets. The increase in subcortical dopaminergic activity occurs in the context of faulty prefrontal regulation and executive dysfunction (40). The striatal and limbic hyperdopaminergic state can be facilitated by opioid peptides and consequently inhibited by opioid antagonists, explaining the therapeutic action of naltrexone.
A history of moderate to severe TBI was associated with an increased risk of relapse, probably associated with the prefrontal dysfunction characteristic of this condition. Both TBI and addictive disorders demonstrate prefrontal cortex abnormalities and may lead to disruption of neural circuits that, originating from the PFC and basolateral amygdala, converge in the ventral striatum. These additive changes may lead to to relapse to heavy drinking following a period of abstinence (47–49). Thus, they may play an important etiological role in the development of more severe and treatment refractory forms of AUD.
It is also well known that stress and negative affect may precipitate relapse into heavy drinking (50). However, we did not observe a clear-cut indication that such mechanisms played a decisive role in determining relapse in our group of veterans. For instance, PTSD status and the severity of depressive and anxiety symptoms were not associated with increased likelihood of relapse.
In summary, intensive outpatient programs constitute an efficacious alternative to treat AUD among U.S. veterans. However, the role of pharmacological treatment is not completely elucidated. Glutamatergic agents appear to be less effective than opiate antagonists to prevent relapse into heavy drinking and to increase cumulative abstinence. Future studies need to examine new pharmacological and nonpharmacological options, including noninvasive brain stimulation techniques. Furthermore, we need to identify subgroups of alcoholic patients that may respond to a specific form of treatment but not to others (e.g., study subjects with a history of moderate to severe TBI or study subjects with known genetic polymorphisms of opiate receptors).
1 : Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry 2015; 72:757–766Crossref, Medline, Google Scholar
2 : The epidemiology of substance use disorders in US veterans: a systematic review and analysis of assessment methods. Am J Addict 2016; 25:7–24Crossref, Medline, Google Scholar
3 : Mild traumatic brain injury in US soldiers returning from Iraq. N Engl J Med 2008; 358:453–463Crossref, Medline, Google Scholar
4 : Does traumatic brain injury increase risk for substance abuse? J Neurotrauma 2009; 26:1077–1082Crossref, Medline, Google Scholar
5 : The natural history of drinking and alcohol-related problems after traumatic brain injury. Arch Phys Med Rehabil 2003; 84:185–191Crossref, Medline, Google Scholar
6 : Screening substance abuse treatment clients for traumatic brain injury: prevalence and characteristics. J Head Trauma Rehabil 2007; 22:360–367Crossref, Medline, Google Scholar
7 : The presence and impact of traumatic brain injury among clients in treatment for co-occurring mental illness and substance abuse. Brain Inj 2008; 22:223–231Crossref, Medline, Google Scholar
8 : Traumatic brain injury in the United States: a public health perspective. J Head Trauma Rehabil 1999; 14:602–615Crossref, Medline, Google Scholar
9 : Psychiatric illness following traumatic brain injury in an adult health maintenance organization population. Arch Gen Psychiatry 2004; 61:53–61Crossref, Medline, Google Scholar
10 : Axis I psychopathology in individuals with traumatic brain injury. J Head Trauma Rehabil 1998; 13:24–39Crossref, Medline, Google Scholar
11 : Recent advances in neurotrauma. J Neuropathol Exp Neurol 2000; 59:641–651Crossref, Medline, Google Scholar
12 : The molecular and cellular sequelae of experimental traumatic brain injury: pathogenetic mechanisms. Neuropathol Appl Neurobiol 1998; 24:251–267Crossref, Medline, Google Scholar
13 : Comparison of prefrontal cell pathology between depression and alcohol dependence. J Psychiatr Res 2003; 37:411–420Crossref, Medline, Google Scholar
14 : A controlled study of cortical gray matter and ventricular changes in alcoholic men over a 5-year interval. Arch Gen Psychiatry 1998; 55:905–912Crossref, Medline, Google Scholar
15 : Alcoholism: allostasis and beyond. Alcohol Clin Exp Res 2003; 27:232–243Crossref, Medline, Google Scholar
16 : Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann N Y Acad Sci 2004; 1032:1–7Crossref, Medline, Google Scholar
17 : Serotonin transporter genetic variation and the response of the human amygdala. Science 2002; 297:400–403Crossref, Medline, Google Scholar
18 : Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry 2002; 159:1642–1652Crossref, Medline, Google Scholar
19 : The addicted human brain: insights from imaging studies. J Clin Invest 2003; 111:1444–1451Crossref, Medline, Google Scholar
20 : Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA 2006; 295:2003–2017Crossref, Medline, Google Scholar
21 : Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA 2014; 311:1889–1900Crossref, Medline, Google Scholar
22 : Naltrexone in the treatment of alcohol dependence. N Engl J Med 2001; 345:1734–1739Crossref, Medline, Google Scholar
23 : Naltrexone is associated with reduced drinking by alcohol dependent patients receiving antidepressants for mood and anxiety symptoms: results from VA Cooperative Study No 425, “Naltrexone in the treatment of alcoholism”. Alcohol Clin Exp Res 2008; 32:85–91Crossref, Medline, Google Scholar
24 : Noradrenergic and serotonergic mechanisms in the neurobiology of posttraumatic stress disorder and resilience. Brain Res 2009; 1293:13–23Crossref, Medline, Google Scholar
25 : Reduction of affective lability and alcohol use following traumatic brain injury: a clinical pilot study of anti-convulsant medications. Brain Inj 2005; 19:309–313Crossref, Medline, Google Scholar
26 : Prevention of alcohol withdrawal seizures with carbamazepine and valproic acid. Alcohol 1989; 6:223–226Crossref, Medline, Google Scholar
27 : Divalproex sodium (Depakote) for alcohol withdrawal and relapse prevention. J Addict Dis 2002; 21:55–64Crossref, Medline, Google Scholar
28 : Mood stabilizers in the treatment of substance use disorders. CNS Spectr 2010; 15:95–109Crossref, Medline, Google Scholar
29 : Efficacy of valproate maintenance in patients with bipolar disorder and alcoholism: a double-blind placebo-controlled study. Arch Gen Psychiatry 2005; 62:37–45Crossref, Medline, Google Scholar
30 : Reliability of a timeline method: assessing normal drinkers’ reports of recent drinking and a comparative evaluation across several populations. Br J Addict 1988; 83:393–402Crossref, Medline, Google Scholar
31 : The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998; 59(Suppl 20):22–33, quiz 34–57Medline, Google Scholar
32 : A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62Crossref, Medline, Google Scholar
33 : The assessment of anxiety states by rating. Br J Med Psychol 1959; 32:50–55Crossref, Medline, Google Scholar
34 : The Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5): development and initial psychometric evaluation. J Trauma Stress 2015; 28:489–498Crossref, Medline, Google Scholar
35 : Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption—II. Addiction 1993; 88:791–804Crossref, Medline, Google Scholar
36 : Initial reliability and validity of the Ohio State University TBI Identification Method. J Head Trauma Rehabil 2007; 22:318–329Crossref, Medline, Google Scholar
37 : An intensive outpatient approach for cocaine abuse treatment: the matrix model. J Subst Abuse Treat 1995; 12:117–127Crossref, Medline, Google Scholar
38 : Substance abuse intensive outpatient programs: assessing the evidence. Psychiatr Serv 2014; 65:718–726Link, Google Scholar
39 : Precision medicine in alcohol dependence: a controlled trial testing pharmacotherapy response among reward and relief drinking phenotypes. Neuropsychopharmacology 2018; 43:891–899Crossref, Medline, Google Scholar
40 : Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry 2016; 3:760–773Crossref, Medline, Google Scholar
41 : The nucleus accumbens: mechanisms of addiction across drug classes reflect the importance of glutamate homeostasis. Pharmacol Rev 2016; 68:816–871Crossref, Medline, Google Scholar
42 : Glutamatergic transmission in drug reward: implications for drug addiction. Front Neurosci 2015; 9:404Crossref, Medline, Google Scholar
43 : Addiction therapy: refining deep brain stimulation to emulate optogenetic treatment of synaptic pathology. Science 2015; 347:659–664Crossref, Medline, Google Scholar
44 : The good and bad news about glutamate in drug addiction. J Psychopharmacol 2016; 30:1095–1098Crossref, Medline, Google Scholar
45 : Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol 2008; 75:218–265Crossref, Medline, Google Scholar
46 : Gq-DREADD selectively initiates glial glutamate release and inhibits cue-induced cocaine seeking. Biol Psychiatry 2015; 78:441–451Crossref, Medline, Google Scholar
47 : DeltaFosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nat Neurosci 2010; 13:745–752Crossref, Medline, Google Scholar
48 : The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci 2009; 10:561–572Crossref, Medline, Google Scholar
49 : Glutamate and reinstatement. Curr Opin Pharmacol 2009; 9:59–64Crossref, Medline, Google Scholar
50 : Neurobiologic advances from the brain disease model of addiction. N Engl J Med 2016; 374:363–371Crossref, Medline, Google Scholar



