Outpatient CBT for Motor Functional Neurological Disorder and Other Neuropsychiatric Conditions: A Retrospective Case Comparison
Abstract
Objective:
No gold-standard treatment exists for motor functional neurological disorder (mFND), and limited evidence has been found for the effectiveness of cognitive-behavioral therapy (CBT) in treating the disorder. This study examined sociodemographic and clinical characteristics, treatment outcomes, and treatment dropout among patients with and without mFND who received CBT in a neuropsychiatric outpatient clinic in the United Kingdom.
Methods:
Data from a large anonymized psychiatric register were used to identify patients who received outpatient CBT in a neuropsychiatry clinic between 2006 and 2016 and who had either mFND (N=98) or other neuropsychiatric conditions (ONP) (N=76, control group). The study examined sociodemographic characteristics, physical symptom improvement, and changes in clinical outcome and scores on three instruments measuring psychological distress, psychiatric sequelae of brain injury, and depression.
Results:
The most common mFND symptoms were weakness, pain, and tremors. A logistic regression analysis found no sociodemographic differences between patients with mFND who dropped out early and those who completed CBT. Pre- and post-CBT scores on the three instruments were available for only a small subset of patients; both mFND and ONP patients showed significant improvements in overall scores. A logistic regression analysis found only a single predictor of symptom improvement in the mFND group: acceptance of a psychological explanation of symptoms prior to treatment.
Conclusions:
Improvements in physical and psychological functioning were similar for patients with mFND and patients with ONP who were treated in a specialist CBT clinic. This study provides evidence that CBT is feasible and effective for some patients with mFND.
Functional neurological disorder with motor symptoms (mFND) refers to a spectrum of neurological symptoms not explained by standard neurological disease (1). The disorder comprises a wide range of symptoms, including weakness, numbness, tremor, gait disorders, and paralysis.
There is no gold-standard treatment, and the development of manualized treatments has been hindered by a lack of consensus on the definitions, classification, and diagnosis of the disorder. Randomized controlled trials (RCTs) of physiotherapy show promising results (2–5), and there is evidence from smaller studies and case series that a multidisciplinary rehabilitative approach is effective (6–9).
Cognitive-behavioral therapy (CBT) is one element of the multidisciplinary approach and emphasizes the importance of cognition and behavior in maintaining the disorder. Maladaptive cognitions, such as dysfunctional automatic thoughts, somatic misinterpretations, and illness beliefs, are challenged in a bid to modify behavior (10). Techniques such as muscle relaxation and psychoeducation may be employed to help correct maladaptive patterns of symptom formation and maintenance.
Evidence regarding the effectiveness of CBT in treating mFND is limited. A case study reported benefits of up to a year for a patient with a functional dystonia after 12 CBT sessions (11). A small pilot study reported improvements after CBT and adjunctive physical therapy, compared with standard medical care (12). A number of trials have shown improvements when CBT is used for patients with nonepileptic seizures and with mixed FND (13–16). There is evidence of its effectiveness in other somatic symptom disorders (17–24), although one RCT comparing CBT to care from a primary physician found no significant difference in outcome (25).
This evidence lends support to an a priori assumption that CBT will improve mFND symptoms, although such treatment may pose challenges because patients may be resistant to psychological accounts of symptoms, and such resistance would affect its uptake and effectiveness. No RCTs have tested the effectiveness of CBT for mFND.
The aim of this study was to evaluate the outcomes of patients with mFND who received a course of CBT at an outpatient neuropsychiatry clinic in South London and Maudsley (SLaM) National Health Service (NHS) Foundation Trust. Because the study was observational and based on clinical practice within a single mental health NHS trust, we included a comparison group. This group comprised patients treated with CBT by the same clinical team for neuropsychiatric and behavioral manifestations of other neurological conditions. We compared sociodemographic characteristics, treatment dropout, and clinical outcomes. We hoped to establish evidence that could inform a future RCT.
Methods
Design and Source of Clinical Data
This was a retrospective comparison of two cohorts: patients with mFND and patients with other neuropsychiatric conditions (ONP) treated in a SLaM neuropsychiatry outpatient clinic between January 1, 2006, and December 31, 2016. Data were obtained from SLaM’s Biomedical Research Centre Clinical Records Interactive Search (CRIS) database. CRIS holds anonymized records for more than 250,000 individuals referred to SLaM services (26). This online system contains all patient information, medication, diagnoses, correspondence, and clinical outcome scores. Records can be retrieved by using searches of the database’s structured fields, such as age, gender, and diagnosis, or of free-text clinical notes and correspondence.
Study Setting and Participants
The outpatient neuropsychiatry services at SLaM treat patients with psychological complications associated with neurological disorders and functional and dissociative disorders. Patients receiving a CBT referral are offered an assessment after which they may be recommended a formal CBT course. A common treatment course is 12–15 sessions, usually occurring weekly.
The core components of CBT for FND are psychoeducation, cognitive and behavioral techniques, and relapse prevention strategies. Therapists challenge cognitive distortions that affect motivation, attempting to build insight so that patients may learn to accept a psychological understanding of symptoms. Some patients may be encouraged to link past and present experiences with physical symptoms, but this is not always the case. Other techniques include mood and thought diaries and relaxation and graded-exposure techniques to reduce behavioral avoidances.
Patients with mFND in this study were aged ≥18 with a primary or secondary diagnosis of conversion disorder with motor symptom or deficit (ICD-10 code F44.4) or those without a formal F44.4 diagnosis who received treatment for functional motor or movement symptoms. In these cases, mFND symptom management and improvement formed a large part of the focus of CBT. Patients received diagnoses in differing medical and psychiatric contexts, but all diagnoses were robust and were confirmed by a senior neurologist or neuropsychiatrist.
Patients in the comparison group were included if they were aged ≥18 treated in the same CBT clinic for psychiatric and behavioral manifestations of other neurological or neuropsychiatric conditions, and had no evidence of mFND or nonepileptic seizure symptoms. Some had comorbid functional somatic symptoms, such as irritable bowel syndrome, chronic fatigue, and fibromyalgia.
Patients in both groups were excluded if they had a CBT assessment but their treatment had not begun. We included patients if CBT had begun but the course was not complete.
Ethical Approval
The CRIS database received ethical approval from the Oxfordshire Research Ethics Committee as an anonymized data set for mental health research.
Outcome Measures
We extracted information on year of birth, gender, race/ethnicity, marital status, employment, housing status, receipt of benefits, use of walking aids, having a caregiver or being a caregiver, physical comorbidity, lifetime experience of sexual or physical abuse, age at onset of any psychological symptom (any symptom, not exclusive to FND), CBT assessment date, and acceptance of psychological formulations before and after CBT. Acceptance of psychological formulations was assessed by using information from detailed case notes written by CBT therapists or case notes and referral letters from patients' consultant neuropsychiatrists.
CBT attendance was calculated as the number of sessions attended out of the number of sessions offered. Information on treatment dropout was recorded.
We created a 3-point scale to measure patient improvement: symptoms improved, remained the same, or got worse. Improvements of patients in the ONP group were based on the goal set by the patient and therapist at the start of therapy. Assessment of improvement was based on the view expressed by CBT therapists in their case notes or referral letter to the consultant neuropsychiatrist at the end of treatment. Neuropsychiatric symptom management and improvement was a goal common to both groups, but only in the FND group did we expect to observe motor function improvement.
We collected scores on the following instruments: Clinical Outcomes in Routine Evaluation–Outcome Measure (CORE-OM), Health of the Nation–Acquired Brain Injury (HoNOS-ABI), and nine-item Patient Health Questionnaire (PHQ-9). The CORE-OM is a 34-item self-report questionnaire measuring psychological distress. It has good criterion validity with other mood scales and has high internal consistency for secondary care (α=0.95) (27). HoNOS-ABI assesses the neuropsychiatric factors linked to brain damage. The scale correlates with established outcome measures—for example, postinjury employment (28)—and has good interrater reliability (29). The PHQ-9 assesses depression severity and demonstrates good internal consistency, a test-retest reliability of 0.87, and criterion validity with the Beck Depression Inventory of 0.79 (30). On each of these three instruments, lower scores indicate better outcomes.
Pre-CBT and post-CBT scores were classified as those taken nearest the CBT assessment date and the final CBT treatment or follow-up session. Scores were included if they were measured within 180 days of the date in question. A comparison of pre- and post-CBT scores assessed change over time and response to treatment.
Statistical Analysis
We used means, standard deviations, and frequency data to assess differences between the mFND and ONP groups. Chi-square analyses were used for frequency data, Mann-Whitney U calculations were used for nonnormally distributed score comparisons, and proportions were used to describe categorical data. An exact McNemar’s test was used to determine the change in the proportion of patients with mFND who accepted psychological formulations before and after CBT. A repeated-measures analysis of variance (ANOVA) assessed the change in CORE-OM scores and their associations with sociodemographic variables. We conducted a binary logistic regression analysis to assess associations between sociodemographic variables and symptom improvement in patients with mFND. Using a simultaneous selection model, we adjusted for age, gender, race/ethnicity, marital status, employment, caregiver status, receipt of benefits, disability, acceptance of psychological formulations, and experience of abuse. The following software was employed: SPSS for Windows, version 21.0; Microsoft Excel, version 14.0.7015.1000; and GraphPad Prism, version 5.01.
Results
Patient Characteristics
Our search returned 941 patients, of whom 573 were patients with functional disorders with nonepileptic seizures but no evidence of motor symptoms. A further 21 did not meet other study criteria (e.g., age <18). A total of 102 individuals were excluded from the mFND group and 71 from the ONP group because they had not begun treatment. The most common reasons for not beginning CBT in the mFND group were referral to the Trust’s inpatient neuropsychiatry ward (36.3% versus 19.7% in the ONP group; χ2=5.5, 95% confidence interval [CI]=2.1–30.0, p<0.05), nonattendance at appointments (19.0% versus 26.8%; χ2=1.6, 95% CI=–4.2 to 21.0, p>0.05), and treatment refusal (13.7% versus 2.8%; χ2=5.9,95% CI=2.2–19.2, p<0.02).
A total of 98 mFND patients and 76 ONP patients began CBT, and they formed our study sample. In the ONP group, epilepsy was the most common disease (N=43, 46.2%), followed by Tourette’s syndrome (N=16, 17.4%), sleep disorders (N=7, 7.6%), multiple sclerosis (N=5, 5.4%), and other neurological diseases (N=22, 23.9%). The most common neuropsychiatric difficulties for which patients in the ONP group received treatment were depression (N=29, 22.7%), anxiety (N=25, 19.5%), low mood (N=20, 15.6%), sleep disorders (N=9, 7.0%), obsessional thoughts and compulsions (N=10, 7.8%), self-harm and suicidal ideation (N=10, 7.8%), panic disorders (N=7, 5.5%), low self-esteem (N=7, 5.5%), and anger management, agoraphobia, and personality changes (N=11, 8.6% total). Most patients had more than one complaint, and there was considerable overlap between symptoms. Data on sociodemographic characteristics, experience of abuse, and lifetime prevalence of fatigue, anxiety and depression in both groups are presented in Table 1.
| mFND (N=98) | ONP (N=76) | ||||||
|---|---|---|---|---|---|---|---|
| Characteristic | N | % | N | % | χ2 | 95% CI | p |
| Gender | |||||||
| Female | 71 | 72.4 | 34 | 44.7 | 13.64 | 12.2, 41.9 | 0.001 |
| Male | 27 | 27.6 | 42 | 55.3 | |||
| Race/ethnicity | |||||||
| White British | 66 | 67.3 | 54 | 71.1 | 0.29 | –10.9, 18.0 | 0.60 |
| White of any other nationality | 15 | 15.3 | 11 | 14.5 | 0.02 | –10.4, 11.3 | 0.88 |
| Any other background (black, Asian, African, Caribbean, Indian)b | 17 | 17.3 | 11 | 14.5 | 0.24 | –8.7, 13.5 | 0.61 |
| Marital status | |||||||
| Single | 42 | 42.9 | 44 | 57.9 | 3.83 | –0.8, 30.0 | 0.051 |
| Married, civil partner, or cohabiting | 45 | 45.9 | 27 | 35.5 | 1.89 | –4.3, 24.3 | 0.17 |
| Divorced, separated, or widowed | 11 | 11.2 | 5 | 6.6 | 1.08 | –4.7, 13.2 | 0.30 |
| Housing type | |||||||
| Council tenant, supported housing, temporary housing | 11 | 11.2 | 8 | 10.5 | 0.02 | –9.4, 10.0 | 0.88 |
| Living with family | 20 | 25.6 | 12 | 19.0 | 0.86 | –8.4, 20.8 | 0.35 |
| Privately owned, privately rented | 47 | 60.3 | 43 | 68.3 | 0.96 | –7.9, 23.0 | 0.33 |
| Not known | 20 | 20.4 | 13 | 17.1 | |||
| Employment and benefit and caregiver status | |||||||
| Employed | 33 | 34.0 | 37 | 48.7 | 3.82 | –0.8, 29.7 | 0.051 |
| Unemployed | 51 | 52.6 | 27 | 35.5 | 5.03 | 2.2, 30.8 | 0.03 |
| Otherc | 14 | 14.3 | 12 | 15.8 | 0.08 | –9.0, 12.8 | 0.78 |
| Employment status unknown | 1 | 1 | 0 | 0 | |||
| Receives benefits | 36 | 39.6 | 25 | 35.7 | 0.25 | –12.1, 19.4 | 0.61 |
| Benefits received unknown | 7 | 7.1 | 6 | 7.9 | |||
| Is a health care or social care worker | 20 | 21.3 | 11 | 14.9 | 1.16 | –6.4, 18.5 | 0.29 |
| Health care or social care worker status unknown | 4 | 4.1 | 2 | 2.6 | |||
| Has a caregiver | 24 | 27.6 | 10 | 14.3 | 4.42 | 0.9, 24.7 | 0.04 |
| Caregiver not known | 11 | 11.2 | 6 | 7.9 | |||
| Has a current physical health condition | |||||||
| Any | 76 | 79.2 | 76 | 100 | 0.30 | –9.0, 16.0 | 0.62 |
| Not known | 2 | 2.0 | 0 | 0 | |||
| Has a condition that could cause neuropsychiatric symptoms | 8 | 8.2 | 76 | 100 | 143.63 | 83.2., 95.8 | 0.001 |
| Comorbid functional symptomsd | 38 | 38.8 | 12 | 15.8 | 10.99 | 9.6, 34.8 | 0.001 |
| Irritable bowel syndrome | 17 | 37.8 | — | — | — | — | — |
| Headache, migraine | 21 | 46.7 | — | — | — | — | — |
| Fibromyalgia, chronic fatigue syndrome | 7 | 15.6 | — | — | — | — | — |
| History of depression, low mood, suicidal thoughts and ideation | 84 | 85.7 | 67 | 88.2 | 0.23 | –8.8, 13.1 | 0.63 |
| History of anxiety | 77 | 80.2 | 67 | 91.8 | 4.40 | 0.07, 22.3 | 0.04 |
| History of anxiety unknown | 2 | 2.2 | 3 | 3.9 | |||
| History of fatigue | 57 | 72.2 | 36 | 55.4 | 4.37 | 0.12, 32.8 | 0.04 |
| History of fatigue unknown | 19 | 19.4 | 11 | 14.5 | |||
| Abuse history | |||||||
| Childhood sexual abuse | 19 | 23.8 | 5 | 8.2 | 7.35 | 4.5, 25.9 | 0.007 |
| Childhood sexual abuse unknown | 18 | 18.4 | 15 | 19.7 | |||
| Childhood physical abuse | 23 | 28.4 | 13 | 21.0 | 1.24 | –5.7, 19.7 | 0.27 |
| Childhood physical abuse unknown | 17 | 17.3 | 14 | 18.4 | |||
| Sexual or physical abuse as an adult | 19 | 23.8 | 19 | 16.1 | 1.55 | –4.6, 19.1 | 0.21 |
| Sexual or physical abuse as an adult unknown | 18 | 18.4 | 14 | 18.4 | |||
| History of family mental health problems | 51 | 63.8 | 51 | 63.8 | 0.009 | –13.6, 14.6 | 0.92 |
| History of family mental health problems unknown | 18 | 18.4 | 14 | 18.4 | |||
| Age at analysis (M±SD)e | 44.5±12.0 | 45.4±13.0 | 1.3 | –1.4, 7.4 | 0.19 | ||
| Age at symptom onset (M±SD)e | 30±14 | 28±15 | 3,105.5 | 0.27 | |||
| Age at CBT assessment (M±SD)e | 40.3±13.0 | 40.7±13.0 | 3,669 | 0.87 | |||
| Time between symptom onset and CBT assessment (M±SD years) | 9.9±9.6 | 12.9±11.6 | –1.9 | –3.1, 1.7 | 0.06 | ||
| Time between formal diagnosis and start of CBT treatment (M±SD years) | 1.9±2.1 | 1.7±2.2 | 0.57 | 0.3. –0.4 | 0.57 | ||
| CBT attendance | |||||||
| Attended all sessions | 55 | 56.1 | 43 | 56.6 | 0.004 | –15.0, 15.9 | 0.95 |
| Dropped out early | 28 | 28.6 | 20 | 26.3 | 0.11 | –12.0, 16.1 | 0.74 |
| Therapist discontinued sessions or sessions were ongoing | 15 | 15.3 | 13 | 17.1 | 0.10 | –9.0, 13.4 | 0.75 |
TABLE 1. Characteristics of patients with motor functional neurological disorder (mFND) and a control group with other neuropsychiatric conditions (ONP) receiving outpatient cognitive-behavioral therapy (CBT)a
The most common mFND symptom was weakness (N=47, 26.9%), most frequently in the leg or entire body, followed by pain (N=46, 26.3%) and tremor, shakes, jerking, or dystonia (N=43, 24.6% total). All patients in the mFND group had at least one motor symptom: 83.7% (N=82) experienced two motor symptoms, 40.8% (N=40) experienced three, and 12.2% (N=12) experienced four. Of patients in the mFND group, 22.4% (N=22) had a history of nonepileptic seizures. Rates of comorbid physical health conditions and other comorbid functional symptoms in both groups are presented in Table 1.
Openness to a Psychological Formulation
Of the 98 patients with mFND, prior to therapy commencement, 49 (49.0%) accepted a psychological formulation, 27 (27.6%) did not, and 13 (13.3%) were unsure. In 10 cases, no data were available or a psychological account was not applicable. After therapy, we assessed acceptance of a psychological formulation in 95 patients: 68 (71.6%) accepted a psychological account, 17 (17.9%) did not, and five (5.3%) were unsure. In eight cases (8.3%), this was not known. A significant increase was noted in the proportion of patients accepting a psychological account after CBT (McNemar’s test, p=0.004).
Treatment Attendance
The mean number of treatment sessions attended was 14.1 (SD=8.00; range, 1–46) for patients in the mFND group and 13.4 (SD=7.3; range, 1−40) for patients in the ONP group. Rates of attendance are presented in Table 1.
In total, 28 patients in the mFND group ended treatment early, and in six cases the therapist discontinued the sessions early. Reasons for dropout or early cessation were as follows: five believed a physical cause explained their symptoms, three believed therapy was ineffective or worsened symptoms, three reported that the clinic was too far away, two reported that their symptoms improved, two had a physical health problem that impeded attendance, and four reported miscellaneous reasons. No information on dropout was available for 15 mFND patients. In the ONP group, 20 patients ended treatment early, and two patients’ therapists discontinued the sessions early. Three believed therapy was ineffective or harmful, and five reported miscellaneous reasons. No information on dropout was available for 14 ONP patients.
A comparison of patients in the mFND group who dropped out and those who completed therapy indicated no statistically significant differences in age, gender, marital status, race/ethnicity, employment, acceptance of psychological explanations, walking aid use, or treatment outcome scores. However, in the mFND group, the percentage who were victims of childhood sexual abuse was larger among those who dropped out than among those who completed therapy (36.7% versus 16.3%; χ2=3.9, 95% CI=–1.7 to 42.0, p=0.05).
Outcomes
To avoid bias in our analysis of symptom improvement, we included all 98 mFND and 76 ONP patients. This included patients for whom therapy was ongoing at the time of data collection, patients who did not complete the full course of therapy, and patients whose clinicians prematurely ceased sessions. Because all patients had completed an extensive course of treatment, an assessment of outcome was possible.
Table 2 presents data on improvement rates for patients in the mFND group. In total, 44 (49.4%) patients with mFND showed symptomatic improvement, compared with 40 ONP patients (58.0%) (a nonsignificant difference). Eight mFND patients (8.2%) got worse, compared with nine ONP patients (11.8%). No information on patients’ improvement was available for nine mFND patients (9.2%) and seven ONP patients (9.2%).
| Symptoms improved (N=44, 49.4%) | Symptoms worsened or remained same (N=45, 50.6%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | N | % | N | % | Unadjusted Odds ratio | 95% CI | p | Adjusted Odds ratiob | 95% CI | p |
| Gender | 0.97 | 0.4–2.5 | 0.95 | 0.34 | 0.02–5.2 | 0.44 | ||||
| Female | 32 | 72.7 | 33 | 73.3 | ||||||
| Male (reference) | 12 | 27.3 | 12 | 26.7 | ||||||
| Ethnicity | 1.6 | 0.7–4.0 | 0.30 | 0.20 | 0.01–3.0 | 0.24 | ||||
| White British | 32 | 72.7 | 28 | 62.2 | ||||||
| Other (reference) | 12 | 27.3 | 17 | 37.8 | ||||||
| Marital status | 1.05 | 0.5–2.4 | 0.91 | 1.7 | 0.1–25.2 | 0.72 | ||||
| Single, divorced, widowed, or separated | 24 | 54.5 | 24 | 53.3 | ||||||
| Married, civil partner, or cohabiting (reference) | 20 | 45.5 | 21 | 46.7 | ||||||
| Work | 2.5 | 1.0–6.2 | 0.05 | 1 | 0.4–23.0 | 1 | ||||
| Employed | 20 | 45.5 | 11 | 25.0 | ||||||
| Unemployed, retired, or sick leave (reference) | 24 | 54.5 | 33 | 75.0 | ||||||
| Is a health or social care worker | 3.1 | 1.1–9.0 | 0.04 | 21.1 | 0.3–1,596.0 | 0.17 | ||||
| Yes | 14 | 33.3 | 6 | 14.0 | ||||||
| No (reference) | 28 | 66.7 | 37 | 86.0 | ||||||
| Caregiver status | 1 | 0.3–3.7 | 1.0 | 0.06 | 0.01–5.6 | 0.22 | ||||
| Is a family caregiver | ||||||||||
| Yes | 5 | 11.9 | 5 | 11.9 | ||||||
| No (reference) | 37 | 88.1 | 37 | 88.1 | ||||||
| Has a caregiver | 0.5 | 0.2–1.4 | 0.18 | 0.15 | 0.01–2.5 | 0.19 | ||||
| Yes | 8 | 20.0 | 13 | 33.3 | ||||||
| No (reference) | 32 | 80.0 | 26 | 66.7 | ||||||
| Receives benefits | 0.2 | 0.09–0.6 | 0.002 | 0.22 | 0.01–7.2 | 0.40 | ||||
| Yes | 9 | 22.0 | 24 | 44.2 | ||||||
| No (reference) | 32 | 78.0 | 19 | 55.8 | ||||||
| Disability | 0.3 | 0.14–0.8 | 0.02 | 0.94 | 0.1–10.0 | 0.96 | ||||
| Uses wheelchair or walking aid | 15 | 36.6 | 26 | 63.4 | ||||||
| Does not use wheelchair or walking aid (reference) | 26 | 63.4 | 15 | 36.6 | ||||||
| Acceptance of a psychological explanation of symptoms before therapy | 4.6 | 1.5–13.9 | 0.007 | 36.7 | 2.1–627.0 | 0.02 | ||||
| Yes | 26 | 81.3 | 17 | 48.6 | ||||||
| No (reference) | 6 | 18.8 | 18 | 51.4 | ||||||
| History of childhood sexual abuse | 1.2 | 0.4–3.6 | 0.76 | 4.2 | 0.1–135.0 | 0.41 | ||||
| Yes | 8 | 23.5 | 8 | 20.5 | ||||||
| No (reference) | 26 | 76.5 | 31 | 79.5 | ||||||
| History of childhood physical abuse | 2.2 | 0.8–6.2 | 0.14 | 14.5 | 0.36–592.0 | 0.16 | ||||
| Yes | 13 | 63.9 | 8 | 20.5 | ||||||
| No (reference) | 23 | 36.1 | 31 | 79.5 | ||||||
TABLE 2. Logistic regression model assessing variables as predictors of symptom improvement among patients with motor functional neurological disorder (mFND) after receipt of cognitive-behavioral therapy (CBT)a
For patients in the mFND group, we compared sociodemographic characteristics of those whose symptoms improved and those whose symptoms stayed the same or got worse. Table 2 presents data for adjusted and unadjusted comparisons. In the logistic regression model, the only significant predictor of improvement was acceptance of a psychological formulation before therapy. The model explained 63% (Nagelkerke R2) of the variance in symptom improvement between those whose symptoms improved and those whose symptoms stayed the same and correctly classified 50% of cases.
The results below are based on subsets of the 98 mFND patients and 76 ONP patients for whom pre- and post-CBT scores were available for the CORE-OM, HoNOS-ABI, and PHQ-9 (Figure 1; see the figure footnote for the sample sizes). Among patients in the mFND group, the mean global CORE-OM score decreased from a mean of 15.5 (SD=6.2) (clinically moderate) pre-CBT to a mean of 10.0 (SD=6.6) (clinically low) post-CBT (t=3.9, df=23, 95% CI=2.6–8.3, two-tailed p=0.001). Scores of patients in the ONP group also decreased significantly, from 16.3 (SD=6.8) (clinically moderate) to 12.8 (SD=6.6) (clinically mild) (t=2.9, df=23, 95% CI=1.06–5.9, two-tailed p=0.007).
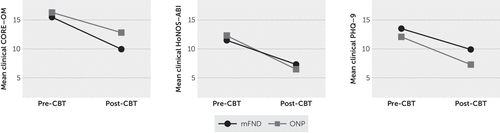
FIGURE 1. Mean scores on three instruments for two patient groups before and after receipt of outpatient cognitive-behavioral therapy (CBT)a
a Pre- and post-CBT scores were available as follows for subsets of patients with motor functional neurological disorder (mFND) and patients with other neuropsychiatric conditions (ONP): Clinical Outcomes in Routine Evaluation–Outcome Measure (CORE-OM), mFND, N=24; ONP, N=24; Health of the Nation–Acquired Brain Injury (HoNOS-ABI), mFND, N=22; ONP, N=15; and nine-item Patient Health Questionnaire (PHQ-9), mFND, N=16; ONP, N=10. CORE-OM scores range from 0 to 40 as follows: healthy, 0–5; low level, 5–10; mild, 10–15; moderate, 15–20; moderate to severe, 20–25; and severe, 25–40. HoNOS-ABI scores range from 0 to 48, with higher scores indicating greater severity. PHQ-9 scores range from 0 to 27 as follows: no depression, 0–4; mild, 5–9; moderate, 10–14, moderately severe, 15–19; and severe, 20–27.
We conducted a repeated-measures (pre- versus post-CBT) ANOVA on CORE-OM scores, with patient group (mFND versus ONP) as a fixed factor. The main effect was statistically significant (F1=24.3, p=0.001, partial η2=0.35). The Bonferroni-corrected interaction between mFND and ONP groups and the change over time (pre- versus post-CBT) was not significant (F1,46=1.13, p=0.30, partial η2=0.02).
In the mFND group, the mean HoNOS-ABI score dropped significantly, from 11.5 (SD=6.0) to 7.3 (SD=5.0) (Z=–3.1, p=0.002). In the ONP group, the mean HoNOS-ABI score also decreased significantly, from 12.3 (SD=7.0) to 6.5 (SD=4.0) (Z=–3.0, p=0.003). A two-way repeated measures ANOVA found a significant main effect (F1=22.6, p=0.001, partial η2=0.39); however, no significant interaction was found between the groups’ changes in pre- and post-CBT scores (F1,35=0.58, p=0.45, partial η2=0.02).
In the mFND group, a statistically significant decrease in PHQ-9 scores was observed post-CBT, from 13.5 (SD=7.0) to 9.9 (SD=6.0) (t=2.6, df=15, 95% CI=0.6–6.5, two-tailed p=0.02). A repeated-measures two-way ANOVA found a significant main effect (F1=10.1, p=0.01, partial η2=0.29); however, the interaction between the mFND and ONP groups and the change in pre- and post-CBT scores on the PHQ-9 was not statistically significant (F1,24=0.22, p=0.64, partial η2=0.01).
CORE-OM scores were significantly correlated with HoNOS-ABI scores (r=0.68, p=0.01) but not with PHQ-9 scores (r=0.78, p=0.06). HoNOS-ABI and PHQ-9 scores were not significantly correlated (r=0.26, p=0.47).
For all three measures, we compared sociodemographic characteristics of patients with available scores and those with no available scores. No significant differences were noted.
Discussion
In this small, retrospective chart review, results suggested that outpatient CBT treatment for mFND had positive effects on motor symptoms, distress, depression, general health, and social functioning. Among the patients in the mFND group, half saw improvements in their physical symptoms, and only a small proportion saw their symptoms worsen (8.2%).
We evaluated whether specific characteristics contributed to symptom improvement. Previous positive prognostic factors in FND include being married (31, 32) and younger age at onset (33, 34). One study found that females were more likely than males to recover (3), but this has not been reported elsewhere (31, 35–37). We found no effect of gender, race/ethnicity, marital status, sexual abuse, or age at symptom onset on symptom improvement. However, the long delay we observed between symptom onset and the offer of treatment is a concern for NHS services.
Our regression analysis revealed a strong predictive variable in symptom improvement: acceptance of psychological accounts of symptoms prior to receipt of CBT, which corroborates previous findings (32, 38, 39). By “psychological,” we mean an information-processing account that invokes attentional processes, attribution errors, and behavioral avoidance and that acknowledges the temporal relationships between symptoms and “stress,” mood, anxiety, or dissociation. When patients do not accept a psychological formulation, CBT therapists may invest more time in explaining this perspective, patients may be less likely to use therapeutic tools outside the clinic, and building a therapeutic alliance may be more challenging.
Symptom severity might independently explain symptom improvement and patients’ acceptance of a psychological formulation prior to CBT. In our analysis, we used patients’ use of a walking aid as a proxy of symptom severity, and pre-CBT psychological acceptance remained a significant predictor of improvement. However, it should be noted that use of walking aids is a broad measure and is relevant to most, but not all, of the mFND patients in this study.
Although pre-CBT acceptance of psychological explanations predicted patient improvement, three patients with mFND did not accept this explanation after receipt of CBT but nonetheless experienced symptomatic improvements. Saifee et al. (39) argued that psychological attributions could be used as a criterion to select patients for CBT. Our findings suggest that improvement may be possible regardless of attribution, albeit in a small proportion of patients. Although symptom reattribution is an important part of CBT, other techniques, such as mindfulness, establishing a sleep routine, and helping solve obstacles to recovery, may be as effective.
That only half the mFND group experienced physical symptom improvements might appear low, but previous literature indicates that FND prognosis is poor. A systematic review found that 39% of patients with mFND had the same or worse symptoms at follow-up, and only 20% had complete remission (40). An RCT testing CBT for patients with medically unexplained symptoms (N=79) reported that 51% of patients maintained improvement at 12-month follow-up, a finding comparable to our own (17).
The goal of CBT in functional disorders may not always be the immediate reduction of physical symptoms but rather improvement in cognitions and behaviors associated with symptoms. Patients’ goals are commonly discussed and agreed upon at the start of therapy. Had our symptom score derived purely from the goals set at the start of therapy, it is possible that a higher proportion of patients would have been classed as improved.
The psychometric measures showed significant improvements in both groups. The HoNOS-ABI is clinician rated, and it is possible that clinicians give more favorable scores at the end of treatment. Many NHS services, however, implement quality control measures, such as independent assessors. The CORE-OM and PHQ-9 are self-report scales and thus are not subject to clinician bias. Only a minority of patients in both groups had a complete set of pre- and posttreatment scores, which may represent a biased sample. To account for this, we compared sociodemographic differences on all three measures between patients for whom pre- and post-CBT scores were available and patients whose pre- or post-CBT scores or both were missing. No differences were found. We conducted a further analysis, assessing pre- and post-CBT scores according to the treating clinician and found no differences.
There are several weaknesses inherent in this observational study. The observed improvement in measures may be explained by a placebo effect; a regression-to-the-mean phenomenon; nonspecific effects of psychotherapy, such as the therapeutic relationship; or other therapeutic inputs, such as medication, physiotherapy, or occupational therapy. The focus of CBT sessions may have been different for mFND and ONP patients, which may have accounted for a proportion of the observed differences. However, our findings suggest that CBT in patients with functional disorders is at least as effective as CBT for patients with other neuropsychiatric conditions and significant psychological comorbidities. In fact, the results for patients in our comparison group make a unique contribution to the literature on the range of disorders responsive to a tailored CBT intervention.
The numbers in our study were relatively small, and our use of a medical register means that clerical errors could not be corrected. We could not blind the data collector. Because of the lack of availability of outcome scores, we included scores available within 180 days of the assessment or final CBT session—a long window of time in which psychological symptoms could have fluctuated. Interpretations and extrapolations from our regression analysis are also limited because of the small sample and the large number of observations. This study represented patients willing to accept a referral. The national referral status of the clinic may restrict attendance of patients living further from the clinic, a specific concern in a group with chronic motor deficits. We do not know whether the observed improvements were sustained over a longer period.
Finally, unlike a traditional RCT, in our study clinicians were not following a treatment manual. However, our naturalistic results do not reflect the imposition of strict selection criteria, which can limit generalizability. Instead this study offers useful information on the practicalities of delivering CBT in the NHS. Our results also provide information on CBT refusal, findings potentially pertinent to future service planning.
Conclusions
This study provided evidence that CBT is feasible and effective for some patients with mFND. The findings may provide a basis for rejecting the therapeutic nihilism sometimes associated with FND. Future research could compare the outcomes of patients with nonepileptic seizures and patients with mFND treated with CBT. An RCT with extensive follow-up would help confirm (or refute) our preliminary results, account for the placebo effect and the effect of other concurrent treatment interventions, establish which elements of CBT are most effective, and estimate how long patients might expect to benefit after therapy cessation.
1 : Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC, American Psychiatric Association, 2013Crossref, Google Scholar
2 : Randomised feasibility study of physiotherapy for patients with functional motor symptoms. J Neurol Neurosurg Psychiatry 2017; 88:484–490Crossref, Medline, Google Scholar
3 : Functional movement disorders: successful treatment with a physical therapy rehabilitation protocol. Parkinsonism Relat Disord 2012; 18:247–251Crossref, Medline, Google Scholar
4 : Physiotherapists and patients with functional (psychogenic) motor symptoms: a survey of attitudes and interest. J Neurol Neurosurg Psychiatry 2012; 83:655–658Crossref, Medline, Google Scholar
5 : Inpatient physiotherapy for functional (psychogenic) gait disorder: a case series of 35 patients. Mov Disord Clin Pract 2016; 3:603–606Crossref, Medline, Google Scholar
6 : Specialist inpatient treatment for severe motor conversion disorder: a retrospective comparative study. J Neurol Neurosurg Psychiatry 2014; 85:895–900Crossref, Medline, Google Scholar
7 : Diagnosis and rehabilitation strategies for patients with hysterical hemiparesis: a report of four cases. Arch Phys Med Rehabil 1998; 79:709–714Crossref, Medline, Google Scholar
8 : Successful rehabilitation in conversion paralysis. Br Med J (Clin Res Ed) 1986; 292:1730–1731Crossref, Medline, Google Scholar
9 : Multidisciplinary clinic for functional movement disorders (FMD): 1-year experience from a single centre. J Neurol Neurosurg Psychiatry 2018; 89:1011–1012Crossref, Medline, Google Scholar
10 : The efficacy of cognitive behavioral therapy: a review of meta-analyses. Cognit Ther Res 2012; 36:427–440Crossref, Medline, Google Scholar
11 : Cognitive behavioral therapy for psychogenic movement disorder. Mov Disord 2009; 24:1856–1857Crossref, Medline, Google Scholar
12 : Cognitive behavioural therapy and adjunctive physical activity for functional movement disorders (conversion disorder): a pilot, single-blinded, randomized study. Psychother Psychosom 2016; 85:381–383Crossref, Medline, Google Scholar
13 : Cognitive-behavioral therapy for psychogenic nonepileptic seizures: a pilot RCT. Neurology 2010; 74:1986–1994Crossref, Medline, Google Scholar
14 : Guided self-help for functional (psychogenic) symptoms: a randomized controlled efficacy trial. Neurology 2011; 77:564–572Crossref, Medline, Google Scholar
15 : Cognitive behavioral therapy for psychogenic nonepileptic seizures. Epilepsy Behav 2009; 14:591–596Crossref, Medline, Google Scholar
16 : Multicenter pilot treatment trial for psychogenic nonepileptic seizures: a randomized clinical trial. JAMA Psychiatry 2014; 71:997–1005Crossref, Medline, Google Scholar
17 : Cognitive behavioural therapy for medically unexplained physical symptoms: a randomised controlled trial. BMJ 1995; 311:1328–1332Crossref, Medline, Google Scholar
18 : Efficacy of treatment for somatoform disorders: a review of randomized controlled trials. Psychosom Med 2007; 69:881–888Crossref, Medline, Google Scholar
19 : Cognitive behaviour therapy for chronic fatigue syndrome in adults. Cochrane Database Syst Rev 2008; (3):
20 : Cognitive-behavioral therapy for somatization and symptom syndromes: a critical review of controlled clinical trials. Psychother Psychosom 2000; 69:205–215Crossref, Medline, Google Scholar
21 : Cognitive behavioural therapy for medically unexplained symptoms: a critical review of the treatment. Behav Ther 2001; 32:537–538Crossref, Google Scholar
22 : Behavioral medicine approaches to somatoform disorders. J Consult Clin Psychol 2002; 70:810–827Crossref, Medline, Google Scholar
23 : Non-pharmacological interventions for somatoform disorders and medically unexplained physical symptoms (MUPS) in adults. Cochrane Database Syst Rev 2014; (11):
24 : Interventions for the treatment and management of chronic fatigue syndrome: a systematic review. JAMA 2001; 286:1360–1368Crossref, Medline, Google Scholar
25 : Cognitive-behavioural therapy v structured care for medically unexplained symptoms: randomised controlled trial. Br J Psychiatry 2008; 193:51–59Crossref, Medline, Google Scholar
26 : Cohort profile of the South London and Maudsley NHS Foundation Trust Biomedical Research Centre (SLaM BRC) case register: current status and recent enhancement of an electronic mental health record-derived data resource. BMJ Open 2016; 6:
27 : Suitability and utility of the CORE-OM and CORE-A for assessing severity of presenting problems in psychological therapy services based in primary and secondary care settings. Br J Psychiatry 2005; 186:239–246Crossref, Medline, Google Scholar
28 : HoNOS-ABI; a clinically useful outcome measure? Psychiatr Bull 2001; 25:421–422Crossref, Google Scholar
29 : HoNOS-ABI: a reliable outcome measure of neuropsychiatric sequelae to brain injury? Psychiatr Bull 2005; 29:53–55Crossref, Google Scholar
30 : Validity and reliability of Patient Health Questionnaire-9 and Patient Health Questionnaire-2 to screen for depression among college students in China. Asia-Pacific Psychiatry 2013; 5:268–275Crossref, Medline, Google Scholar
31 : Slater revisited: 6 year follow up study of patients with medically unexplained motor symptoms. BMJ 1998; 316:582–586Crossref, Medline, Google Scholar
32 : Psychiatric outcome in patients with a psychogenic movement disorder: a prospective study. Neuropsychiatry Neuropsychol Behav Neurol 2001; 14:169–176Medline, Google Scholar
33 : The 12 year prognosis of unilateral functional weakness and sensory disturbance. J Neurol Neurosurg Psychiatry 2003; 74:591–596Crossref, Medline, Google Scholar
34 : Organic syndromes diagnosed as conversion disorder: identification and frequency in a study of 85 patients. J Psychosom Res 2000; 49:7–12Crossref, Medline, Google Scholar
35 : The prognosis of fixed dystonia: a follow-up study. Parkinsonism Relat Disord 2009; 15:592–597Crossref, Medline, Google Scholar
36 : Long-term prognosis of patients with psychogenic movement disorders. Parkinsonism Relat Disord 2006; 12:382–387Crossref, Medline, Google Scholar
37 : Motor conversion disorder: a prospective 2- to 5-year follow-up study. Psychosomatics 1998; 39:519–527Crossref, Medline, Google Scholar
38 : Neurology out-patients with symptoms unexplained by disease: illness beliefs and financial benefits predict 1-year outcome. Psychol Med 2010; 40:689–698Crossref, Medline, Google Scholar
39 : Inpatient treatment of functional motor symptoms: a long-term follow-up study. J Neurol 2012; 259:1958–1963Crossref, Medline, Google Scholar
40 : The prognosis of functional (psychogenic) motor symptoms: a systematic review. J Neurol Neurosurg Psychiatry 2014; 85:220–226Crossref, Medline, Google Scholar



