Temporal Processing: Neural Correlates and Clinical Relevance
The ability to track time over a wide range of durations (i.e., milliseconds, seconds, minutes, hours, days, months, years) is essential for optimizing interactions with a constantly changing complex world (9, 10, 16–19). Timing is a crucial factor in the sensory-motor processing that generates the intricate, precisely controlled movements required to play a musical instrument, catch a ball, or dance. Accurate timing of complex sequences is required for both generating and understanding language. Timing of multiple potentially salient events over longer periods of time is essential for predicting when and where something is likely to happen, in order to respond quickly when opportunities or dangers arise. The importance of timing and temporal processing in movement disorders is well established (2). Alterations in aspects of temporal processing and timing have also been identified in many other neuropsychiatric conditions (1, 20–24). The therapeutic value of temporally based interventions (e.g., rhythmic cueing, slow rhythmic drumming) has been demonstrated for multiple neuropsychiatric conditions (25–28).
Unlike physical senses, the sense of internal time does not have a specific, dedicated brain region. Temporal perception (inner experience of time) is an abstract concept, constructed within the brain based on available information (external and internal sensory, memory) by a network of areas that likely are specialized for different aspects of timing (Figure 1 and Cover) (2, 3, 5–10). Multiple factors can alter how passage of time is perceived (temporal distortion), indicating the subjective nature of experienced time (3, 8, 19, 29–34). In general, rewarding or pleasant circumstances are associated with shortening (time compression) and aversive circumstances (e.g. threatening, painful, stressful, boring) are associated with lengthening (time dilation) of the perceived passage of time.
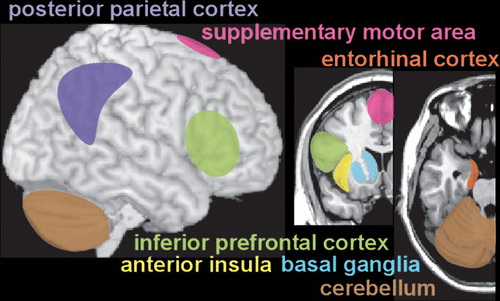
FIGURE 1 and COVER. Unlike physical senses, the “sense” of internal time does not have a specific dedicated brain region. Temporal perception (inner experience of time) is an abstract concept, constructed within the brain based on available information (external and internal sensory, memory) by a network of areas that are variably recruited depending on specific task demands (1–10). The approximate locations of commonly implicated cortical and subcortical areas are shown on representative MRI.
Memory is fundamental to capturing and using temporal information to guide behavior (5, 7, 19, 35–37). It has been known for some time that processing pathways for spatial information (where) and contextual information (what) converge in the medial temporal area. Recent functional MRI (fMRI) studies in healthy adults have implicated the perirhinal cortex and anterolateral entorhinal cortex (ventral stream) in providing temporal order information (when) to hippocampal encoding of memories (Figure 1 and Cover) (38–41). Although the temporal flow of experience is continuous, it is segmented into discrete episodes during encoding (41–44). One major hypothesis is that changes in multidimensional context (e.g., turning a corner or stepping through a doorway, beginning a new section in a narrative, changing a behavioral goal) form boundaries. The binding together of events that occur within boundaries creates a single episode. There is evidence that events that occurred in different episodes are perceived as happening farther apart in time (time dilation), and events occurring within an episode are perceived as happening closer in time (time compression) (36, 42, 44). Increased attention when context changes enough to evoke a boundary may contribute to time dilation across boundaries (41–44).
A major approach to identifying the neural underpinnings of temporality and time perception is based on classification of dimensions (taxonomy) (12, 17, 35, 45). Although details vary, generally agreed-upon dimensions capture duration, pattern, and modality. The critical distinction in duration (i.e., short versus long intervals) is subsecond versus suprasecond timing, as they are believed to have different neural correlates. Patterns of timing are most commonly divided into simple intervals versus complex intervals. An important distinction is whether the timing is of a standard interval or duration (even if repeated) or requires decoding of temporal sequencing (e.g., pattern of events, order of events, location on a mental timeline). A related dimension is whether events are episodic (e.g., isolated intervals) or continuous (e.g., rhythmic, cyclic). Modality is generally divided into sensory (perceptual) versus motor by whether the task-related decision depends on temporal aspects of the sensory input (e.g., judging which stimulus is longer) or developing an internally timed motor response. Another dimension that has been proposed is based on whether use of temporal information is explicitly part of the task (e.g., duration estimate) or can be used implicitly to facilitate performance. Tasks may also differ by whether prospective memory (e.g., participants informed in advance that they will be required to provide duration judgments) or retrospective memory (e.g., participants not informed in advance so they must reconstruct duration information) is required.
Reviews and meta-analyses of functional imaging (fMRI, positron emission tomography [PET]) studies have probed the neural correlates of many aspects of temporal processing in healthy individuals (1–11, 13–15). Overall, these studies support the presence of a widespread network of cortical (supplementary motor area, premotor cortex, inferior frontal gyrus, anterior insula, inferior parietal cortex, posterior superior temporal gyrus/sulcus) and subcortical (basal ganglia, thalamus, cerebellum) areas that are variably recruited based on specific task parameters and demands (Figure 1). Meta-analyses of functional imaging studies of temporal processing tasks have identified some dimension-related differences in neural correlates. A recent meta-analysis grouped fMRI and PET studies based on the dimensions of modality (motor, perceptual) and duration (subsecond, suprasecond) (11). Although the extent and laterality of activations differed across the four conditions, all recruited a relatively similar set of cortical (supplementary motor area, inferior frontal gyrus, anterior insula) and subcortical (basal ganglia) areas (Figure 2). Activation of the supplementary motor area by all conditions is consistent with its proposed role as a higher level area representing amodal (i.e., regardless of information source) duration. A recent fMRI study in healthy individuals demonstrated a duration-sensitive topographic organization (chronotopic map) for this area (46). The cerebellum was activated only by the subsecond motor condition, consistent with its role in precise timing (12). A meta-analysis that compared fMRI studies in which task timing was either provided by external cues (e.g., cued finger tapping, rhythm reproduction or monitoring, temporal prediction) or had to be generated internally (e.g., duration discrimination, interval production or reproduction, interval bisection, self-initiated) also found that both conditions activated a similar network of cortical and subcortical areas (Figure 2) (13). A few areas were associated only with internally timed tasks (right: intraparietal sulcus; midline: inferior olive) or externally cued tasks (left: precentral gyrus; right: posterior superior temporal sulcus/gyrus). The contrast analysis identified several areas (bilateral: inferior frontal gyrus/anterior insula; left: supplementary motor area, precentral gyrus) that were activated more in the externally cued tasks (Figure 2). Overall, these results support some dimension-related differences but not fully dissociable networks (11, 13).
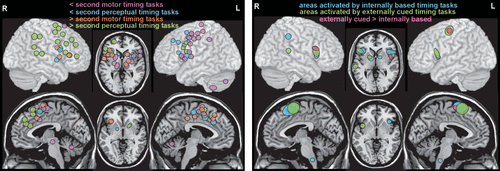
FIGURE 2. Meta-analyses of functional imaging studies have explored the neural correlates of specific aspects of temporal processing. Activations are color-coded based on specific task parameters and demands. Left: One meta-analysis grouped studies based on whether the task was motor or nonmotor (perceptual) and by duration (subsecond, suprasecond) (11). Although the extent and laterality differed, all four conditions were associated with activation of the supplementary motor area, inferior frontal cortex, anterior insula, and basal ganglia. Cerebellum was activated only by the subsecond motor condition, consistent with its role in precise timing (12). Right: A meta-analysis comparing studies in which task timing was either provided by external cues or had to be generated internally also reported differential activation of only a few areas (13). Overall, these results support some differences based on dimensions of temporal processing but not fully dissociable networks.
The high dimensionality of temporal processing makes it challenging to combine data across studies in order to identify neural correlates of any single dimension, as multiple other dimensions are likely to be merged in the resulting groupings. Another approach is to focus on how temporal information is used. One meta-analysis combined fMRI and PET studies in three functional domains (music, language, action perception) to test the hypothesis that there is a cohesive network of areas supporting higher level predictive processing (14). Convergence of activations identified a widespread network of cortical (bilateral: anterior insula, inferior frontal gyrus, ventral premotor cortex; right: presupplementary motor cortex, middle frontal gyrus, supramarginal gyrus; left: posterior superior temporal sulcus/gyrus) and subcortical (right: subthalamus; left: caudate, cerebellum) areas (Figure 3). Activations associated with prediction formation (i.e., generating an expectation for a future event) and prediction violation (i.e., expected event does not occur) were also compared with test the hypothesis that they are processed by the same network. A contrast analysis between prediction formation and prediction violation identified only a single area (posterior superior temporal sulcus/gyrus) as possibly different, indicating that both primarily engage the same network. Although these results also imply the presence of domain-general (what, when, where) predictive processing, the authors noted that confirmation would require more studies (14). A recent study tested the hypothesis that subcortical predictive processing differs by source of timing information by comparing patients with Parkinson’s disease (PD) or spinocerebellar ataxia on two subsecond temporal prediction tasks (47). Performance of the PD group was improved by single interval cues but not by rhythmic cues. In contrast, performance of the spinocerebellar ataxia group was improved by rhythmic cues but not by single interval cues. As the authors discussed, these results are further support for basal ganglia processing of temporal predictions based on rhythm and cerebellar processing of temporal predictions based on intervals (47).
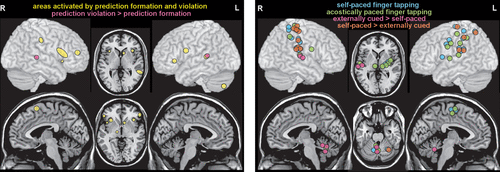
FIGURE 3. Another approach is to focus on how temporal information is used or acquired. Left: One meta-analysis combined functional imaging studies in three different domains (music, language, and action perception) to test the hypothesis that there is a cohesive network of areas supporting predictive processing (14). Convergence of activations identified a widespread network of cortical and subcortical areas. Comparison of areas activated by prediction formation (i.e., generating an expectation) and prediction violation (i.e., expected event does not occur) identified only a single area as possibly different, indicating that both primarily engage the same network. Although these results also imply the presence of domain-general (what, when, where) predictive processing, the authors noted that confirmation will require more studies (14). Right: Another meta-analysis of functional imaging studies compared acoustically paced and self-paced finger tapping to identify the neural basis of sensorimotor synchronization (15). Movement cued by repetitive sensory stimuli induces motor entrainment to the beat (sensorimotor synchronization). A comparison to memory-based finger tapping was also included. Externally cued and self-paced conditions both activated the expected widespread network of cortical and subcortical areas. Many of these areas were more activated in the self-paced condition. In contrast, the cerebellar vermis was activated in the acoustically paced and memory-based (not shown) conditions but not the self-paced condition. As discussed by the authors, these results support the hypothesis that different subcortical networks are preferentially recruited when external cuing of movement induces sensorimotor entrainment compared to when movements are internally initiated (15).
Another meta-analysis combining fMRI and PET studies compared acoustically paced and self-paced finger tapping to identify the neural basis of sensorimotor synchronization (15). Movement cued by repetitive sensory stimuli induces motor entrainment to the beat (sensorimotor synchronization) (15). A comparison to memory-based finger tapping was also included. Convergence of activations for the externally cued and self-paced conditions identified the expected widespread network of both cortical (bilateral: sensorimotor cortex, supplementary motor cortex, inferior parietal cortex; left: ventral and dorsal premotor cortex) and subcortical (bilateral: lateral cerebellum; left: putamen, globus pallidus, thalamus) areas (Figure 3). Many of these areas were more activated in the self-paced condition. In contrast, the cerebellar vermis was activated in the acoustically paced and memory-based conditions, but not the self-paced condition (Figure 3). As discussed by the authors, these results support the hypothesis that different subcortical networks are preferentially recruited when external cuing of movement induces sensorimotor entrainment compared with when movements are internally initiated (15). The therapeutic benefits of incorporating rhythmic auditory stimulation (rhythmic auditory cuing) into motor rehabilitation of patients with PD are well established (26, 28). The presumed mechanism of action is that induction of sensorimotor synchronization facilitates motor preparation and initiation. The contrast in patients with PD between preservation of one use of rhythm-based timing information and loss of another (i.e., temporal prediction) provides further evidence that neural correlates of temporal processing may differ depending on how the temporal information is used. Although rhythmic auditory stimulation has been primarily incorporated into treatment of PD, it has also been demonstrated to be of benefit in other acquired brain injury conditions (e.g., stroke, traumatic brain injury, multiple sclerosis, cerebral palsy) and developmental disorders (e.g., autism spectrum disorder) (27, 28).
In summary, although there is still much to be discovered regarding the neural correlates of temporal processing, evidence to date supports some insights. fMRI and PET indicate that multiple cortical and subcortical structures participate. Depending on its intended use, there is parallel and presumably simultaneous processing of timing information. Impairments in specific aspects of temporal processing can be associated with different neuropsychiatric disorders, and successes in rhythm-based interventions support an emerging relevance in clinical management.
1 : Time dysperception perspective for acquired brain injury. Front Neurol 2014; 4:217–217Crossref, Medline, Google Scholar
2 : Time processing and motor control in movement disorders. Front Hum Neurosci 2016; 10:631Crossref, Medline, Google Scholar
3 : Temporal cognition: connecting subjective time to perception, attention, and memory. Psychol Bull 2016; 142:865–907Crossref, Medline, Google Scholar
4 : Interactive roles of the cerebellum and striatum in sub-second and supra-second timing: support for an initiation, continuation, adjustment, and termination (ICAT) model of temporal processing. Neurosci Biobehav Rev 2016; 71:739–755Crossref, Medline, Google Scholar
5 : Space and time in the brain. Science 2017; 358:482–485Crossref, Medline, Google Scholar
6 : On the integration of space, time, and memory. Neuron 2017; 95:1007–1018Crossref, Medline, Google Scholar
7 : Explicit understanding of duration develops implicitly through action. Trends Cogn Sci 2018; 22:923–937Crossref, Medline, Google Scholar
8 : Intertwined facets of subjective time. Curr Dir Psychol Sci 2018; 27:422–428Crossref, Google Scholar
9 : Metastable states of multiscale brain networks are keys to crack the timing problem. Front Comput Neurosci 2018; 12:75Crossref, Medline, Google Scholar
10 : Anticipated moments: temporal structure in attention. Nat Rev Neurosci 2018; 19:34–48Crossref, Medline, Google Scholar
11 : The neural correlates of time: a meta-analysis of neuroimaging studies. J Cogn Neurosci 2019; 31:1796–1826Crossref, Medline, Google Scholar
12 : Consensus paper: decoding the contributions of the cerebellum as a time machine: from neurons to clinical applications. Cerebellum 2019; 18:266–286Crossref, Medline, Google Scholar
13 : Neural substrates of internally-based and externally-cued timing: an activation likelihood estimation (ALE) meta-analysis of fMRI studies. Neurosci Biobehav Rev 2019; 96:197–209Crossref, Medline, Google Scholar
14 : Is there a prediction network? meta-analytic evidence for a cortical-subcortical network likely subserving prediction. Neurosci Biobehav Rev 2019; 105:262–275Crossref, Medline, Google Scholar
15 : The neural basis of audiomotor entrainment: an ALE meta-analysis. Front Hum Neurosci 2014; 8:776Medline, Google Scholar
16 : Thy mind, thy brain and time. Trends Neurosci 2018; 41:641–643Crossref, Medline, Google Scholar
17 : The neural basis of timing: distributed mechanisms for diverse functions. Neuron 2018; 98:687–705Crossref, Medline, Google Scholar
18 : Towards ecologically valid interval timing. Trends Cogn Sci 2018; 22:850–852Crossref, Medline, Google Scholar
19 : How and why the brain evolves time. Behav Brain Res 2020; 377:112071Crossref, Medline, Google Scholar
20 : Meta-analysis of time perception and temporal processing in schizophrenia: differential effects on precision and accuracy. Clin Psychol Rev 2017; 54:44–64Crossref, Medline, Google Scholar
21 : Predictive timing disturbance is a precise marker of schizophrenia. Schizophr Res Cogn 2018; 12:42–49Crossref, Medline, Google Scholar
22 : Editorial: Time perception and dysfunction: clinical and practical implications. Front Hum Neurosci 2018; 12:435Crossref, Medline, Google Scholar
23 : Positive symptoms and time perception in schizophrenia: a meta-analysis. Schizophr Res Cogn 2018; 13:3–6Crossref, Medline, Google Scholar
24 : Time perception and autistic spectrum condition: a systematic review. Autism Res 2019; 12:1440–1462Crossref, Medline, Google Scholar
25 : Therapeutic applications for integrating rhythm and reflection in support of people with co-occurring drug and alcohol, and mental health issues. Dual Diagn Open Acc 2018; 3:4Crossref, Google Scholar
26 : Future perspectives on neural mechanisms underlying rhythm and music based neurorehabilitation in Parkinson’s disease. Ageing Res Rev 2018; 47:133–139Crossref, Medline, Google Scholar
27 : The potential role of rhythmic entrainment and music therapy intervention for individuals with autism spectrum disorders. J Exerc Rehabil 2019; 15:180–186Crossref, Medline, Google Scholar
28 : A review on the relationship between sound and movement in sports and rehabilitation. Front Psychol 2019; 10:244Crossref, Medline, Google Scholar
29 : Psychological time as information: the case of boredom. Front Psychol 2014; 5:917–917Crossref, Medline, Google Scholar
30 : Emotional modulation of interval timing and time perception. Neurosci Biobehav Rev 2016; 64:403–420Crossref, Medline, Google Scholar
31 : Pain dilates time perception. Sci Rep 2017; 7:15682Crossref, Medline, Google Scholar
32 : The influence of social stress on time perception and psychophysiological reactivity. Psychophysiology 2017; 54:706–712Crossref, Medline, Google Scholar
33 : Feel the time: time perception as a function of interoceptive processing. Front Hum Neurosci 2018; 12:74Crossref, Medline, Google Scholar
34 : Time distortion under threat: sympathetic arousal predicts time distortion only in the context of negative, highly arousing stimuli. PLoS One 2019; 14:e0216704Crossref, Medline, Google Scholar
35 : The functional anatomy of time: What and when in the brain. Trends Cogn Sci 2016; 20:500–511Crossref, Medline, Google Scholar
36 : The hippocampus: a special place for time. Ann N Y Acad Sci 2016; 1369:93–110Crossref, Medline, Google Scholar
37 : Premembering experience: a hierarchy of time-scales for proactive attention. Neuron 2019; 104:132–146Crossref, Medline, Google Scholar
38 : Brain activity related to working memory for temporal order and object information. Behav Brain Res 2018; 354:55–63Crossref, Medline, Google Scholar
39 : Mapping sequence structure in the human lateral entorhinal cortex. eLife 2019; 8:458133Crossref, Google Scholar
40 : Precise temporal memories are supported by the lateral entorhinal cortex in humans. Nat Neurosci 2019; 22:284–288Crossref, Medline, Google Scholar
41 : Time is just a memory. Nat Neurosci 2019; 22:151–153Crossref, Medline, Google Scholar
42 : The ebb and flow of experience determines the temporal structure of memory. Curr Opin Behav Sci 2017; 17:186–193Crossref, Medline, Google Scholar
43 : Boundaries shape cognitive representations of spaces and events. Trends Cogn Sci 2018; 22:637–650Crossref, Medline, Google Scholar
44 : Transcending time in the brain: how event memories are constructed from experience. Hippocampus 2019; 29:162–183Crossref, Medline, Google Scholar
45 : Understanding time perception through non-invasive brain stimulation techniques: a review of studies. Behav Brain Res 2020; 377:112232Crossref, Medline, Google Scholar
46 : Chronotopic maps in human supplementary motor area. PLoS Biol 2019; 17:e3000026Crossref, Medline, Google Scholar
47 : Double dissociation of single-interval and rhythmic temporal prediction in cerebellar degeneration and Parkinson’s disease. Proc Natl Acad Sci USA 2018; 115:12283–12288Crossref, Medline, Google Scholar



