Visual Hallucinations and the Role of Medications in Parkinson’s Disease: Triggers, Pathophysiology, and Management
Abstract
Visual hallucinations, which are part of the syndrome of Parkinson’s disease (PD) psychosis, affect patients’ quality of life and increase the likelihood of residential aged-care placement. The association between visual hallucinations and dopaminergic and other medications that are necessary for the symptomatic management of motor and other symptoms of PD is a common clinical dilemma. While dopaminergic medications have long been associated with PD psychosis, a clear causal link has not been established, and other neurotransmitter systems, particularly noradrenaline, serotonin, and acetylcholine, are implicated and important. A diverse range of demographic and disease-related risk factors, some being modifiable, highlight the complexity of potential underlying pathophysiological processes but also broaden practical options for prevention and treatment that can be multifaceted and individualized. The investigators reviewed the clinical features and epidemiology of visual hallucinations and PD, explored the pathological evidence for dysfunction of multiple neurotransmitter systems that may be relevant to these phenomena, and addressed the potential of medications commonly used in PD to either trigger or treat these symptoms.
Parkinson’s disease (PD) is the second most common age-related neurodegenerative disease worldwide (1), with an increasing prevalence that makes it the fastest growing neurologic cause of disability (2). In the absence of disease-modifying therapies, symptom-based treatments with a range of medications, including dopaminergic therapies, are required. However, medication use has been associated with a range of complications, including PD psychosis (3). While PD and dementia with Lewy bodies (DLB), as part of the Lewy body disease (LBD) spectrum, are both associated with visual hallucinations, they represent different clinical diagnostic entities with different patterns of medication use. Therefore, although we refer to DLB, the principal focus of the present review is PD.
PD-associated psychosis is defined as at least one of the following: illusions, false sense of presence, hallucinations, or delusions; meeting the United Kingdom Brain Bank criteria for PD; symptom occurrence after the onset of PD; recurrent or continuous symptoms for at least 1 month; and exclusion of other causes (4). Visual hallucinations are the most common symptom of PD psychosis (5) and occur in up to 70% of patients with advanced PD (6). They generally evolve throughout the course of the disease, with periods of remission and relapse (5). Most significantly, the presence of psychosis in PD is associated with higher mortality, caregiver distress, and nursing home placement (4, 7).
Auditory, tactile, olfactory, and gustatory hallucinations also occur in PD but are less common. Delusions, defined as fixed false beliefs that are maintained despite evidence to the contrary, are typically a later symptom of PD psychosis (4, 8). Experts have proposed that PD psychosis may represent a set of symptoms with distinct pathophysiological mechanisms rather than a single pathophysiological syndrome with a spectrum of severity (8). Therefore, we focus on visual hallucinations in the present article.
Minor phenomena, including pareidolia (seeing faces or objects in formless visual stimuli, such as clouds), illusions such as viewing inanimate objects as living beings or parts of living beings, passage hallucinations (the perception of a person or animal passing through the peripheral visual field), and presence hallucinations (the sense of a presence), typically occur early in the course of the disease (6). Formed (i.e., complex) visual hallucinations, typically of people or animals, have been proposed to occur later in the disease course (8). Visual hallucinations in PD are usually associated with a clear sensorium (9), are not disturbing to the patient (6). Patients’ insight is typically preserved until the advanced stages (6, 9).
Visual hallucinations may also occur in the context of delirium, defined as an acute disturbance in attention and awareness accompanied by cognitive deficits, typically with a fluctuating course (10). Patients with PD are at increased risk of developing delirium (11), which may arise at any point in the course of PD and has risk factors similar to those for PD psychosis, including an association with medications, particularly those with anticholinergic properties (11).
While visual hallucinations have been reported with the use of dopaminergic medications since levodopa was first introduced (12), evidence for a causal link is inconclusive, and disease-related factors, particularly disease duration, have consistently been identified as better predictors of the emergence of this psychosis symptom (8). Abnormalities in several other interrelated neurotransmitter systems, namely the noradrenergic, serotonergic, and cholinergic systems, are also observed in PD. Medications that modulate these systems are commonly used in PD, and there is some evidence that cholinesterase inhibitors and serotonin receptor-blocking agents may be beneficial in the treatment of visual hallucinations. Other less frequently examined neurotransmitters that may be relevant include GABA, glutamate, and melatonin.
Here, we aim to describe the key epidemiological associations and identified pathophysiological processes underlying visual hallucinations in PD as they relate to the symptomatic management of the condition and medications that are commonly used. The more that is understood about these phenomena, the clearer it becomes that visual hallucinations in PD cannot be attributed to dysfunction involving a single chemical, anatomical area or neural system and that the combination of disease characteristics, medication profiles, and risk factors likely vary between patients. This has practical implications for prevention and treatment, which require a comprehensive assessment and, if needed, pharmacological therapy tailored to the individual patient.
Risk Factors and Conceptual Models for Visual Hallucinations in PD
Several disease-related and other factors have been linked to visual hallucinations in PD. The presence of visual hallucinations is associated with the duration of PD (13, 14), older age, disease severity, and the presence of motor fluctuations (13–15). Other established risk factors include female sex (15), visual impairment (16), cognitive impairment (17), REM sleep behavior disorder (18), autonomic dysfunction (15), depression (19), apathy (20), and anxiety (20). A bidirectional association between visual hallucinations and cognitive impairment has been observed in PD, with the presence of minor visual hallucinations often preceding any significant cognitive decline (6, 8) and visual hallucinations representing a risk factor for later development of PD dementia (21).
Studies of the etiological basis of visual hallucinations in PD psychosis have focused on both the role of individual risk factors and the interrelationships between multiple factors. Explanatory models have emphasized dysfunction of components of attentional and perceptual processing or dream imagery intrusion with dopaminergic and other medications as likely playing a modulating role (8).
The attentional control hypothesis attributes visual hallucinations to dysfunctional integration between three key neural networks: the dorsal attention network (DAN), the ventral attention network (VAN), and the default mode network (DMN) (22). The DMN comprises the posterior cingulate cortex, precuneus, medial prefrontal cortex, and medial temporal lobe and is involved with introspection, episodic memory, and mind wandering. The VAN comprises the right basolateral amygdala and ventral frontal cortex and mediates activation of other networks and engages attention to salient stimuli. The DAN comprises the striatum and dorsolateral prefrontal cortex and is involved with goal-directed voluntary orienting in response to external stimuli (22). The attentional control hypothesis postulates that in the context of ambiguous visual input, there is a failure to engage the DAN and a subsequent intrusion of default mode contents into perceptual consciousness (22). There is evidence of neurodegeneration of components of these attentional networks in patients with PD who have visual hallucinations. Additionally, studies have demonstrated that dopamine-, serotonin-, and acetylcholine-modulating medications have effects on the activity of and connectivity between these networks (23, 24).
Dysfunctional attentional network interactions have also been demonstrated in DLB (25), which may be associated with the generation of visual hallucinations (25). In addition, thalamo-cortical dysfunction, with decoupling between thalamic nuclei and the DMN, has been proposed to underly psychosis in DLB and PD (26).
Pathophysiology of PD Psychosis
The precise pathophysiology of visual hallucinations in PD remains unclear; however, neuropathological and structural imaging studies have provided some insight regarding the type and distribution of pathologic change associated with these phenomena. Neuronal loss in multiple brain regions, with associated LBs containing alpha-synuclein, is the principle pathological finding in Parkinson’s disease (27). However, characteristic Alzheimer’s disease (AD) pathology (β-amyloid plaques and tau-containing neurofibrillary tangles) can be found in the brains of patients with PD and is associated with earlier onset of dementia (28). Cognitive impairment is a risk factor for visual hallucinations, and concomitant AD pathology has been linked with the phenomena (28).
Dopamine cell loss in the substantia nigra is a required feature for the pathological confirmation of PD, but this is not the earliest affected area (29). According to Braak’s hypothesis, LBD pathology begins in the olfactory regions and lower brain stem (stages I and II), progresses to the midbrain substantia nigra (stage III), and then to the basal forebrain, hypothalamus, thalamus, and hippocampus (stage IV) before spreading to higher-order cortical association areas (stages V and VI) (29). The evolution of visual hallucinations in PD has been conceptualized to mirror this spreading pathology with minor experiences indicating brainstem pathology, formed visual hallucinations with insight indicating basal forebrain involvement, and multimodality hallucinations with associated loss of insight and delusions indicating widespread cortical LBD pathology (8).
Pathological studies have demonstrated higher total LB density (28), as well as greater LB deposition in limbic structures, regions involved in visual processing and executive function in PD patients with visual hallucinations. Specifically, in patients with PD who experience visual hallucinations, higher LB densities have been reported in the basolateral nucleus of the amygdala (30), parahippocampal and inferior temporal cortices (31), and frontal and parietal cortical areas (32). These brain areas are involved in the attentional networks proposed to be dysfunctional in PD psychosis (22, 33). Additionally, greater comorbid AD pathology is seen in PD patients with visual hallucinations on the basis of neuropathological studies (28) and CSF amyloid Aβ1–42 (34).
Neuroimaging studies have revealed gray matter atrophy in multiple regions in patients with PD who experience visual hallucinations. The most prominent changes have been reported across areas involved in visual perception, the hippocampus, and several cholinergic brain structures, such as the substantia innominata and pedunculopontine nucleus (35, 36).
Pathology of Neurotransmitter Pathways in PD Psychosis and Relevance of Medications
The distribution of pathology in PD involves key components of dopaminergic and other important neurotransmitter pathways implicated in PD psychosis. Dopamine is synthesized in the substantia nigra, with projections to the striatum, limbic system and frontal lobe (37, 38), and five recognized dopamine receptors (D1–D5) with broad distribution throughout the brain and peripheries at varying levels of expression (39). It is known that dopaminergic medications have differential actions across these receptors, and, similarly, antipsychotic medications display different binding affinities for individual dopamine receptors (38).
While loss of dopaminergic neurons within the substantia nigra is a central pathological feature of PD (27, 29), neuronal loss also occurs in multiple other subcortical nuclei with projections to cortical, limbic, and basal ganglia regions. These include the dopaminergic nuclei of the ventral tegmentum, noradrenergic locus coeruleus, serotonergic raphe nuclei, histaminergic tuberomammillary nucleus of the hypothalamus, and cholinergic nucleus basalis of Meynert and the pedunculopontine nucleus (27). LB deposition and neuronal loss in the locus coeruleus occurs earlier and is more prominent than PD pathology in the substantia nigra, with some evidence that pathology in this region may contribute to subsequent dopaminergic cell loss, because noradrenergic neurons directly innervate the substantia nigra (40, 41).
Additionally, the amygdala is a common site of pathology in PD (30), with higher LB deposition in this region in patients with visual hallucinations. The amygdala has been conceptualized as a major hub in brain networks, with a role in sensory processing (42) that is particularly relevant to visual hallucinations. Sensory modulation functions of the amygdala are supported by a complex interplay of multiple neurotransmitters, including glutamatergic excitatory neurons and GABAergic inhibitory neurons (42). GABAergic neurons are in turn inhibited by dopamine (42). The amygdala is also innervated by serotonergic (43) and cholinergic (44) neurons with complex excitatory and inhibitory modulatory effects.
A postmortem study quantifying regional deficits in innervation from PD-affected subcortical nuclei through measurement of neurotransmitter and neurotransmitter proteins in individuals with associated dementia and control subjects revealed widespread deficits in dopaminergic, serotonergic, and noradrenergic innervation of cortical, limbic, and basal ganglia regions in the PD group (45). Reduced dopaminergic innervation was seen in the caudate, hippocampus, amygdala, inferior parietal lobule, precuneus, and visual association cortex. Reduced serotonergic innervation was observed in the caudate, middle frontal gyrus, inferior parietal lobule, and visual association cortex. Reduced noradrenergic innervation was found in all brain regions tested, including the caudate, middle frontal gyrus, anterior cingulate gyrus, hippocampus, amygdala, inferior parietal lobule, precuneus, and visual association cortex (45). Another postmortem study comparing PD subjects with and without dementia and control subjects revealed reduced choline acetyltransferase activity in the frontal cortex in all PD subjects and additional reduced hippocampal cholinergic activity in those with dementia (46).
In addition, dopaminergic, serotonergic, and cholinergic projections involve neural regions that comprise attentional networks, and medications that modulate these neurotransmitters systems have been demonstrated to alter connectivity within attentional networks. Dopamine has been shown to increase connectivity within the VAN and reduce connectivity within the DMN in healthy adults (23). Conversely, increased serotonin signaling was associated with increased connectivity within the DMN (23). In patients with AD, acetylcholinesterase inhibitors and selective serotonin reuptake inhibitors (SSRIs) have both been shown to increase DMN connectivity (24). The direct relevance of these alterations in connectivity of attentional networks to visual hallucinations in PD is unclear currently, but further research will likely provide better understanding.
It is noteworthy that patterns of neurodegeneration involving key neurotransmitter pathways in PD differ between individual patients and may underly differing behavioral presentations and response to medications that modulate these pathways (47). Genetic factors are also important. For example, there is an association between glucocerebrosidase mutations and psychosis, but this may be confounded by its association with cognitive impairment and more rapid disease progression (8). Associations between other genes and visual hallucinations in PD are equivocal, and it is likely that the genetic underpinnings of PD psychosis are complex, with involvement of multiple genes, each with a small effect size (8, 48).
Proposed abnormalities in key neurotransmitter pathways in PD psychosis include an imbalance in dopaminergic activity between subcortical and frontal circuits, a relative hypocholinergic state, and an upregulation and overstimulation of cortical serotonin receptors. Other neurotransmitter systems are less well studied; however, the interconnectedness of the noradrenergic and dopaminergic systems and observations of psychotic symptoms associated with a range of prescription and recreational drugs suggest that alterations in noradrenaline, GABA, glutamate, and melatonin are also likely to be relevant. Below, we discuss the interplay between commonly prescribed medications in PD and their effect on the major neurotransmitter systems.
Dopamine
Abnormalities in dopaminergic signaling have long been central to theories of the pathophysiology of PD with its range of manifestations, including psychotic symptoms. The dopamine hypothesis of schizophrenia was conceptualized in the 1970s and posits that psychotic symptoms are related to dysregulation of dopaminergic activity, with excessive transmission at dopamine receptors (49, 50). A refinement of this theory held that the etiology of schizophrenia was linked to increased dopaminergic activity subcortically and decreased activity prefrontally, resulting behaviorally in the attribution of aberrant salience to stimuli (50). Initial evidence for the dopamine hypothesis arose from the observation that prodopaminergic drugs appeared to elicit psychotic symptoms, including hallucinations, and that antidopaminergic medications appeared to be effective in treating them (49). Additionally, psychostimulant drugs of abuse are known to induce hallucinations through activation of dopamine D2 receptors (51). The relevance of the dopamine hypothesis to PD is the observation that initiation or changes in dosing of dopaminergic therapy may precipitate or terminate hallucinations (12, 52).
Dopaminergic medications used in PD include levodopa, dopamine agonists, and monoamine oxidase B (MAO-B) and catechol-O-methyltransferase (COMT) inhibitors. These agents replace dopamine, stimulate dopamine receptors, or reduce the metabolism of dopamine, resulting in increased dopamine levels (53, 54). Amantadine enhances dopamine release indirectly via antagonism of N-methyl-d-aspartate (NMDA) receptors (38). Because it has a slightly different mechanism, the relationship of amantadine to PD psychosis is discussed in a later section of this article. COMT inhibitors and amantadine are typically used when motor fluctuations develop, for wearing off and dyskinesias, respectively (55).
All dopaminergic agents have been associated with incident visual hallucinations (12, 56–59). However, associations are inconsistent, and most compellingly, visual hallucinations have been reported in drug-naive patients with PD (60). Furthermore, previous work demonstrated that an experimental intravenous infusion of levodopa did not exacerbate hallucinations in a cohort of PD patients with this symptom (61). Finally, large studies have demonstrated that incident visual hallucinations are unrelated to dopaminergic medications (62, 63), and it is clear that many studies that have demonstrated a positive association did not control for other established risk factors, notably age, disease duration, severity, and the presence of dementia (4).
Advanced therapies employed later in the course of PD include apomorphine, levodopa carbidopa intestinal gel, and deep brain stimulation (DBS). Importantly, these approaches have all been associated with a reduction in visual hallucinations in large trials. The recently published EuroInf 2 multicenter study (64), which compared motor and nonmotor outcomes in a cohort of patients commencing subthalamic nucleus (STN) deep brain stimulation (DBS), apomorphine infusion, or levodopa carbidopa intestinal gel demonstrated that perceptual problems and hallucinations were reduced in all study groups, most significantly for patients undergoing DBS or commencing apomorphine. In the case of STN-DBS, this was thought to be related to a corresponding reduction in the levodopa equivalent dose (64). Visual hallucinations have been reported less frequently with apomorphine (65), and a reduction in psychotic symptoms with its use may be related to its differing chemical structure in relation to other dopamine agonists and its effects on serotonin receptors (66). Apomorphine has a piperidine moiety (shared with many antipsychotics), exhibits serotonin receptor antagonist effects similar to newer second-generation antipsychotics, and has agonist effects at both D1 and D2 receptors (34, 66).
Noradrenaline
Despite evidence that noradrenergic deficiency is significant in PD, its role in the pathophysiology of visual hallucinations is relatively unexplored. The regulation of noradrenaline and dopamine is highly interrelated with corelease in response to various stimulations, a shared reuptake transporter, colocalized receptors, and common signaling pathways (67). Noradrenaline is a potent agonist for all D2-like receptor subtypes (68). Locus coeruleus innervation activates acetylcholine neurons in the basal forebrain, and therefore pathology in this noradrenergic nucleus may contribute to reduced cortical acetylcholine levels (69). Noradrenaline has a wide array of functions. Most relevant to the known risk factors for the pathophysiology of visual hallucinations in PD, noradrenergic innervation of subcortical and brainstem structures is related to vigilance, arousal/wakefulness, attentional set-shifting, and REM sleep behavior (41). Noradrenaline reuptake inhibitors (NRIs), such as atomoxetine and combined serotonin and noradrenaline reuptake inhibitors (SNRIs), have some evidence for improving global cognition and treating depression in PD, respectively (41).
Acetylcholine
Observations regarding the distribution of pathology in PD and response to various medications have led to the theory of a relative hypocholinergic state favoring the emergence of visual hallucinations (7, 8). The dopaminergic and cholinergic systems are closely related. Ehringer and Hornykiewicz (70) first postulated the idea of a functional equilibrium in the striatum between the excitatory activity of acetylcholine and the inhibitory activity of dopamine. It has been demonstrated in animal models that corticostriatal neurons synapse upon cholinergic interneurons which modulate dopamine neurons mediating the corticostriatal control of striatal dopamine release (71). In turn, dopamine inputs regulate cholinergic interneurons by driving pauses in the firing of dorsomedial cholinergic interneurons (72). This notion has been supported by the observation that parkinsonism is aggravated by centrally acting cholinergic medications and improved by anticholinergic medications (73).
Similarly, while anticholinergic medications, such as trihexyphenidyl, are used in PD particularly for tremor (55), they are known to impact negatively on cognition (74) and are associated with an increased frequency of visual hallucinations (75). Therefore, it should perhaps not be surprising that improvements in cognition and a reduced frequency of visual hallucinations have been demonstrated in PD patients with cognitive impairment using cholinesterase inhibitors, such as rivastigmine (76). Finally, the activity of high-order thalamic nuclei, which exhibit functional connections with the DMN, is under the control of cholinergic neurons (26), supporting the role of cholinergic drugs in treating the thalamocortical dysfunction that may underly visual hallucinations in PD (26).
Serotonin
There is a complex interplay between dopaminergic (DA) and serotonergic (5-HT) systems in PD, and again, it has been conceptualized that a serotonin-dopamine imbalance syndrome may play a role in PD psychosis (77). LB accumulation in the cerebral cortex with progression of PD is associated with degeneration of pyramidal neurons containing serotonin receptors, which is accompanied by a compensatory upregulation of serotonergic 5-HT2A receptors. This upregulation of 5-HT2A receptors has been demonstrated in the cortical areas involved in visual perception in patients with PD psychosis, and together with an increase in raphe nucleus serotonin levels resulting in increased activity within visual pathways, may hypothetically underpin visual hallucinations (77). This proposal has been supported by the observation that hallucinogenic drugs stimulate these same 5HT2A receptors (78).
Further evidence regarding a serotonergic role in PD visual hallucinations relates to the use of pimavanserin, a 5-HT2A inverse agonist with no dopamine D2 receptor antagonist properties. This treatment has been shown to reduce psychotic symptoms in PD without worsening of motor symptoms (79). Clozapine and quetiapine have also been evaluated for the treatment of PD psychosis given their lower propensity to worsen parkinsonism compared with traditional antipsychotics. It is worth noting that both of these agents are nonselective 5-HT2A antagonists (77). However, the evidence for efficacy is considerably more robust for clozapine (80).
The role of commonly used antidepressants in PD visual hallucinations is poorly understood. Antidepressants, including selective serotonin reuptake inhibitors (SSRIs), serotonin noradrenaline reuptake inhibitors (SNRIs), and tricyclic antidepressants (TCAs), are usually reported as well tolerated in large treatment trials for PD (81). TCAs are effective for PD depression but have multiple other unwanted pharmacological actions, including antihistaminergic and anticholinergic effects (38), likely underlying their well-known association with delirium (82). Unsurprisingly, there are case reports of visual hallucinations associated with the use of TCAs (83). However, visual hallucinations have not been consistently reported in larger treatment trials including TCAs (84). Similarly, while SSRIs and SNRIs are generally better tolerated than TCAs, visual hallucinations have also been reported with their use (83) but not in large PD depression treatment trials (81, 84). It is noteworthy that these larger trials do not clearly reflect real-world use of these agents for patients with PD, given that participants who had ever experienced psychotic symptoms, participants with cognitive impairment, and older patients were not included (85). There is some evidence of benefit with antidepressants used in the context of PD psychosis. A small open-label study of escitalopram demonstrated improvement in visual hallucinations in patients with PD (86).
Glutamate
Abnormalities in glutamatergic neurotransmission have been implicated in psychosis, particularly involving NMDA receptors and especially in the context of schizophrenia (87), where NMDA receptor modulation with glycine or d-serine improves psychotic symptoms (87). Amantadine exhibits NMDA receptor antagonism, a mechanism shared with memantine, amphetamines, and ketamine, which have been shown to produce psychotic symptoms in patients with AD (88) and in healthy individuals (89). Amantadine is typically used for dyskinesias in PD. Riluzole, a potent antiglutamatergic drug, was recently demonstrated to effectively treat hallucinations in a patient with 22q11.2 deletion syndrome, whereby reduced proline dehydrogenase results in increased proline, which is converted to excessive glutamate (90). Thus, the glutamatergic system may offer a novel therapeutic target for the symptomatic treatment of visual hallucinations in PD.
GABA
There is some evidence that modulation of GABA neurotransmission may be relevant to visual hallucinations in PD. For example, reduced GABA concentrations have recently been demonstrated using magnetic resonance spectroscopy in the visual cortex of PD patients with complex visual hallucinations compared with PD patients without hallucinations (91). In an earlier study, the investigators reported that the antispasmodic baclofen, a GABA agonist, can trigger visual hallucinations in PD (92). While interesting, the potential mechanisms by which GABA may be operating in the pathophysiology of visual hallucinations in PD remain unclear.
Melatonin
There has been recent interest in the use of melatonin in the treatment of delirium, an altered state of consciousness with some similarities to PD psychosis (11). While melatonin has been used for many years as a treatment for REM sleep behavior disorder, there are no adequate placebo-controlled trial data to support its use (93). However, in a recent case report, a patient with PD experiencing visual hallucinations exhibited reduction in the frequency of nocturnal hallucinations with melatonin treatment (94). Interestingly, no alterations were made to this dopaminergic therapy. This suggests that modulation of melatonin is a further avenue for investigation in the management of visual hallucinations in PD.
Management of Visual Hallucinations in PD
Understanding the complex interactions between neurotransmitter systems in the context of neurodegeneration secondary to PD assists in efforts to treat psychotic symptoms as they arise. Management is complex and varies between patients. The first consideration should most obviously be prevention. When these symptoms occur acutely, systemic illness should be considered and treated. When symptoms persist, an increasing array of treatment options are available and can be tailored to the individual patient. A treatment algorithm is presented in Figure 1.
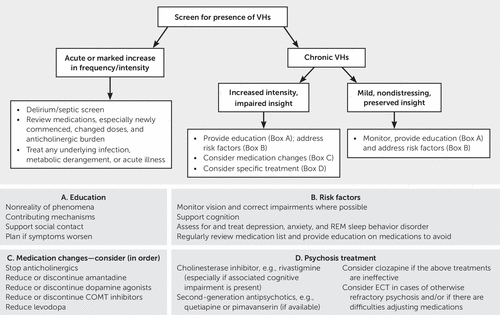
FIGURE 1. Management flow chart for visual hallucinations (VHs) in Parkinson’s diseasea
a COMT=catechol-O-methyltransferase.
Prevention
An assessment of risk factors for visual hallucinations and targeting of those that are modifiable may either prevent or reduce visual hallucinations in individual patients. Providing aids for visual impairment or corrective surgery, treating REM sleep behavior disorder through avoidance of triggering factors, treating comorbid sleep apnea, considering pharmacological agents such as melatonin, supporting cognition, and treating mood disorders and anxiety are all important goals. To date, evidence for these types of interventions is limited (95). Other proposed nonpharmacological strategies include improving lighting, reducing visual triggers, and increasing social contact, particularly during times of the day when visual hallucinations may be more frequent (95, 96).
Any medication used in PD should be introduced gradually, with vigilance for potential drug interactions and avoidance of agents with anticholinergic properties. The anticholinergic burden of individual medications can be reviewed from sources such as the Beers criteria (97). There is also a potential role for pharmacological modulation of a number of risk factors for visual hallucinations, such as acetylcholinesterase inhibitors and potentially NRIs for cognitive impairment and SSRIs and SNRIs for depression. Such medications should be introduced cautiously, with vigilance for drug interactions given their reported side effects and variability in sensitivity among individual patients with different disease profiles, risk factors, and existing medication combinations. TCAs should generally be avoided (95).
Early in the disease, when visual hallucinations are usually accompanied by insight and are not distressing, specific treatment is not usually required, and explanation and reassurance to the patient may be sufficient (9). Simple techniques such as turning on a light, asking the patient to look more closely at or approach the visual hallucinations, asking the patient to wait for the hallucinations to disappear, or asking a family member or caregiver to check the nonreality of the phenomenon may be useful (9). Patients should be monitored for increased frequency of visual hallucinations, change in phenomenology, and impairment of insight. If needed, pharmacological manipulation can be guided by the patient’s disease, risk factor, and medication profiles.
Acute Management
When visual hallucinations occur acutely in PD or become more prominent or distressing in a patient with established visual hallucinations, consideration must be given to whether these symptoms are related to an acute systemic illness or whether a recent change in medication may be a precipitant (8). For delirium, provoking factors, including infection, dehydration, constipation, and metabolic disturbances, should be systematically identified and treated (95). Next, the patient’s medication list should be reviewed for agents that could contribute to psychosis, with the goal of ceasing nonessential, potentially contributory medications, particularly those most recently added, and reducing polypharmacy (80). There is no consensus regarding the order of reduction in medications, but experts tend to advocate reducing or discontinuing anticholinergics, amantadine, dopamine agonists, MAO-B inhibitors, and COMT inhibitors before considering a reduction in levodopa (80). Any withdrawal of dopaminergic agents, specifically, should be achieved slowly to avoid rebound motor symptoms or dopamine agonist withdrawal syndrome (11, 80). Clearly, managing psychotic symptoms through reductions in dopaminergic medication needs to be carefully balanced with preserving good motor function (95).
The cholinesterase inhibitor rivastigmine has been shown to improve psychotic symptoms in patients with cognitive impairment and visual hallucinations and has minimal side effects (8, 76, 81). Experts suggest that this should be considered particularly when there is comorbid dementia (81). There is also some evidence that donepezil reduces psychotic symptoms, including visual hallucinations, in patients with PD (98).
Ongoing Management
If visual hallucinations are significant enough to cause distress and persist despite treatment of acute precipitants, consideration should be given to use of a low-dose antipsychotic with low-risk of parkinsonism, such as pimavanserin, quetiapine, or clozapine. However, the increased mortality associated with antipsychotic use among older patients should be carefully considered and discussed with family members (99). While this risk is concerning and other treatment options should always be considered, it should also be balanced with the real quality of life implications of untreated psychosis and association with institutionalization (4, 34). Many patients and families may be comfortable with this risk, particularly if a short-term trial of antipsychotic medication can be achieved. However, chronic use is often required. Nevertheless, antipsychotic treatment should be reviewed regularly and reduced or discontinued when possible (95).
Clozapine is typically used for significant psychosis that does not respond to quetiapine, given the potential for serious hematological and cardiac adverse reactions (80) and the need for monitoring (81). Quetiapine is relatively safe in patients with parkinsonism and widely used for PD psychosis, but evidence for its efficacy is limited (8, 80). Pimavanserin is unfortunately not available in all countries. Other second-generation antipsychotics, such as olanzapine and risperidone, have demonstrated mixed evidence for efficacy in PD psychosis and have been shown to worsen motor function (80, 81).
ECT has been demonstrated to improve both motor and psychotic symptoms in PD psychosis (100) and could be considered if medication strategies are ineffective.
Lastly, current international guidelines suggest avoiding device-aided therapies with the potential exception of levodopa carbidopa intestinal gel infusion for patients with significant psychotic symptoms and for patients with dementia (101, 102). However, given the evidence that the institution of all advanced therapies has been associated with a net reduction in visual hallucinations in large trials, a decision to proceed with any of these therapies in patients with visual hallucinations should be individualized, and future recommendations may change.
Conclusions
Abnormalities in diverse neurotransmitter systems are implicated in visual hallucinations in PD and reflect both the distribution of neurodegenerative pathology and the complex interactions between neurotransmitters. In the absence of an effective preventive or disease-modifying therapy for PD, patients will continue to require symptomatic treatment with medications that have the potential to either exacerbate or reduce visual hallucinations. Individual patients have differing risk-factor profiles and disease characteristics and are managed with different medication combinations, highlighting the heterogeneity of patterns of neurodegeneration in PD associated with visual hallucinations.
While there is a substantive literature linking visual hallucinations to dopaminergic medications, differing study methodologies and failure to effectively disentangle disease-related and medication-induced symptoms make the literature difficult to interpret. This is reflected in the current diagnostic criteria, which emphasize that medications are neither sufficient nor necessary contributors to PD psychosis (4).
However, studies thus far have provided some insight into the mechanisms of medication effects, as well as the identification of agents, such as pimavanserin, that have shown evidence of treating PD psychosis without worsening motor symptoms. Future research may expound further potential roles for medications with glutamatergic, cholinergic, noradrenergic, serotonergic, and melatonergic properties in the treatment of PD psychosis.
The complex pathophysiology underlying visual hallucinations in PD can make treatment difficult but also indicates that a broad range of approaches to prevention and management can be considered and highlights the need for individualized therapy. The presence of other syndromes, such as cognitive impairment, REM sleep behavior disorder, or depression, may guide such therapy.
1 : Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology 2007; 68:384–386Crossref, Medline, Google Scholar
2 : Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2019; 18:459–480Crossref, Medline, Google Scholar
3 : Behavioral effects of levodopa. Mov Disord 2015; 30:90–102Crossref, Medline, Google Scholar
4 : Diagnostic criteria for psychosis in Parkinson’s disease: report of an NINDS, NIMH work group. Mov Disord 2007; 22:1061–1068Crossref, Medline, Google Scholar
5 : Onset and remission of psychosis in Parkinson’s disease: pharmacologic and motoric markers. Mov Disord Clin Pract (Hoboken) 2018; 5:31–38Crossref, Medline, Google Scholar
6 : Neuropsychological correlates of mild to severe hallucinations in Parkinson’s disease. Mov Disord 2010; 25:2785–2791Crossref, Medline, Google Scholar
7 : Psychosis in Parkinson’s disease: from the soft signs to the hard science. J Neurol Sci 2017; 379:169–176Crossref, Medline, Google Scholar
8 : The psychosis spectrum in Parkinson disease. Nat Rev Neurol 2017; 13:81–95Crossref, Medline, Google Scholar
9 : Hallucinations in Parkinson disease. Nat Rev Neurol 2009; 5:331–342Crossref, Medline, Google Scholar
10 : Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC, American Psychiatric Association, 2013Crossref, Google Scholar
11 : Management of delirium in Parkinson’s disease. J Neural Transm (Vienna) 2019; 126:905–912Crossref, Medline, Google Scholar
12 : L-dopa therapy in Parkinson’s disease: a critical review of nine years’ experience. Can Med Assoc J 1969; 101:59–68Medline, Google Scholar
13 : Epidemiology of psychosis in Parkinson’s disease. J Neurol Sci 2010; 289:12–17Crossref, Medline, Google Scholar
14 : Frequency, prevalence, incidence and risk factors associated with visual hallucinations in a sample of patients with Parkinson’s disease: a longitudinal 4-year study. Int J Geriatr Psychiatry 2013; 28:626–631Crossref, Medline, Google Scholar
15 : Risk factors for hallucinations in Parkinson’s disease: results from a large prospective cohort study. Mov Disord 2013; 28:755–762Crossref, Medline, Google Scholar
16 : Poor visual discrimination and visual hallucinations in Parkinson’s disease. Clin Neuropharmacol 1998; 21:289–295Medline, Google Scholar
17 : REM sleep behavior disorder, hallucinations, and cognitive impairment in Parkinson’s disease. Mov Disord 2006; 21:462–466Crossref, Medline, Google Scholar
18 : Hallucinations and sleep disorders in PD: ten-year prospective longitudinal study. Neurology 2010; 75:1773–1779Crossref, Medline, Google Scholar
19 : Hallucinations in Parkinson’s disease: prevalence, phenomenology and risk factors. Brain 2000; 123:733–745Crossref, Medline, Google Scholar
20 : Characteristics of visual hallucinations in Parkinson disease dementia and dementia with Lewy bodies. Am J Geriatr Psychiatry 2006; 14:153–160Crossref, Medline, Google Scholar
21 : Incidence of and risk factors for cognitive impairment in an early Parkinson disease clinical trial cohort. Neurology 2009; 73:1469–1477Crossref, Medline, Google Scholar
22 : Visual misperceptions and hallucinations in Parkinson’s disease: dysfunction of attentional control networks? Mov Disord 2011; 26:2154–2159Crossref, Medline, Google Scholar
23 : Opposite effects of dopamine and serotonin on resting-state networks: review and implications for psychiatric disorders. Mol Psychiatry 2019Medline, Google Scholar
24 : Cholinergic and serotonergic modulation of resting state functional brain connectivity in Alzheimer’s disease. Neuroimage 2019; 199:143–152Crossref, Medline, Google Scholar
25 : Divergent functional connectivity during attentional processing in Lewy body dementia and Alzheimer’s disease. Cortex 2017; 92:8–18Crossref, Medline, Google Scholar
26 : Hallucinations, somatic-functional disorders of PD-DLB as expressions of thalamic dysfunction. Mov Disord 2019; 34:1100–1111Crossref, Medline, Google Scholar
27 : Pathoanatomy of Parkinson’s disease. J Neurol 2000; 247(Suppl 2):II3–II10Crossref, Medline, Google Scholar
28 : Plaques and tangles as well as Lewy-type alpha synucleinopathy are associated with formed visual hallucinations. Parkinsonism Relat Disord 2014; 20:1009–1014Crossref, Medline, Google Scholar
29 : Evaluation of the Braak hypothesis: how far can it explain the pathogenesis of Parkinson’s disease? Expert Rev Neurother 2012; 12:673–686Crossref, Medline, Google Scholar
30 : Clinical correlates of selective pathology in the amygdala of patients with Parkinson’s disease. Brain 2002; 125:2431–2445Crossref, Medline, Google Scholar
31 : Visual hallucinations in Lewy body disease relate to Lewy bodies in the temporal lobe. Brain 2002; 125:391–403Crossref, Medline, Google Scholar
32 : Cortical and amygdalar Lewy body burden in Parkinson’s disease patients with visual hallucinations. Parkinsonism Relat Disord 2006; 12:253–256Crossref, Medline, Google Scholar
33 : Resting-state functional MRI in Parkinsonian syndromes. Mov Disord Clin Pract (Hoboken) 2019; 6:104–117Crossref, Medline, Google Scholar
34 : Risk factors for early psychosis in PD: insights from the Parkinson’s Progression Markers Initiative. J Neurol Neurosurg Psychiatry 2017; 88:325–331Crossref, Medline, Google Scholar
35 : The pedunculopontine nucleus is related to visual hallucinations in Parkinson’s disease: preliminary results of a voxel-based morphometry study. J Neurol 2012; 259:147–154Crossref, Medline, Google Scholar
36 : Neuroanatomical substrates of visual hallucinations in patients with non-demented Parkinson’s disease. J Neurol Neurosurg Psychiatry 2012; 83:1155–1161Crossref, Medline, Google Scholar
37 : Mayo Clinic Medical Neurosciences Organised by Neurological Systems and Levels, 5th ed. Rochester, Minn, Mayo Clinic Scientific Press, 2008Crossref, Google Scholar
38 : Stahl’s Essential Psychopharmacology: Neuroscientific Basis and Practical Applications, 3rd ed. New York, Cambridge University Press, 2011Google Scholar
39 : The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev 2011; 63:182–217Crossref, Medline, Google Scholar
40 : The locus ceruleus norepinephrine system: functional organization and potential clinical significance. Neurology 2009; 73:1699–1704Crossref, Medline, Google Scholar
41 : Targeting the norepinephrinergic system in Parkinson’s disease and related disorders: the locus coeruleus story. Neurochem Int 2017; 102:22–32Crossref, Medline, Google Scholar
42 : Failing as doorman and disc jockey at the same time: amygdalar dysfunction in Parkinson’s disease. Mov Disord 2016; 31:11–22Crossref, Medline, Google Scholar
43 : Control of amygdala circuits by 5-HT neurons via 5-HT and glutamate cotransmission. J Neurosci 2017; 37:1785–1796Crossref, Medline, Google Scholar
44 : Basal forebrain and brainstem cholinergic neurons differentially impact amygdala circuits and learning-related behavior. Curr Biol 2018; 28:2557–2569.e4Crossref, Medline, Google Scholar
45 : Dopaminergic, serotonergic, and noradrenergic deficits in Parkinson disease. Ann Clin Transl Neurol 2015; 2:949–959Crossref, Medline, Google Scholar
46 : Hippocampal Lewy pathology and cholinergic dysfunction are associated with dementia in Parkinson’s disease. Brain 2014; 137:2493–2508Crossref, Medline, Google Scholar
47 : Cognition in Parkinson’s disease. Int Rev Neurobiol 2017; 133:557–583Crossref, Medline, Google Scholar
48 : Genes and nonmotor symptoms in Parkinson’s disease. Int Rev Neurobiol 2017; 133:111–127Crossref, Medline, Google Scholar
49 : Dopamine receptors and the dopamine hypothesis of schizophrenia. Synapse 1987; 1:133–152Crossref, Medline, Google Scholar
50 : The dopamine hypothesis of schizophrenia: version III: the final common pathway. Schizophr Bull 2009; 35:549–562Crossref, Medline, Google Scholar
51 : Pharmacology of hallucinations: several mechanisms for one single symptom? BioMed Res Int 2014; 2014:
52 : Pharmacology of hallucinations induced by long-term drug therapy. Am J Psychiatry 1982; 139:494–497Crossref, Medline, Google Scholar
53 : A review of the mechanisms and role of monoamine oxidase inhibitors in Parkinson’s disease. Neurology 2004; 63(Suppl 2):S32–S35Crossref, Medline, Google Scholar
54 : Clinical pharmacology, therapeutic use and potential of COMT inhibitors in Parkinson’s disease. Drugs 2000; 59:1233–1250Crossref, Medline, Google Scholar
55 : Parkinson’s disease. Lancet 2015; 386:896–912Crossref, Medline, Google Scholar
56 : Psychosis and other psychiatric manifestations of levodopa therapy. Arch Neurol 1970; 23:193–200Crossref, Medline, Google Scholar
57 : Tolerability and safety of ropinirole versus other dopamine agonists and levodopa in the treatment of Parkinson’s disease: meta-analysis of randomized controlled trials. Drug Saf 2010; 33:147–161Crossref, Medline, Google Scholar
58 : Adverse drug reactions to selegiline: a review of the French pharmacovigilance database. Clin Neuropharmacol 2000; 23:271–275Crossref, Medline, Google Scholar
59 : ADS-5102 (amantadine) extended-release capsules for levodopa-induced dyskinesia in Parkinson disease (EASE LID study): a randomized clinical trial. JAMA Neurol 2017; 74:941–949Crossref, Medline, Google Scholar
60 : Minor hallucinations occur in drug-naive Parkinson’s disease patients, even from the premotor phase. Mov Disord 2016; 31:45–52Crossref, Medline, Google Scholar
61 : Intravenous levodopa in hallucinating Parkinson’s disease patients: high-dose challenge does not precipitate hallucinations. Neurology 1998; 50:515–517Crossref, Medline, Google Scholar
62 : Factors associated with drug-induced visual hallucinations in Parkinson’s disease. J Neurol 2005; 252:1223–1228Crossref, Medline, Google Scholar
63 : Antiparkinsonian medication is not a risk factor for the development of hallucinations in Parkinson’s disease. J Neural Transm (Vienna) 2004; 111:1447–1453Crossref, Medline, Google Scholar
64 : EuroInf 2: subthalamic stimulation, apomorphine, and levodopa infusion in Parkinson’s disease. Mov Disord 2019; 34:353–365Crossref, Medline, Google Scholar
65 : A 10 year retrospective audit of long-term apomorphine use in Parkinson’s disease. J Neurol 2004; 251:1370–1374Crossref, Medline, Google Scholar
66 : Parkinson’s disease, visual hallucinations and apomorphine: a review of the available evidence. Parkinsonism Relat Disord 2016; 27:35–40Crossref, Medline, Google Scholar
67 : Norepinephrine versus dopamine and their interaction in modulating synaptic function in the prefrontal cortex. Brain Res 2016; 1641(Pt B):217–233Crossref, Medline, Google Scholar
68 : Evidence for noncanonical neurotransmitter activation: norepinephrine as a dopamine D2-like receptor agonist. Mol Pharmacol 2016; 89:457–466Crossref, Medline, Google Scholar
69 : The emerging role of norepinephrine in cognitive dysfunctions of Parkinson’s disease. Front Behav Neurosci 2012; 6:48Crossref, Medline, Google Scholar
70 : Distribution of noradrenaline and dopamine (3-hydroxytyramine) in the human brain and their behavior in diseases of the extrapyramidal system. Parkinsonism Relat Disord 1998; 4:53–57Crossref, Medline, Google Scholar
71 : Cortical control of striatal dopamine transmission via striatal cholinergic interneurons. Cereb Cortex 2016; 26:4160–4169Crossref, Medline, Google Scholar
72 : Dopamine cells differentially regulate striatal cholinergic transmission across regions through corelease of dopamine and glutamate. Cell Rep 2018; 25:3148–3157.e3Crossref, Medline, Google Scholar
73 : Adams and Victor’s Principles of Neurology, 10th ed. New York, McGraw-Hill, 2014Google Scholar
74 : Use of drugs with anticholinergic effect and impact on cognition in Parkinson’s disease: a cohort study. J Neurol Neurosurg Psychiatry 2010; 81:160–165Crossref, Medline, Google Scholar
75 : Trigger medications and patient-related risk factors for Parkinson disease psychosis requiring anti-psychotic drugs: a retrospective cohort study. BMC Neurol 2013; 13:145Crossref, Medline, Google Scholar
76 : Rivastigmine in the treatment of parkinsonian psychosis and cognitive impairment: preliminary findings from an open trial. Mov Disord 2001; 16:1171–1174Crossref, Medline, Google Scholar
77 : Parkinson’s disease psychosis as a serotonin-dopamine imbalance syndrome. CNS Spectr 2016; 21:355–359Crossref, Medline, Google Scholar
78 : Hallucinogenic drug interactions at human brain 5-HT2 receptors: implications for treating LSD-induced hallucinogenesis. Psychopharmacology (Berl) 1989; 98:495–499Crossref, Medline, Google Scholar
79 : Pimavanserin for patients with Parkinson’s disease psychosis: a randomised, placebo-controlled phase 3 trial. Lancet 2014; 383:533–540Crossref, Medline, Google Scholar
80 : Pharmacological interventions for psychosis in Parkinson’s disease patients. Expert Opin Pharmacother 2018; 19:499–505Crossref, Medline, Google Scholar
81 : Update on treatments for nonmotor symptoms of Parkinson’s disease-an evidence-based medicine review. Mov Disord 2019; 34:180–198Crossref, Medline, Google Scholar
82 : Which medications to avoid in people at risk of delirium: a systematic review. Age Ageing 2011; 40:23–29Crossref, Medline, Google Scholar
83 : Factors associated with complex visual hallucinations during antidepressant treatment. Hum Psychopharmacol 2004; 19:577–584Crossref, Medline, Google Scholar
84 : Antidepressive treatments for Parkinson’s disease: a systematic review and meta-analysis. Parkinsonism Relat Disord 2015; 21:833–842, discussion 833Crossref, Medline, Google Scholar
85 : A randomized, double-blind, placebo-controlled trial of antidepressants in Parkinson disease. Neurology 2012; 78:1229–1236Crossref, Medline, Google Scholar
86 : Successful use of escitalopram for the treatment of visual hallucinations in patients with Parkinson disease. Clin Neuropharmacol 2017; 40:246–250Crossref, Medline, Google Scholar
87 : Glutamate and dopamine in schizophrenia: an update for the 21st century. J Psychopharmacol 2015; 29:97–115Crossref, Medline, Google Scholar
88 : Visual hallucinations and agitation in Alzheimer’s disease due to memantine: report of three cases. J Neurol Neurosurg Psychiatry 2007; 78:546Crossref, Medline, Google Scholar
89 : Comparative and interactive human psychopharmacologic effects of ketamine and amphetamine: implications for glutamatergic and dopaminergic model psychoses and cognitive function. Arch Gen Psychiatry 2005; 62:985–994Crossref, Medline, Google Scholar
90 : Riluzole effectively treats psychotic symptoms and improves cognition in 22q11.2 deletion syndrome: a clinical case. Eur J Med Genet 2019; 62:
91 : Reduced occipital GABA in Parkinson disease with visual hallucinations. Neurology 2018; 91:e675–e685Crossref, Medline, Google Scholar
92 : Baclofen in Parkinson’s disease. J Neurol Neurosurg Psychiatry 1978; 41:707–708Crossref, Medline, Google Scholar
93 : Melatonin for rapid eye movement sleep behaviour disorder in Parkinson’s disease: a double blind, randomized controlled trial. Mov Disord 2020; 35:344–349Crossref, Medline, Google Scholar
94 : Melatonin treatment of visual hallucinations in Parkinson disease. J Clin Psychopharmacol 2018; 38:532–534Crossref, Medline, Google Scholar
95 : Management of visual hallucinations in dementia and Parkinson’s disease. Int Psychogeriatr 2019; 31:815–836Crossref, Medline, Google Scholar
96 : Advances in the treatment of visual hallucinations in neurodegenerative diseases. Future Neurol 2013; 8:433–444Crossref, Medline, Google Scholar
97 By the American Geriatrics Society 2015 Beers Criteria Update Expert Panel: American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc 2015; 63:2227–2246Crossref, Medline, Google Scholar
98 : Donepezil for dementia in Parkinson’s disease: a randomised, double blind, placebo controlled, crossover study. J Neurol Neurosurg Psychiatry 2005; 76:934–939Crossref, Medline, Google Scholar
99 : Association of antipsychotic use with mortality risk in patients with Parkinson disease. JAMA Neurol 2016; 73:535–541Crossref, Medline, Google Scholar
100 : Improvements in both psychosis and motor signs in Parkinson’s disease, and changes in regional cerebral blood flow after electroconvulsive therapy. Prog Neuropsychopharmacol Biol Psychiatry 2011; 35:1704–1708Crossref, Medline, Google Scholar
101 : Collective physician perspectives on non-oral medication approaches for the management of clinically relevant unresolved issues in Parkinson’s disease: consensus from an international survey and discussion program. Parkinsonism Relat Disord 2015; 21:1133–1144Crossref, Medline, Google Scholar
102 : Practical approaches to commencing device-assisted therapies for Parkinson disease in Australia. Intern Med J 2017; 47:1107–1113Crossref, Medline, Google Scholar



