Functional Stroke Symptoms: A Narrative Review and Conceptual Model
Abstract
Stroke services have been reconfigured in recent years to facilitate early intervention. Throughout stroke settings, some patients present with functional symptoms that cannot be attributed to a structural cause. Emphasis on fast diagnosis and treatment means that a proportion of patients entering the care pathway present with functional symptoms that mimic stroke or have functional symptoms in addition to vascular stroke. There is limited understanding of mechanisms underlying functional stroke symptoms, how the treatment of such patients should be managed, and no referral pathway or treatment. Predisposing factors vary between individuals, and symptoms are heterogeneous: onset can be acute or insidious, and duration can be short-lived or chronic in the context of new or recurrent illness cognitions and behaviors. This article proposes a conceptual model of functional symptoms identified in stroke services and some hypotheses based on a narrative review of the functional neurological disorder literature. Predisposing factors may include illness experiences, stressors, and chronic autonomic nervous system arousal. Following the onset of distressing symptoms, perpetuating factors may include implicit cognitive processes, classical and operant conditioning, illness beliefs, and behavioral responses, which could form the basis of treatment targets. The proposed model will inform the development of theory-based interventions as well as a functional stroke care pathway.
Stroke is a medical emergency requiring identification and treatment within a critical time frame. Many stroke services have undergone reconfiguration to improve clinical outcomes. For example, in 2010, the National Health Service in England centralized stroke services in large metropolitan areas, leading to decreases in mortality and reductions in health care costs (1). The time-critical nature of stroke intervention results in a low threshold for patient admissions; consequently, a percentage of patients in stroke care pathways will not have stroke but conditions that mimic stroke. A proportion of these are “medical mimics,” with symptoms attributable to medical conditions, such as Bell’s palsy, migraine, and syncope. The others can be classified as “functional stroke mimics” (FSMs), with symptoms inconsistent with structural damage or physical disease that is attributable to a functional neurological condition (2). This includes a subset of patients with symptoms that match a DSM diagnosis of conversion disorder (DSM-5 300.11) or an ICD-10 diagnosis of dissociative (conversion) disorders (F44), but who are unlikely to receive such diagnoses in U.K. stroke settings.
Evidence from large single-center studies suggests that up to 26% of patients with suspected stroke have conditions that mimic stroke (3), and up to 8% are FSMs (2, 4). Functional presentations to stroke settings are an underrecognized and underresearched health care issue. Importantly, functional stroke symptoms can also occur in patients with vascular stroke. A previous study reported that a quarter of patients with functional motor symptoms had a comorbid organic movement disorder (5), and a review of evidence concluded that a third have history of neurological disease (6).
Identification of Functional Stroke Symptoms
Generally, functional symptoms are more common in women, and a recent meta-analysis confirmed this pattern in FSMs (2). Patients with functional stroke are, on average, younger than those with vascular stroke and younger than medical stroke mimics (2, 7), but they are older than patients in neurology clinics with other functional syndromes (8). A recently published meta-analysis indicates that patients with functional stroke presentations are more often identified in acute stroke services, compared with community settings or ambulance services (9). Once patients are in the stroke care pathway, multidisciplinary stroke experts investigate symptoms by using detailed neuroimaging techniques, clinical examinations, and physiofunctional assessments before reaching any diagnosis. Analysis of stroke admission data has shown that approximately a quarter of FSMs report a history of functional complaints (10). Patients with functional stroke present with a full range of symptoms, compared with patients with vascular stroke (2). Gargalas et al. (11) found that FSMs present with more weakness and more speech and sensory disturbance, but symptomatic features were not a reliable way of distinguishing FSMs from those with vascular stroke. It is not known whether vascular stroke patients with comorbid functional symptoms present with similar clinical features.
Heterogeneity
FSMs are a heterogeneous patient group. Presenting symptoms range in form and severity and can occur in one or multiple modalities (2). Case series and retrospective studies (12, 13) have shown that functional stroke-like symptoms can have a sudden or more insidious onset, with acute onsets more often associated with anxiety. Moreover, symptoms may be transient or develop into a more chronic condition. This heterogeneity is a challenge when developing a conceptual model of functional stroke symptoms. Further evidence for these patterns is expected from a recently completed prospective study. Predisposing and precipitating factors may vary between patients who have only functional presentations versus those who have significant functional symptoms alongside a vascular stroke and between individuals with acute versus chronic syndromes. The extent of such variation is poorly quantified.
Rationale
Across all health specialties, functional symptoms are poorly understood by patients and practitioners. Patients often enter a medical “no man’s land,” never receiving a full explanation for their symptoms, which negatively affects health outcomes (14, 15). In neurological populations, patients with functional seizures have been found to wait an average of 7.2 years for a diagnosis (16), although this evidence requires updating. Clinicians report that they avoid giving functional diagnoses because of a lack of perceived expertise and concern that consultations could become unpleasant and confrontational (17). Although pursuing the “ultimate cause” of symptoms is not always helpful or necessary for successful recovery, patients should expect a robust explanation for their symptoms; instead, patients report worries that they will be perceived as time wasting, and some believe that their clinicians lack knowledge and awareness of their condition (18). These themes may be amplified within specialized acute stroke services, where there is less time to engage and build doctor-patient rapport. Compared with other patients on the stroke ward (11, 19), those with functional stroke presentations have shorter lengths of stay (20). Löwe and Gerloff (20) argued that this limits the chance of patients’ receipt of a thorough assessment, a positive diagnosis, and time to adjust to distressing neurological symptoms. These issues indicate a need for an accessible and efficient formulation that clinicians can adopt to engage in meaningful conversations about patients’ symptoms and that can guide referral decision making.
Many explanatory models for functional symptoms are available (21). However, because of significant heterogeneity between different functional disorders and individual differences within syndromes (22), there is a demand for functional symptom–specific models. Understanding and approaching FSMs requires some unique considerations: namely, the acute context of their presentation and the strong social and medical response to symptoms. Moreover, clinicians are performing assessments and providing diagnoses not in outpatient or clinic settings but in emergency settings or on wards with an intense turnover of patients. Therefore, an explanatory model of functional symptoms adapted to this specific medical context would be valuable.
This article presents a review of the literature relevant to understanding functional stroke symptoms. Concordant with the Research Domain Criteria Initiative (23), we present a transdiagnostic model of functional symptoms in stroke, drawing on multiple domains. We describe predisposing and perpetuating factors that could inform future research and propose a possible care pathway. The heterogeneity of patients with functional symptoms will be considered throughout.
Predisposing Factors
Life Events and Trauma
Illness experiences are framed by cultural and social contexts (24). Historically, functional symptoms were associated with psychological trauma and stress, and for a proportion of patients, these are important risk factors. A systematic review and meta-analysis of case-control studies reported an association between functional motor disorder (FMD) and maltreatment (25). Likewise, prospective studies have reported that functional neurological disorders (FND) have stronger associations with childhood sexual abuse and physical neglect than do organic disorders (26–28). Experiences of sexual abuse are more common (though not invariable) in patients with FND, compared with patients diagnosed as having depression and healthy control subjects, and a greater proportion of patients diagnosed as having FND report severe life events in the month prior to symptom onset (29). Certain events may be more common in these patients—for example, experiences of emotional neglect, bullying, and other interpersonal problems (25–27, 30). Changes to the FND diagnostic criteria reflect findings that stress, abuse, or trauma are sufficient but not necessary for the occurrence of functional symptoms (31). Supporting this change are findings from a recent study using medical records from a large psychiatric organization in London, which reported similar rates of childhood sexual abuse in FND patients and those with general psychiatric diagnoses (27). Therefore, although a thorough patient history is always important, evidence of past trauma or psychiatric comorbidity is not necessary for the diagnosis of functional stroke symptoms.
Illness-Relevant Experiences and Social Environment
A review of evidence suggests that experiences of organic illness can influence later functional symptoms (32). A high proportion of FMD patients and patients with functional seizures have a history of organic neurological disorders, physical injury, or surgery (6, 33), and injury to the affected limb has been found to be associated with acute onset of functional weakness (34). A systematic review of functional motor and sensory symptoms reported physical injury in 37% of patients prior to symptom onset (35). It is noteworthy that injury was more likely to be reported prior to functional paraparesis (35), which might indicate that different triggers are relevant to lateralized or hemiplegic presentations, as seen in FSMs. Analyses of data from a national birth cohort study showed that poor parental health during adolescence was longitudinally associated with medically unexplained symptoms occurring in adulthood (36). This research indicated a role for symptom modeling in FND etiology, which we expect applies to functional stroke.
Exposure to stroke or cardiovascular disease, in immediate social circles or in oneself, may predispose individuals to functional stroke symptoms, because a lay understanding of bodily function informs presenting symptomology and interpretation. Hypothetically, a history of stroke may bias a patient’s response to harmless sensory symptoms, which could contribute to the occurrence of functional stroke symptoms. Another likely predisposing factor is the presence of known stroke risk factors, such as hypertension (11, 19, 32). Individuals (and physicians) may be primed to expect stroke symptoms if told that their risk of stroke is increased and may misinterpret bodily sensations accordingly (see the section below on cognitive biases). A contemporary model outlines how illness experiences and expectations are neurally encoded and then key to the generation of functional symptoms, which may explain why particular symptoms occur in an individual patient (37). Exposure to stroke-related illness needs to be assessed in FSMs.
The wider social context may also act as a predisposing factor for functional stroke. Löwe and Gerloff (20) suggested that cultural beliefs are a vulnerability factor in the development of functional symptoms and use of health care services. In a consecutive case series in a Middle Eastern hospital, patients of Arab and African ethnicity reported twice the rate of functional stroke, compared with those who were Eastern, Asian, or Western, although this effect may also be partly explained by socioeconomic status (38).
Public health campaigns that aim to raise awareness of stroke symptoms have been shown to result in increased visits to stroke websites, calls to helplines, visits to emergency departments for stroke symptoms, and rates of thrombolysis treatment (39). Although such effects may be short term (40), it is plausible that such campaigns influence presentations of functional stroke symptoms. Careful prospective controlled studies of such campaigns are needed to test this hypothesis scientifically.
Chronic Activation of Stress Pathways
Biological stress pathways are implicated as risk factors for functional symptoms. Aybek et al. (41) reported a correlation between life events, cortisol, and α-amylase levels in FND patients but not in control subjects. Furthermore, FND patients have higher baseline cortisol and α-amylase levels, compared with healthy control subjects (41), suggesting chronic activation of biological stress pathways, which may precede symptom onset. Activation of stress pathways may therefore have a similar role in predisposing individuals to functional stroke symptoms, although evidence is currently limited.
Other Potential Predisposing Factors
Across the functional disorder literature, there is a growing consensus that in addition to the social and biological factors mentioned, patients are predisposed to functional symptoms by premorbid cognitive biases (37, 42, 43) and aberrant emotion processing (44). In their integrative conceptual model of functional seizures, Reuber and Brown (45) suggested that preexisting mental representations of symptoms form a “scaffold” for functional symptoms, which is activated when symptoms occur. We suggest that a similar process occurs in functional stroke, where, for example, a memory of a past ischemic event may evoke a pattern of stroke-like symptoms. However, in this review, we emphasize the role of cognitive and emotional processes as perpetuating factors that become salient after the occurrence of symptoms, rather than predisposing symptom onset (Figure 1).
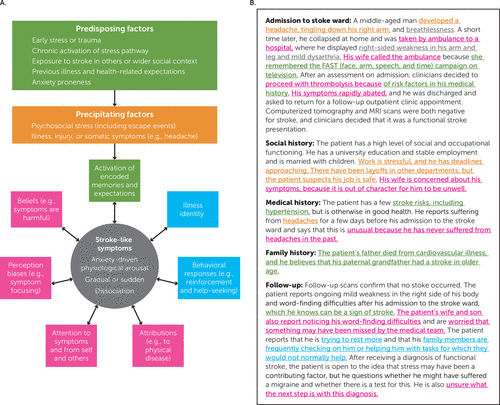
FIGURE 1. Conceptual model of functional stroke symptomsa
a Panel A shows examples of perpetuating factors (encircling stroke-like symptoms), and a corresponding hypothetical clinical vignette is shown in panel B.
Precipitating Factors
Acute Stressors
Compared with patients with depression or healthy control subjects, patients with functional diagnoses report more life events prior to symptom onset (29). Keynejad et al. (31) suggested that patients with high biological propensity for functional symptoms may be at risk following a relatively minor stressor, compared with individuals with low biological susceptibility, who may be at risk only after exposure to a severe stressor. In addition to acting as predisposing risk factors, physical illnesses may act as a precipitating event for a proportion of FSMs. In interviews, 80% of FMD patients reported physical events, such as illness, injury, or infection, in the preceding 3 months, and physical injury to the symptomatic limb was reported by 20% of patients with sudden-onset weakness (12, 33). Such events are acute stressors in themselves, and they also influence the interpretation of symptoms, potentially increasing the likelihood of perceiving symptoms as threatening.
Physiological Arousal
Initial stroke-like symptoms are likely a result of physiological arousal, which is misinterpreted and drives further physical symptoms (Figure 1). In retrospective interviews, 59% of patients with sudden-onset functional weakness reported symptoms of panic (34). In another retrospective study, 38% of FMD patients reported a panic attack at the onset of symptoms (33). Persons experiencing such symptoms would likely believe that they were indicative of a serious medical disease, particularly given increasing public knowledge about stroke. Anxiety triggers further autonomic system arousal, adding to initial symptoms. This leads to the testable hypothesis that anxiety symptoms are a more frequent accompaniment to the onset of functional stroke symptoms, compared with vascular stroke and potentially other functional presentations.
Dissociative Processes
Compartmentalization, a form of dissociation, may also play a role in the emergence of functional symptoms. In this account, symptoms result from disturbances in processes underlying consciousness and self-control, such as memory retrieval. Applying this theory, stroke-like symptoms arise from “the retrieval of inappropriate perceptual hypotheses from memory during the creation of primary (mental) representations” (46). For example, unexplained weakness could arise as a result of the automatic memory selection of a movement task leading to inappropriate motor action.
Perpetuating Factors
Cognitive Biases
Perception.
Previous models of “unexplained” symptoms suggest that autopoietic cognitive and attentional processes are central to functional symptom maintenance (47). For instance, Deary et al. (47) suggested that persons with “medically unexplained” symptoms perceive somatic symptoms as threatening, noxious, and unpleasant—a bias termed “somatosensory amplification” (48). Observational studies have found associations between somatosensory amplification and the number of somatic symptoms and have reported that somatosensory amplification may mediate the association between symptoms and psychological distress (48). Similarly, perceptions of symptoms as threatening and severe and low in personal control are associated with poor health-related quality of life in patients with functional seizures (49). Recent longitudinal studies have provided evidence that cognitive biases can precede functional symptoms. In an epidemiological study of women, catastrophic misperceptions of ambiguous bodily reactions were associated with greater risk of developing a somatoform disorder (43). However, a systematic review found that such cognitive biases had descriptive but not predictive validity, because most supporting research was limited to cross-sectional designs and small samples (42). Overall, evidence currently implicates perception biases as important symptom-perpetuating factors. In the case of FSMs, biases in the initial perception of functional stoke symptoms are likely to affect the interpretation of ongoing symptoms and help-seeking behavior.
Attributions and beliefs.
Relatedly, patients with functional versus organic disorders differ in their symptom attributions. Patients with functional motor symptoms have a more external locus of control (attribution style), compared with patients with weakness from neurological conditions (50). A case-control study revealed that patients with functional weakness were less than half as likely as those with organic weakness to attribute symptoms to stress (51). In functional stroke, the experience of distressing symptoms, followed by referral and admission to acute stroke services, coupled with intense medical attention and diagnostic tests, may shape organic illness attributions. Moreover, we have observed that patients seldom receive a clear, positive diagnosis of functional disorder; consequently, they are left with a poor understanding of their condition or a high level of uncertainty about their symptoms that can increase existing anxiety.
Attention and expectation.
Vigilance and attention to somatic symptoms can exacerbate functional symptoms by reinforcing perceptual and attributional biases (52). For instance, patients with functional tremor reported greater attention to their tremor, compared with patients with organic tremor (53). Patients with functional tremor also demonstrated a greater expectation of tremor symptoms, reporting tremor to be present 85% of the waking day, whereas actigraphy recordings revealed tremor to occur 3.9% of the time (54). Patients with organic tremor, however, reported symptoms as occurring 57% of the day, when they occurred only 24.8% of the time, according to actigraphy monitoring. These differences may well influence the effect that the symptoms have on the person’s well-being and his or her beliefs about symptom severity. A systematic review of attention biases in persons with FND, chronic fatigue syndrome, and fibromyalgia reported decreased external-oriented attention, compared with healthy control subjects, indicated by difficulties with divided attention, multitasking, and information processing (55). Individuals with functional seizures experience increased attention to negative social cues or threatening stimuli, which can lead to avoidance behaviors that ultimately perpetuate symptoms (see below) (56). Henningsen et al. (57) suggested that as symptoms become more chronic, symptom expectations become represented in neural pathways; subsequently, autonomic nervous system arousal produces sensory input to reflect predictions in order to reduce prediction errors and maintain homeostasis. Applying these concepts to functional stroke, we suggest a multilateral relationship between symptoms and cognitive biases informed by predisposing factors, whereby symptoms can elicit cognitive biases and be initiated or perpetuated by them (bidirectional arrows in Figure 1).
Emotion Processing
A significant proportion of research emphasizes the role of emotion processing in functional disorder etiology. Compared with healthy control subjects, patients with functional seizures experience difficulties in emotion identification, selection and implementation of regulation strategies, and accurately appraising external emotion information (58). A systematic review of experimental studies provided neurobiological support for these findings; during emotion experimental tasks, functional symptoms were found to be associated with increased limbic and motor region activation and aberrant prefrontal and paralimbic activation functionally connected to motor areas (44). Compared with healthy control subjects, FND patients showed increased activity and connectivity between the amygdala and supplementary motor area during the recall of stressful events or when responding to negative emotions or emotive faces (59, 60). Subsequently, Pick et al. (44) suggested that aberrant emotion processing mediates associations between environmental risk factors and functional symptoms. Moreover, such aberrant neural activity has shown associations with symptom duration and severity (59), indicating a neurophysiological pathway for persistent functional symptoms. Overall, findings suggest that emotion-related processes play a key role in functional symptom maintenance and form an important treatment target, but this has not been explored in FSMs.
The three-systems model (TSM) provides a framework to understand the mechanism through which functional stroke symptoms are reactive to external events via emotional behavior (61–64). The TSM suggests that individual emotional responses reflect the unique pattern of three discordant components: subjective cognitive states, physical arousal, and behavioral responses, with each component having a different loading (65). We postulate that individuals with functional stroke symptoms demonstrate an emotional response pattern loaded heavily on physiological components. As such, functional stroke symptoms can be conceptualized as autonomic reactions to a life event or social context, with varying degrees of concordance from cognitive (e.g., worry, catastrophizing) and behavioral (e.g., avoidance, help seeking) components. The TSM can account for the heterogeneity of patients with functional symptoms. Those whose emotional responses are dominated by physiological and behavioral components may be more likely to interpret symptoms as a deterioration in physical health, because they do not experience corresponding subjective cognitions (e.g., “I feel anxious”). Patients with concordant cognitive components may be more likely to attribute symptoms to stress or emotional states. These differences have important implications for treatment (66).
Stress Response
As symptoms continue and interfere with daily life, classical conditioning offers an explanation for how stress and functional symptoms can become coupled (32, 61). Physiological responses to stress (e.g., somatic symptoms) can become a conditioned response, and over time, the threshold for the physiological response falls until symptoms occur in response to minor stressors or even the memory of an event (32).
Social Context and Response to Symptoms
Stroke-like symptoms are understandably associated with a set of acute responses from patients, caregivers, and medical health professionals concerned about the possibility of serious cerebrovascular events. In the immediate aftermath of functional stroke symptoms, patients may experience intense medical attention, emergency hospital admission, and extensive physical assessments and treatment (13). Our systematic review (9) and previous studies (38) have reported that rates of functional symptoms are higher in acute medical services, compared with community settings, and are identified to a greater extent in samples receiving thrombolysis, compared with samples not receiving thrombolysis. Learning theory can provide an explanation for how such responses perpetuate symptoms. Symptom-related beliefs and behaviors are easily and quickly internalized as they are associated with positive reinforcers and negative consequences (67). Urgent responses from health professionals reinforce the perception of symptoms as serious and threatening and induce further anxiety. Care and attention received from family and friends can act as a positive reinforcer (68, 69). There is some evidence that the belief that stress is the cause of the symptoms is more common among the relatives of patients with FND than the patients themselves, meaning that relatives may encourage the patient to get more rest (70). It is also possible that to avoid further upsetting the patient, caregivers support patients’ views and coping behaviors, even if they disagree. Relatives have been found to be more pessimistic regarding the potential duration of symptoms and the possible emotional impact of the symptoms (70). Levy et al. (67) reported that children whose parents had more anxious, attentive responses to somatic symptoms complained of more symptoms than did children whose parents gave less solicitous responses. Wives of men who had experienced mild stroke reported taking on a hypervigilant role, which can sustain anxiety around symptoms and prevent full recovery (71).
In response to ongoing symptoms and social reinforcement, patients can develop an illness identity that contributes to symptom maintenance (72). This could be relevant for patients with more chronic functional stroke symptoms. Strong illness identities have been found to be associated with greater disability and poorer psychological outcomes in other functional patient groups, making illness identity an important treatment consideration (73).
Illness Behaviors
Following illness or injury, patients adapt their behavior to reduce the risk of further harm, but such a process can inhibit recovery. In a prospective study of anxiety after diagnosis of a stroke or transient ischemic attack, phobic anxiety disorders were the most common anxiety subtype (74). All anxious patients reported phobic avoidance in physical, social, and daily activities, and avoidance was associated with poorer quality of life, compared with patients who did not report avoidance (74). The associations between avoidance and functional symptoms suggest that avoidance is a perpetuating factor that reinforces illness-related beliefs and illness identity (75, 76). Observational studies have shown that patients admitted to a hospital for stroke spent almost 50% of time resting in bed and only 13% of time doing activities to support physical mobility (77). Inactivity before and after discharge can lead to problems such as postural hypotension, muscle atrophy, and loss of strength (78, 79). Caregivers may also contribute to inactivity when they assist patients with daily tasks or encourage rest. These issues are also relevant to functional stroke symptoms. Patients who experience unilateral weakness avoid using the affected side, even when symptoms have subsided, which leads to loss of strength. Subsequently, subjective weakness and soreness occur when the muscles are used, which reinforces beliefs of physical damage and increases attention to any weakness in the limbs. Similarly, patients may experience lightheadedness when active after prolonged rest, which can be perceived as a possible sign of stroke and can lead to further activity avoidance. Finally, patients may become more alert to unilateral symptoms, checking for facial asymmetry or being hypervigilant of any potential stroke-like symptoms.
Summary of the Model for Understanding Functional Stroke
Symptoms of physiological arousal, such as dizziness, palpitations, transient numbness, weakness, or fatigue, might ordinarily be normalized. However, in the context of particular predisposing and precipitating factors (i.e., illness-related experiences and significant life stressors), symptoms can be perceived as more sinister and can be attributed to physical injury or disease. Concurrently, increased attention is given to symptoms, and the reactions of caregivers and others reinforce beliefs that symptoms are indicative of a serious event. All these responses increase anxiety, which elicits further symptoms, help-seeking behavior, and other behavioral responses (45). In chronic cases, a cycle of symptoms, attention, anxiety, expectation, and cognitive and behavioral responses perpetuates symptoms and, in some patients, contributes to disability. It is likely that only parts of the suggested model (Figure 1) will apply to any individual patient, and during assessment, the model should be applied so that the patient is provided with an explanation that best reflects his or her experience.
Implications
Stepped-Care Pathway
Our model provides a preliminary theoretical framework with which to begin the design of a functional stroke care pathway, which could also benefit patients with stroke. Figure 2 shows a potential stepped-care pathway, similar to that previously recommended by the National Health Service Scotland (80), which we have adapted for functional stroke symptoms.
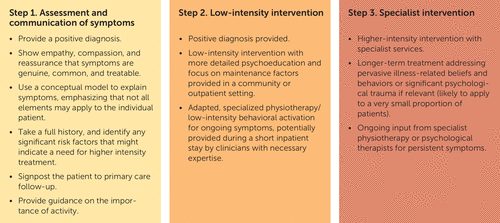
FIGURE 2. Suggested stepped-care pathway for functional stroke symptoms
Assessment of and Communication About Symptoms
First, our model can be used as an accessible tool for clinicians in stroke services to enhance their knowledge of functional stroke and communicate a model of understanding symptoms to patients. Considerable advice is emerging that is designed to inform neurologists about how best to manage FND, and this needs to be adapted and disseminated among stroke staff who work in relatively unique neurology settings (81–83). When possible, they should show by demonstration (e.g., drift without pronation and Hoover’s sign) and by explanations that avoid the perception that functional symptoms are a “last resort” diagnosis (84). Stroke doctors may be the starting point of the multidisciplinary approach and must be able to give clear, unambiguous explanations that set the tone for later interventions (85), even when patients present with transient, subjective, or sensory symptoms that may be more difficult to demonstrate.
Low-Intensity Intervention
Following clear communication of symptoms, the perpetuating factors outlined in our model could be targeted by interventions with use of cognitive or behavioral techniques in primary care or hospital settings (86). In line with long-standing recommendations (85) and a recent consensus statement (87), these interventions would need to be adapted and specialized to functional symptoms and could be delivered by a range of health professionals, such as psychologists, behavior therapists, and physiotherapists, who have the necessary expertise. Drawing on Chalder and Willis’s (22) comprehensive discussion of transdiagnostic versus specific processes in functional disorders, we suggest that a functional stroke intervention should start by identifying the unique pattern of cognitive, emotional, and behavioral processes relevant to the etiology of a patient’s symptoms. For example, patients who demonstrate a bias for cognitive responses may be more responsive to cognitive techniques, whereas those with a propensity for physiologically oriented responses and behaviors may benefit more from desensitizing interventions aimed at reducing arousal (66). Taking a transdiagnostic approach allows our model to be applicable to both FSMs and those with functional symptoms in addition to a vascular stroke. However, this relies on clinicians discussing with patients that symptoms and disability in stroke are, as in all other conditions, not entirely attributable to physical pathology but are also affected by cognitive and behavioral responses. Cognitively and behaviorally informed approaches have supporting evidence in improving outcomes for patients with functional symptoms (87–89).
Specialist Intervention
A small number of FSM patients may require higher-intensity and longer-term intervention with psychiatric or psychological services, a referral the stroke clinician should suggest in the discharge letter to the general practitioner. Case studies are useful references that provide detailed examples of how such treatments can be implemented with patients who have acute-onset functional symptoms (90). Our model posits that factors evident during assessment can determine the appropriate referral pathway for an individual patient; for example, anxiety, strong illness-related beliefs, and avoidance behavior are likely important treatment targets in any treatment pathway, whereas significant trauma history may require earlier input from more specialist services. Some evidence suggests that patients who have high levels of physical dysfunction may benefit from access to intensive, specialized physiotherapy and that this is especially effective if accessed during the acute onset of symptoms (91). Neurophysiotherapists in stroke or neurology wards are more likely to have the skills to provide this intervention, compared with clinicians on general medical wards or in community settings. Therefore, an impetus may exist to allow FSM patients to have a longer inpatient stay.
Considerations Throughout Intervention
Patient involvement.
Patients should be actively involved in the formulation and treatment of their symptoms. Research demonstrates that patients with functional symptoms who have high level of involvement in treatment planning and who self-monitor improvement have better outcomes, compared with patients with low involvement (92).
Overmedicalization.
A proportion of FSMs probably recover without intervention (11). Establishing a comprehensive care pathway could have the counterintuitive effect of reinforcing symptoms by inducing worry and illness beliefs and contributing to an illness identity, particularly when patients have transient symptoms. During assessment, clinicians must use their judgment to identify patients requiring immediate intervention versus those who may benefit from more of a “watchful waiting” approach. Our model suggests that patients who respond to symptoms with high levels of distress, catastrophic interpretations, and high levels of disability—comparable to that of patients with other somatic symptoms/functional disorders (93)—would likely benefit from more immediate access to support. Regardless, it would still be pertinent to give a comprehensive explanation for the patient’s symptoms, including explaining the interaction between psychological processes and physical symptoms (83). Equally important is recording that the patient experienced functional symptoms or, in cases in which there is also organic pathology, that there was a functional element to the presentation, because this is important in identifying a repeated or chronic pattern of symptoms in the future.
Conclusions
In this model, we suggest functional stroke has a multimodal etiology. We hypothesize that functional stroke symptoms have potentially distinct patterns of contributory factors and may not necessarily follow the same chronic course as other functional conditions, because age at onset is, on average, older and the history of symptoms results in referral to emergency stroke settings, rather than to other community or neuropsychiatry services. Instead, the occurrence of distressing physiological symptoms and the subsequent acute social response, including admission to an acute stroke unit, are key precipitating events, which initiate a cascade of responses that can sustain symptoms. Research is needed to test our hypothesized model because existing studies have neglected functional stroke presentations. There is a strong clinical imperative for research to inform a clear care pathway for functional stroke within stroke services, because patients currently have little opportunity to gain a full understanding of their symptoms and stroke clinicians lack guidelines on how to manage these presentations.
1 : Impact of centralising acute stroke services in English metropolitan areas on mortality and length of hospital stay: difference-in-differences analysis. BMJ 2014; 349:g4757Crossref, Medline, Google Scholar
2 : Incidence and outcome of functional stroke mimics admitted to a hyperacute stroke unit. J Neurol Neurosurg Psychiatry 2017; 88:2–6Crossref, Medline, Google Scholar
3 : The differential diagnosis of suspected stroke: a systematic review. J R Coll Physicians Edinb 2013; 43:114–118Crossref, Medline, Google Scholar
4 : The incidence of stroke mimics among stroke department admissions in relation to age group. J Stroke Cerebrovasc Dis 2008; 17:418–422Crossref, Medline, Google Scholar
5 : Psychogenic movement disorders: frequency, clinical profile, and characteristics. J Neurol Neurosurg Psychiatry 1995; 59:406–412Crossref, Medline, Google Scholar
6 : Psychogenic explanations of physical illness: time to examine the evidence. Perspect Psychol Sci 2016; 11:606–631Crossref, Medline, Google Scholar
7 : The incidence of stroke mimics among stroke department admissions in relation to age group. J Stroke Cerebrovasc Dis 2008; 17:418–422Crossref, Medline, Google Scholar
8 : Who is referred to neurology clinics? The diagnoses made in 3781 new patients. Clin Neurol Neurosurg 2010; 112:747–751Crossref, Medline, Google Scholar
9 : Epidemiology of functional stroke mimic patients: a systematic review and meta-analysis. Eur J Neurol 2019; Epub ahead of printCrossref, Google Scholar
10 : Clinical features associated with medically unexplained stroke-like symptoms presenting to an acute stroke unit. Eur J Neurol 2005; 12:81–85Crossref, Medline, Google Scholar
11 : Incidence and outcome of functional stroke mimics admitted to a hyperacute stroke unit. J Neurol Neurosurg Psychiatry 2017; 88:2–6Crossref, Medline, Google Scholar
12 : Functional weakness: clues to mechanism from the nature of onset. J Neurol Neurosurg Psychiatry 2012; 83:67–69Crossref, Medline, Google Scholar
13 : Safety of tPA in stroke mimics and neuroimaging-negative cerebral ischemia. Neurology 2010; 74:1340–1345Crossref, Medline, Google Scholar
14 : The prognosis of functional (psychogenic) motor symptoms: a systematic review. J Neurol Neurosurg Psychiatry 2014; 85:220–226Crossref, Medline, Google Scholar
15 : Enigmatic illness: narratives of patients who live with medically unexplained symptoms. Soc Theory Health 2004; 2:47–66Crossref, Google Scholar
16 : Diagnostic delay in psychogenic nonepileptic seizures. Neurology 2002; 58:493–495Crossref, Medline, Google Scholar
17 : Are there interactional reasons why doctors may find it hard to tell patients that their physical symptoms may have emotional causes? A conversation analytic study in neurology outpatients. Patient Educ Couns 2011; 85:e189–e200Crossref, Medline, Google Scholar
18 : "Blaming, shaming, humiliation": stigmatising medical interactions among people with non-epileptic seizures. Wellcome Open Res 2017; 2:55Crossref, Medline, Google Scholar
19 : Stroke mimic diagnoses presenting to a hyperacute stroke unit. Clin Med 2016; 16:423–426Crossref, Medline, Google Scholar
20 : Functional somatic symptoms across cultures: perceptual and health care issues. Psychosom Med 2018; 80:412–415Crossref, Medline, Google Scholar
21 , et al: Explanatory models of medically unexplained symptoms: a qualitative analysis of the literature. Ment Health Fam Med 7:223–231Medline, Google Scholar
22 : “Lumping” and “splitting” medically unexplained symptoms: is there a role for a transdiagnostic approach? J Ment Health 2017; 26:187–191Crossref, Medline, Google Scholar
23 : Research Domain Criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry 2010; 167:748–751Crossref, Medline, Google Scholar
24 : Social psychologic factors affecting the presentation of bodily complaints. N Engl J Med 1972; 286:1132–1139Crossref, Medline, Google Scholar
25 : Stressful life events and maltreatment in conversion (functional neurological) disorder: systematic review and meta-analysis of case-control studies. Lancet Psychiatry 2018; 5:307–320Crossref, Medline, Google Scholar
26 : Organic vs functional neurological disorders: the role of childhood psychological trauma. Child Abuse Negl 2017; 63:1–6Crossref, Medline, Google Scholar
27 : Characteristics of patients with motor functional neurological disorder in a large UK mental health service: a case-control study. Psychol Med (Epub ahead of print, Feb 18, 2019)Crossref, Google Scholar
28 : Childhood experiences and personality traits in patients with motor conversion symptoms. Acta Psychiatr Scand 1998; 98:288–295Crossref, Medline, Google Scholar
29 : Life events and escape in conversion disorder. Psychol Med 2016; 46:2617–2626Crossref, Medline, Google Scholar
30 : The impact of early trauma and recent life-events on symptom severity in patients with conversion disorder. J Nerv Ment Dis 2005; 193:508–514Crossref, Medline, Google Scholar
31 : Stress and functional neurological disorders: mechanistic insights. J Neurol Neurosurg Psychiatry 2019; 90:813–821Crossref, Medline, Google Scholar
32 : A review of functional neurological symptom disorder etiology and the integrated etiological summary mode. J Psychiatry Neurosci 2018; 43:170–190Google Scholar
33 : Physical precipitating factors in functional movement disorders. J Neurol Sci 2014; 338:174–177Crossref, Medline, Google Scholar
34 : Functional weakness: clues to mechanism from the nature of onset. J Neurol, Neurosurg Psychiatry 2012; 83:67–69Crossref, Medline, Google Scholar
35 : The role of physical injury in motor and sensory conversion symptoms: a systematic and narrative review. J Psychosom Res 2009; 66:383–390Crossref, Medline, Google Scholar
36 : Childhood risk factors for adults with medically unexplained symptoms: results from a national birth cohort study. Am J Psychiatry 1999; 156:1796–1800Medline, Google Scholar
37 : A Bayesian account of “hysteria”. Brain 2012; 135:3495–3512Crossref, Medline, Google Scholar
38 : Functional stroke mimics: incidence and characteristics at a primary stroke center in the Middle East. Psychosom Med 2018; 80:416–421Crossref, Medline, Google Scholar
39 : A time series evaluation of the FAST National Stroke Awareness Campaign in England. PLoS One 2014; 9:
40 : Can a media campaign change health service use in a population with stroke symptoms? Examination of the first Irish stroke awareness campaign. Emerg Med J 2014; 31:536–540Crossref, Medline, Google Scholar
41 : Objective biomarkers of stress in motor functional neurological (conversion) disorder (P6.211). Neurology 2017; 16:88Medline, Google Scholar
42 : Descriptive and predictive validity of somatic attributions in patients with somatoform disorders: a systematic review of quantitative research. J Psychosom Res 2013; 75:199–210Crossref, Medline, Google Scholar
43 : Catastrophizing misinterpretations predict somatoform-related symptoms and new onsets of somatoform disorders. J Psychosom Res 2016; 81:31–37Crossref, Medline, Google Scholar
44 : Emotional processing in functional neurological disorder: a review, biopsychosocial model and research agenda. J Neurol Neurosurg Psychiatry 2019; 90:704–711Crossref, Medline, Google Scholar
45 : Understanding psychogenic nonepileptic seizures: phenomenology, semiology and the Integrative Cognitive Model. Seizure 2017; 44:199–205Crossref, Medline, Google Scholar
46 : Different types of “dissociation” have different psychological mechanisms. J Trauma Dissociation 2006; 7:7–28Crossref, Medline, Google Scholar
47 : The cognitive behavioural model of medically unexplained symptoms: a theoretical and empirical review. Clin Psychol Rev 2007; 27:781–797Crossref, Medline, Google Scholar
48 : Clinical application of somatosensory amplification in psychosomatic medicine. Biopsychosoc Med 2007; 1:17Crossref, Medline, Google Scholar
49 : Predictors of health-related quality of life in patients with epilepsy and psychogenic nonepileptic seizures. Epilepsy Behav 2017; 68:153–158Crossref, Medline, Google Scholar
50 : Hopelessness and locus of control in patients with motor conversion disorder. Nord J Psychiatry 1999; 53:37–40Crossref, Google Scholar
51 : The symptom of functional weakness: a controlled study of 107 patients. Brain 2010; 133:1537–1551Crossref, Medline, Google Scholar
52 : Psychological mechanisms of medically unexplained symptoms: an integrative conceptual model. Psychol Bull 2004; 130:793–812Crossref, Medline, Google Scholar
53 : Attention to self in psychogenic tremor. Mov Disord 2011; 26:2575–2576Crossref, Medline, Google Scholar
54 : Believing is perceiving: mismatch between self-report and actigraphy in psychogenic tremor. Brain 2012; 135:117–123Crossref, Medline, Google Scholar
55 : A unifying theory for cognitive abnormalities in functional neurological disorders, fibromyalgia and chronic fatigue syndrome: systematic review. J Neurol Neurosurg Psychiatry 2018; 89:1308–1319Crossref, Medline, Google Scholar
56 : Basal cortisol is positively correlated to threat vigilance in patients with psychogenic nonepileptic seizures. Epilepsy Behav 2009; 16:558–560Crossref, Medline, Google Scholar
57 : Persistent physical symptoms as perceptual dysregulation: a neuropsychobehavioral model and its clinical implications. Psychosom Med 2018; 80:422–431Crossref, Medline, Google Scholar
58 : Emotion dysregulation in patients with psychogenic nonepileptic seizures: a systematic review based on the extended process model. Epilepsy Behav 2018; 86:37–48Crossref, Medline, Google Scholar
59 : Grey matter changes in motor conversion disorder. J Neurol Neurosurg Psychiatry 2014; 85:236–238Crossref, Medline, Google Scholar
60 : Neural correlates of recall of life events in conversion disorder. JAMA Psychiatry 2014; 71:52–60Crossref, Medline, Google Scholar
61 : The learning theory model of neurosis: a new approach. Behav Res Ther 1976; 14:251–267Crossref, Medline, Google Scholar
62 : Fear reduction and fear behaviour: problems in treating a construct; in Research in Psychotherapy. Edited by Shilien JM. Washington, DC, American Psychological Association, 1968Crossref, Google Scholar
63 : The Psychophysiology of Emotion. New York, Holt, Rinehart and Winston, 1972Google Scholar
64 : I. Synchrony and desynchrony in fear and avoidance. Behav Res Ther 1974; 12:311–318Crossref, Medline, Google Scholar
65 : Somatic response patterning and stress: some revisions of activation theory; in Psychological Stress. Edited by Appley MH, Trumbell R. New York, Appleton-Century-Crofts, 1967Google Scholar
66 : The three-systems-model of fear and emotion: a critical examination. Behav Res Ther 1981; 19:75–85Crossref, Medline, Google Scholar
67 : Increased somatic complaints and health-care utilization in children: effects of parent IBS status and parent response to gastrointestinal symptoms. Am J Gastroenterol 2004; 99:2442–2451Crossref, Medline, Google Scholar
68 : Conversion disorder: advances in our understanding. CMAJ 2011; 183:915–920Crossref, Medline, Google Scholar
69 : Psychology of medically unexplained symptoms: a practical review. Cogent Psychol 2015; 2:1Crossref, Google Scholar
70 : Differences in relatives’ and patients’ illness perceptions in functional neurological symptom disorders compared with neurological diseases. Epilepsy Behav 2015; 42:159–164Crossref, Medline, Google Scholar
71 : Experiences of male patients and wife-caregivers in the first year post-discharge following minor stroke: a descriptive qualitative study. Int J Nurs Stud 2009; 46:1194–1200Crossref, Medline, Google Scholar
72 : Social roles and long-term illness: is it time to rehabilitate convalescence? Clin Rehabil 2007; 21:291–298Crossref, Medline, Google Scholar
73 : Functioning in chronic fatigue syndrome: do illness perceptions play a regulatory role? Br J Health Psychol 2018; 1:15–25Crossref, Google Scholar
74 : Anxiety after stroke: the importance of subtyping. Stroke 2018; 49:556–564Crossref, Medline, Google Scholar
75 : Cognitive-behavioral treatment for severe and persistent health anxiety (hypochondriasis). Brief Treat Crisis Interv 2003; 3:353–367Crossref, Google Scholar
76 : Anxiety and avoidance in psychogenic nonepileptic seizures: the role of implicit and explicit anxiety. Epilepsy Behav 2014; 33:77–86Crossref, Medline, Google Scholar
77 : Inactive and alone: physical activity within the first 14 days of acute stroke unit care. Stroke 2004; 35:1005–1009Crossref, Medline, Google Scholar
78 : Incidence and outcome of orthostatic hypotension in stroke patients undergoing rehabilitation. Arch Phys Med Rehabil 2003; 84:559–562Crossref, Medline, Google Scholar
79 : Stroke induced sarcopenia: muscle wasting and disability after stroke. Int J Cardiol 2013; 170:89–94Crossref, Medline, Google Scholar
80 Healthcare Improvement Scotland: Stepped care for functional neurological symptoms: a new approach to improving outcomes for a common neurological problem in Scotland. Edinburgh, National Health Service, Healthcare Improvement Scotland, 2012Google Scholar
81 : Functional symptoms in neurology: management. J Neurol Neurosurg Psychiatry 2005; 76(suppl 1):i13–i21Crossref, Medline, Google Scholar
82 : Functional neurological disorders: the neurological assessment as treatment. Pract Neurol 2016; 16:7–17Crossref, Medline, Google Scholar
83 : Functional symptoms and signs in neurology: assessment and diagnosis. J Neurol Neurosurg Psychiatry 2005; 76(suppl 1):i2–i12Crossref, Medline, Google Scholar
84 : Stroke mimic: functional neurological disorder. Br J Neurosci Nurs 2019; 15:148–152Crossref, Google Scholar
85 : Cognitive Behavioural Therapy as a Treatment for Conversion Hysteria. Oxford, United Kingdom, Oxford University Press, 2001Google Scholar
86 : Functional neurological symptoms: welcome to the new normal. Pract Neurol 2016; 16:2–3Crossref, Medline, Google Scholar
87 : Physiotherapy for functional motor disorders: a consensus recommendation. J Neurol Neurosurg Psychiatry 2015; 86:1113–1119Crossref, Medline, Google Scholar
88 : Functional neurological disorders: mechanisms and treatment. J Neurol 2016; 263:611–620Crossref, Medline, Google Scholar
89 : Guided self-help for functional (psychogenic) symptoms: a randomized controlled efficacy trial. Neurology 2011; 77:564–572Crossref, Medline, Google Scholar
90 : Non-epileptic attacks: a cognitive and behavioural approach in a single case with a four-year follow-up. Clin Psychol Psychother 1996; 3:291–297Crossref, Google Scholar
91 : Randomised feasibility study of physiotherapy for patients with functional motor symptoms. J Neurol Neurosurg Psychiatry 2017; 88:484–490Crossref, Medline, Google Scholar
92 : A learning theory model of chronic illness behavior: theory, treatment, and research. Psychosom Med 1978; 40:379–401Crossref, Medline, Google Scholar
93 : Somatic symptom disorder: an important change in DSM. J Psychosom Res 2013; 75:223–228Crossref, Medline, Google Scholar



