A Systematic Review of Neuropsychiatric Symptoms and Functional Capacity in Huntington’s Disease
Abstract
Objective:
Neuropsychiatric symptoms are known to increase caregiver burden and decrease patient quality of life among patients with Huntington’s disease. Functional capacity is an outcome commonly used in Huntington’s disease clinical trials to quantify disease progression or intervention response. Some studies have examined the relationship between neuropsychiatric symptoms and functional capacity; however, this evidence has not been synthesized. The authors reviewed existing evidence on the association between neuropsychiatric symptoms and functional capacity in Huntington’s disease.
Methods:
A systematic review was conducted using PubMed and CINAHL. Articles were included if they described primary research in humans with Huntington’s disease, measured one or more neuropsychiatric symptoms and functional capacity, and reported statistical methods to identify associations between the two concepts. Additional eligible articles were identified through reference mining and review of other relevant literature.
Results:
Fourteen articles were eligible for review. Neuropsychiatric symptoms were measured individually, in clusters (i.e., depression, anxiety, and suicide items contributing to a depression cluster score), or with an overall score. Significant associations with decreased functional capacity were found most commonly with depression (N=7, median r=0.48) and apathy (N=5, median r=0.47). Other neuropsychiatric symptoms, clusters, and overall scores were all associated with functional capacity in three or fewer studies.
Conclusions:
There is some evidence that depression and apathy are associated with decreased functional capacity in Huntington’s disease. Other neuropsychiatric symptoms have been infrequently examined. Further knowledge of the relationships between neuropsychiatric symptoms and functional capacity will identify areas for intervention and improvement of outcomes in patients with Huntington’s disease.
Huntington’s disease is a slowly progressive terminal illness characterized by motor, cognitive, and neuropsychiatric decline (1). Much focus in Huntington’s disease research has been placed on alleviation of motor symptoms, but neuropsychiatric symptoms also contribute to decreased patient quality of life (2–5) and increased caregiver burden (2, 6). Although it is considered a rare disease, at least 200,000 people in the United States are at risk of developing Huntington’s disease (7).
Neuropsychiatric symptoms are common in Huntington’s disease and can include affective symptoms, irritability, obsessive–compulsive symptoms, and delusions, among others. Recent longitudinal studies estimate a 100% prevalence of at least one neuropsychiatric symptom in patients with Huntington’s disease over time, and apathy and irritability-related symptoms are most common (8). Patients and their caregivers have identified “emotional issues” as having a greater impact on their lives than any other symptom (9). Despite the known prevalence and negative impact of neuropsychiatric symptoms, interventional trials for these symptoms are lacking in Huntington’s disease.
Existing interventional trials in Huntington’s disease frequently use functional capacity as an outcome. Functional capacity is defined as the maximum “performance potential for activities of daily living and/or work tasks” (10). Shoulson and Fahn (11) first suggested measuring functional capacity as a way to evaluate care strategies in Huntington’s disease. In 1979, they proposed the Huntington’s Disease Functional Capacity Scale, currently referred to as the Total Functional Capacity Scale, and noted that variables affecting functional capacity are multifactorial (i.e., not only related to motor symptoms) (11). Implications of functional capacity decline include disease progression and loss of independence (12); thus, it has long been used as a gauge of overall patient status that presumably cannot be fully accounted for by measurements of any individual symptom domain. Today, the Total Functional Capacity Scale is a core common data element according to the National Institute of Neurological Disorders and Stroke, meaning that it should be used in all Huntington’s disease studies (13).
Interventional trials are needed to produce high-quality evidence for treatment of neuropsychiatric symptoms in patients with Huntington’s disease. Because functional capacity is a ubiquitous outcome in Huntington’s disease research, it will likely be used in future trials of neuropsychiatric symptoms. Before it can be used effectively in such a trial, researchers must understand what is currently known about the associations between these variables. Thus, we conducted a systematic review to examine the relationship between neuropsychiatric symptoms and functional capacity in Huntington’s disease.
Methods
Theoretical Framework
No conceptual or theoretical model has been previously used to describe the relationship between neuropsychiatric symptoms and functional capacity in Huntington’s disease. We adapted the theory of unpleasant symptoms (14) for use in this context (Figure 1). This theory was first proposed to aid clinician-researchers in understanding how patients experience symptoms (14). It describes physiologic, psychologic, and situational factors that influence multiple symptoms in a disease state, which in turn influence performance of functional, cognitive, and physical activities. The theory of unpleasant symptoms has been used in several neurological disease populations to investigate the effect of symptoms on outcomes such as quality of life (15). We adapted the model to depict the proposed relationships between various neuropsychiatric symptoms and functional capacity.
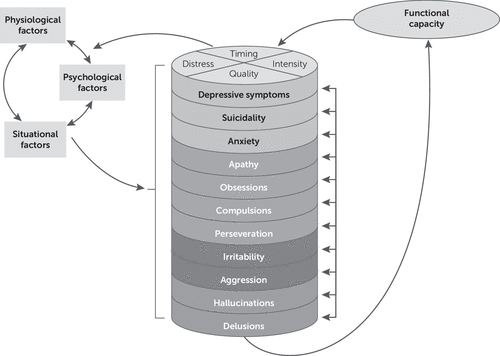
FIGURE 1. Proposed adaptation of the theory of unpleasant symptoms for neuropsychiatric symptoms and functional capacity in Huntington’s diseasea
a Reciprocal effects of functional capacity on the factors and symptoms are shown.
It is believed that physiologic, psychologic, and situational factors contribute to expression of neuropsychiatric symptoms in Huntington’s disease. For example, physiologic factors such as differential loss of GABAA receptors in the striatum and glucose hypometabolism in selected frontal brain regions have been associated with mood disorders and depressive symptoms in Huntington’s disease (16, 17). Psychologic factors certainly contribute to these symptoms, as the symptoms themselves are psychiatric in nature. Excessive worry is a psychologic attribute that may characterize a patient’s experience of anxiety as a neuropsychiatric symptom. Situational factors also influence neuropsychiatric symptoms, as low-income patients may be unable to afford mood-altering medications, and strained patient–caregiver relationships may exacerbate existing neuropsychiatric issues.
In order to adapt the theory of unpleasant symptoms to examine the impact of neuropsychiatric symptoms on functional status in Huntington’s disease, individual neuropsychiatric symptoms have been inserted in the model to replace “symptom 1,” “symptom 2,” and so forth. Factor analyses have identified clusters of neuropsychiatric symptoms in Huntington’s disease (18, 19). In this adapted model, individual symptoms are listed next to other symptoms in their symptom cluster, and each of the four clusters (depression, apathy, irritability, and psychosis) is represented by distinct shading. Performance as an outcome was loosely defined for the original theory of unpleasant symptoms framework as functional, cognitive, and physical activities (14). Functional capacity replaces performance in the adapted model, as this has been precisely defined and is the specific outcome of interest in the present model.
Systematic Review
Literature from PubMed and CINAHL databases was reviewed. A search using the terms Huntington’s disease AND ((psychiatric OR neuropsychiatric OR behavioral OR behavioural) AND symptom*) AND (function OR (functional AND (capacity OR status OR ability OR performance))), filtered for human research only, yielded 628 results. Articles were considered eligible if they reported on primary research in patients with Huntington’s disease, measured one or more neuropsychiatric symptoms and functional capacity, and included statistical analysis of the relationship between the two variables. Literature reviews, animal studies, and studies that used a measure of functional capacity to operationalize disease stage rather than functional capacity were excluded. Nine articles were identified from this search. Two additional articles were identified during a concurrent systematic review of neuropsychiatric symptoms and quality of life in Huntington’s disease. For that review, PubMed and CINAHL were searched using the terms (psych* OR neuropsych* OR depressi* OR anxi* OR irritab* OR compulsiv* OR impulsiv* OR obsess* OR perseverat* OR apath* OR hallucinat* OR delusion* OR suicid*) AND ((Huntington OR Huntington’s) AND disease) AND (qol OR hrqol OR “quality of life”), and the same eligibility criteria were used to identify articles relevant for the current review. Finally, reference mining of all eligible articles revealed three additional articles that met inclusion criteria. In total, 14 articles were identified. Research design, neuropsychiatric symptoms measured, instruments used to measure these symptoms in relation to functional capacity, conceptual terms and instruments used to measure functional capacity, statistical methods, and results are presented in Table 1. Notably, none of the authors reported using a specific theoretical framework to guide their study. We also aimed to identify pertinent qualitative research in the course of this review; all articles were additionally screened for use of qualitative methods and results related to neuropsychiatric symptoms and functional capacity in patients with Huntington’s disease. No pertinent qualitative research was found; thus, only quantitative results are presented here.
| Study | Design | Neuropsychiatric symptoms measured for analysis with function | Instrument for neuropsychiatric measurement | Term used for function | Instrument to measure functional capacity | Statistical method for association between psychiatric and functional variables | Results and conclusions related to neuropsychiatric symptoms |
|---|---|---|---|---|---|---|---|
| Anderson et al. (25) | Cross-sectional, retrospective (HSG data set, patients with Huntington’s disease, N=1,642) | Obsessive and compulsive symptoms | UHDRS-b (single item) | Functional capacity | UHDRS TFC | t test | Patients with obsessive-compulsive symptoms (N=446) had significantly worse functional scores than patients without obsessive-compulsive symptoms (N=1,196) (t=3.47, p<0.001). Patients with high obsessive-compulsive symptoms (highest 25% of the O-C obsessive-compulsive symptoms group, N=121) had significantly worse functional scores than patients with low obsessive-compulsive symptoms (lowest 25% of the obsessive-compulsive symptoms group, N=116) (t=4.99, p<0.001). |
| Banaszkiewicz et al. (2) | Cross-sectional, prospective (patient-caregiver dyads, N=80) | Aggression, apathy, depression, irritability, anxiety, and psychosis | UHDRS-b (measured with single items except for psychosis, which included hallucinations and delusions); HAM-D | Functional disability | UHDRS FAS | Simple linear and multiple regression | On simple regression, depression and apathy were significantly negatively associated with functional score. Irritability, aggression, anxiety, and psychosis subscores were not significantly associated with functional score. On multiple regression, apathy was an independent predictor of functional disability. |
| Beglinger et al. (30) | Cross-sectional, retrospective (HSG data set, patients with Huntington’s disease, N=265) | Depression | UHDRS-b (single-item measure of depression) | Functional capacity | UHDRS TFC and FAS | logistic regression (only with FAS) | Depression was significantly related to loss in ability to engage in usual employment on the FAS (p<0.01). |
| Eddy and Rickards (26) | Cross-sectional, prospective (patients with Huntington’s disease, N=25, healthy control subjects, N=20) | Depression, anxiety, and apathy | PBA subscales (single items) | Functional capacity | UHDRS TFC | Spearman correlations | Depression, anxiety, and apathy scores were not significantly associated with functional capacity scores. |
| Epping et al. (23) | Cross-sectional, retrospective (patients with prodromal Huntington’s disease, N=803, gene negative patients, N=223) | Depression | BDI-II | Functional capacity | Shoulson and Fahn TFC | Analysis of covariance | TFC score decreased with increased depressive symptoms (although the TFC decrease from minimal to severe depression groups was only 1 point). |
| Fritz et al. (3) | Cross-sectional, retrospective (baseline data from larger longitudinal study used; patients with Huntington’s disease: prodromal, N=193, early-stage, N=187, and late-stage, N=91) | Apathy | PBA-s (single item) | Functional status | UHDRS TFC, FAS and IS (composite score of clinician-rated functioning created: scores were recoded to ensure that higher scores indicated better outcomes; individual scores standardized to a mean of 0 and standard deviation of 1; standardized scores were summed for each composite score, then sum scores were scaled with a mean of 0 and standard deviation of 1 so that all composite scores were on the same scale) | Multiple linear regression | There was no statistically significant association between apathy and clinician-rated functioning. |
| Hamilton et al. (32) | Cross-sectional (patients with Huntington’s disease and their informants, N=22) | Apathy, executive dysfunction, and disinhibition | Frontal Lobe Personality Scale | functional decline/ functional disability | HD-ADL, TFC (citations for UHDRS and Shoulson and Fahn TFC) | Simple linear regression | Composite apathy/executive dysfunction score (these subscales were combined because they were highly correlated, r=0.83, p<0.001) was significantly related to decline in activities of daily living, even after controlling for motor and cognitive deficits. |
| Hubers et al. (31) | Cross-sectional, prospective (N=152 patients with Huntington’s disease, gene-negative control subjects, N=56); longitudinal data were gathered but not used to examine relationships of interest | Suicidality | PBA (single item) | Global functioning | UHDRS TFC (Shoulson and Fahn TFC also cited) | Binary logistic regression | The difference in numbers of Huntington’s disease patients who were nonsuicidal and suicidal with TFC scores <10.5 was not statistically significant. |
| Marder et al. (27) | Longitudinal, retrospective (patients with Huntington’s disease, N=960) | Mood, anxiety, aggression, psychosis, and other behavioral abnormalities | UHDRS-b | Functional decline | UHDRS TFC and IS | Mixed-effects model to determine influence of disease characteristics and covariates on change in slope of TFC and IS | Increased depression factor (depression/anxiety) at baseline was associated with faster decline on Independence Scale scores (p=0.03). No other baseline psychiatry-related factors had a significant influence on the slope of the IS or TFC. |
| Mayeux et al. (24) | Cross-sectional, retrospective chart review (patients with Huntington’s disease, N=48); Longitudinal analyses were included but not for relationship of interest to this review | Depression | BDI; BPRS | Functional capacity | Shoulson and Fahn TFC | Simple linear regression, partial correlations | Depression scores from BDI and BPRS were significantly negatively correlated with the functional capacity score on simple regression. On partial correlation, the BDI/BPRS scores (seemingly combined) contributed independently to the determination of functional capacity, but it was no longer associated with functional capacity on reanalysis of the BPRS after removing somatic components. |
| Nehl and Paulsen (1) | Cross-sectional, retrospective (patients with Huntington’s disease, included in regression analysis, N=1,727) | Depression/anxiety, suicidal thoughts, aggressivity, obsessive-compulsive, delusions, and hallucinations | UHDRS-b | Functional capacity | UHDRS TFC (citations for Shoulson and Fahn TFC) | Hierarchical multiple regression | None of the psychiatric symptoms accounted for a statistically significant amount of variance in TFC. |
| Sheppard et al. (33) | Cross-sectional (patients with Huntington’s disease, N=20, control subjects, N=20) | Depression | BDI-II (study subjects <65 years old), GDS-short form (study subjects ≥65 years old) | Instrumental activities of daily living | Lawton and Brody Activities of Daily Living Questionnaire | Spearman’s rank correlations; Depression scores were dichotomized (elevated or nonelevated) | Depression was significantly correlated with the self-report Lawton Instrumental Activities of Daily Living Scale. |
| Sprengelmeyer et al. (28) | Cross-sectional, prospective (patients with Huntington’s disease, N=61, control subjects, N=40) | Depression | HADS-SIS | Ability to cope with demands of daily life | UHDRS TFC | Mann-Whitney test | Depressed and nondepressed Huntington’s disease groups showed no statistically significant difference in TFC scores. |
| van Duijn et al. (29) | Cross-sectional, retrospective (patients with Huntington’s disease, N=1,993) | Neuropsychiatric symptoms (predetermined five subscales: depression, irritability/aggression, obsessive-compulsive behaviors, apathy, and psychosis) | UHDRS-b (predetermined five subscales: depression, irritability/aggression, obsessive-compulsive behaviors, apathy, and psychosis) | Global functioning | UHDRS TFC | One-way between groups analysis of variance or nonparametric Kruskal-Wallis test or univariate analyses; multivariate logistic regression | Patients with Huntington’s disease with each of the five symptoms measured (depression cluster, irritability/aggression, obsessive-compulsive behaviors, apathy, and psychosis) had lower TFC scores than patients without these symptoms (p<0.001 for all), but on multiple regression, TFC scores only statistically significantly correlated with apathy. |
TABLE 1. Results of the systematic review on neuropsychiatric symptoms and functional status in Huntington’s diseasea
Results
First, instruments used to measure the primary outcome, functional capacity, are summarized. Second, evidence for the relationship between neuropsychiatric symptoms and functional capacity in Huntington’s disease is presented in three distinct sections: relationship of a total neuropsychiatric symptom score with functional capacity, relationship of individual neuropsychiatric symptoms with functional capacity, and relationship of neuropsychiatric symptom clusters with functional capacity. Effect sizes for each of these relationships are summarized in Table 2.
| Symptom and neuropsychiatric tool | Functional capacity tool | Effect sizeb | r or β (rounded to the nearest hundredth)c | Study |
|---|---|---|---|---|
| Individual symptoms | ||||
| Depressive symptoms | ||||
| HAM-D | FAS | r=–0.43 | 0.43 | Banaszkiewicz et al. (2) |
| UHDRS-b | FAS | r=0.039 | 0.04 | Beglinger et al. (30) |
| PBA-s | TFC | r=–0.003 | 0.01 | Eddy and Rickards (26) |
| BDI-II | TFC | r=0.52 | 0.52 | Epping et al. (23) |
| BDI | TFC | r=–0.69 | 0.69 | Mayeux et al. (24) |
| BPRS | TFC | r=–0.76 | 0.76 | Mayeux et al. (24) |
| BDI-II | Lawton and Brody ADL | r=–0.57 | 0.57 | Sheppard et al. (33) |
| HADS-SIS | TFC | r=0.03 | 0.03 | Sprengelmeyer et al. (28) |
| Suicidality | ||||
| PBA | TFC | r=0.157 | 0.16 | Hubers et al. (31) |
| UHDRS-b | TFC | β=–0.025 | 0.03 | Nehl and Paulsen (1) |
| Anxiety | ||||
| UHDRS-b | FAS | r=–0.2 | 0.2 | Banaszkiewicz et al. (2) |
| PBA-s | TFC | r=–0.26 | 0.26 | Eddy and Rickards (26) |
| Irritability | ||||
| UHDRS-b | FAS | r=0.02 | –0.02 | Banaszkiewicz et al. (2) |
| Aggression | ||||
| UHDRS-b | FAS | r=–0.19 | 0.19 | Banaszkiewicz et al. (2) |
| UHDRS-b | TFC | β=–0.03 | 0.03 | Nehl and Paulsen (1) |
| Apathy | ||||
| UHDRS-b | FAS | r=–0.47 | 0.47 | Banaszkiewicz et al. (2) |
| PBA-s | TFC | r=–0.096 | 0.1 | Eddy and Rickards (26) |
| PBA-s | Composite of TFC, FAS, and IS | adjusted r2=0.14 | 0.37 | Fritz et al. (3) |
| FLOPS | TFC | r=0.77 | 0.77 | Hamilton et al. (32) |
| HD-ADL (instrumental) | r=0.92 | 0.92 | ||
| HD-ADL (physical) | r=0.83 | 0.83 | ||
| UHDRS-b | TFC | r=0.058 | 0.06 | van Duijn et al. (29) |
| Delusions | ||||
| UHDRS-b | TFC | β=–0.044 | 0.04 | Nehl and Paulsen (1) |
| Hallucinations | ||||
| UHDRS-b | TFC | β=–0.013 | 0.01 | Nehl and Paulsen (1) |
| Symptom clusters | ||||
| Depression cluster 1 (depression and anxiety) | ||||
| UHDRS-b | IS | β=0.061 | 0.06 | Marder et al. (27) |
| UHDRS-b | TFC | β=–0.02 | 0.02 | Nehl and Paulsen (1) |
| Depression cluster 2 | ||||
| UHDRS-b | TFC | r=0.008 | 0.01 | van Duijn et al. (29) |
| Irritability/aggression cluster | ||||
| UHDRS-b | TFC | r=0.008 | 0.01 | van Duijn et al. (29) |
| Obsessive-compulsive cluster | ||||
| UHDRS-b | TFC | r=0.096 and r=0.308d | 0.1 | Anderson et al. (25) |
| 0.31 | ||||
| UHDRS-b | TFC | B=–0.055 | 0.06 | Nehl and Paulsen (1) |
| UHDRS-b | TFC | r=0.017 | 0.02 | van Duijn et al. (29) |
| Psychosis cluster | ||||
| UHDRS-b | FAS | r=–0.25 | 0.25 | Banaszkiewicz et al. (2) |
| UHDRS-b | TFC | r=0.017 | 0.02 | van Duijn et al. (29) |
| Total behavioral score | ||||
| UHDRS-b | FAS | r=–0.35 | 0.35 | Banaszkiewicz et al. (2) |
TABLE 2. Standardized effect sizes for neuropsychiatric symptoms in Huntington’s diseasea
Measurement of Functional Capacity
Functional capacity is the primary outcome in the modified theory of unpleasant symptoms model described above. In the articles included in this review, six instruments were used to measure functional capacity or a related concept: Shoulson and Fahn’s Total Functional Capacity Scale (11, 12), the Unified Huntington’s Disease Rating Scale (UHDRS) Total Functional Capacity Scale (20), the UHDRS Functional Assessment Scale (20), the UHDRS Independence Scale (20), the Lawton and Brody Activities of Daily Living questionnaire (21), and the Huntington’s Disease Activities of Daily Living (HD-ADL) questionnaire (22).
Available validity and reliability data for these measures are summarized in Table 3. As previously mentioned, the Total Functional Capacity Scale is widely used in clinical trials. It is administered via a semistructured interview in which patients are rated in five functional areas (occupation, finances, domestic responsibilities, activities of daily living, and location of patient care) (12, 20). The total score ranges from 0 to 13, with higher scores indicating greater functional capacity. Interestingly, the Huntington Study Group claimed that their UHDRS version of the Total Functional Capacity Scale was the same as Shoulson and Fahn’s original scale (20), when in fact, the description for each item is significantly abridged in the UHDRS version. However, seemingly because the same items, scores, administration method, and interpretation are used for both versions, the application of original Shoulson and Fahn reliability data to the UHDRS version has been accepted in the field.
| Measure | Validity | Reliability |
|---|---|---|
| Shoulson and Fahn TFC (11, 12) | Concurrent validity: measures of caudate atrophy on MRI and metabolism on positron emission tomography significantly correlated with TFC scores (p values for both <0.001). Face validity: created by eight field experts, widely used. | Interrater reliability for agreement within 1 point was 65%, and within 2 points it was 85% (48). |
| UHDRS TFC (20) | Face validity: widely used in Huntington’s disease clinical trials. Concurrent validity: significantly correlated with caudate change (49). Convergent validity: significantly intercorrelated with IS (0.86) and FAS (0.9) (p<0.001) (50). | No Cronbach’s alpha or interrater reliability was reported for this subscale, although HSG (1996) cites reliability data for the Shoulson and Fahn TFC scale. It was highly intercorrelated with UHDRS motor, behavioral, and functional checklist scores (p<0.005), which had Cronbach’s alpha values of 0.95, 0.9, and 0.95, respectively. |
| UHDRS FAS (20) | Convergent validity: intercorrelations with a TFC score of 0.9 and an IS score of 0.91 (p values for both <0.001) (50). | High internal consistency: Cronbach’s alpha was 0.95. |
| UHDRS IS (20) | Convergent validity: intercorrelations with a TFC score of 0.86 and an FAS score of 0.91 (p values for both <0.001) (50). | No Cronbach’s alpha or interrater reliability was reported for this subscale. It was highly intercorrelated with UHDRS motor, behavioral, and functional checklist scores (p<0.005), which had Cronbach’s alpha values of 0.95, 0.9, and 0.95 respectively. |
| Lawton and Brody Activities of Daily Living questionnaire (21) | Not established in Huntington’s disease diagnosis (developed for older adults); used in one Huntington’s disease study. | Interrater reliability showed a correlation of 0.85 between total instrumental ADL scores. |
| HD-ADL (22) | Convergent validity: correlates with Shoulson and Fahn TFC (r=–0.89). Construct validity: PCA identified four factors accounting for 74% of variance (all eigenvalues >1). | High internal consistency: Cronbach’s alpha was 0.91. |
TABLE 3. Reliability and validity of scales identified in this review to measure functional capacity in Huntington’s diseasea
The other scales used lack reliability or validity data in Huntington’s disease (the Independence Scale and the Lawton and Brody Activities of Daily Living Scale), increase patient burden with longer administration time (Functional Assessment Scale), and/or include measurement of neuropsychiatric symptoms that could confound results in a study examining how neuropsychiatric symptoms affect functional status (HD-ADL). Additionally, these alternative scales have been used infrequently in Huntington’s disease studies compared with the Total Functional Capacity Scale.
Only two articles in this review specifically reported using the Shoulson and Fahn Total Functional Capacity Scale (23, 24); others used the UHDRS Total Functional Capacity scale (3, 25–29), and still others cited both Shoulson and Fahn and the Huntington Study Group (UHDRS) (1, 30–32). The UHDRS Functional Assessment Scale was used to measure functional capacity in two studies (2, 30), and the UHDRS Independence Scale and Lawton and Brody Activities of Daily Living scales were each used in one study (27, 33). One article in this review used a composite of the three UHDRS functional assessment components to operationalize functional capacity (3); the method for creating this composite score is described in Table 3.
Relationship of Total Neuropsychiatric Symptoms With Functional Capacity
One study in this review reported evidence for the relationship between a total neuropsychiatric symptom score and functional capacity in Huntington’s disease (2). This cross-sectional study (N=80) measured a total neuropsychiatric score using the Unified Huntington’s Disease Rating Scale Behavioral Subscale (UHDRS-b) (20). The UHDRS-b assesses 10 neuropsychiatric symptoms of HD: sadness/low mood, low self-esteem/guilt, anxiety, suicidal thoughts, disruptive/aggressive behavior, irritable behavior, obsessions, compulsions, delusions, and hallucinations (20). Interestingly, it appears that only five symptoms—apathy, psychosis (combined delusions and hallucinations), irritability, aggression, and anxiety—were included as part of the total neuropsychiatric score (2), bringing into question both reliability and content validity of the tool as used in the study.
Simple linear regression was used to study this relationship, and increased neuropsychiatric symptoms were associated with decreased functional capacity (r=0.35) (2). No other studies looked at the effect of a total neuropsychiatric score on functional capacity.
Relationship of Individual Neuropsychiatric Symptoms With Functional Capacity
Depression.
The relationship between depression and functional capacity was analyzed by more studies in this review than any other proposed relationship (N=7). Study designs were cross-sectional (N=5) and retrospective (N=2), and sample sizes ranged from 40 to 803. Nine different scales were used to measure depression. Scales not specific to Huntington’s disease included the Hamilton Depression Rating Scale (HAM-D) (34), Beck Depression Inventory (BDI) (35), BDI-II (36), Brief Psychiatric Rating Scale (BPRS) (37), Hospital Anxiety and Depression Scale–Snaith Irritability Scale (HADS-SIS) (38), and Geriatric Depression Scale–short form (39) (Table 4).
| Measure | Validity | Reliability | Considerations |
|---|---|---|---|
| UHDRS-b (20) | Construct validity: factor analyses has shown heterogenous factors (19, 42). Convergent validity: shown between the depression item on the UHDRS-b and the “feel sad” item on the BDI (correlation coefficient=0.834, p<0.01), as well as the “depressed mood” item on the HAM-D (r=0.917) (41). Face validity: the longest-used tool for behavioral symptoms in Huntington’s disease. | Internal consistency: Cronbach’s alpha was equal to 0.83. | Although the whole tool has established validity and reliability, single items are often used in isolation to assess only one symptom. Depression has established convergent validity with the BDI and HAM-D, but no other individual item has established validity, and no individual item has reliability data to support its use. |
| HAM-D (34) | Convergent validity: validity with UHDRS-b “depressed mood” item (r=0.917) (41). Face validity: used to measure depression in several Huntington’s disease studies. | Interrater reliability was equal to 0.9. | Used to assess depressive symptoms only. |
| PBA (40) | Content/face validity: created by a panel of Huntington’s disease experts from a list of patient symptoms and complaints and extensive literature review. Construct validity: PCA identified three factors, although these factors only accounted for 40.7% of the total variance. | Interrater reliability was >0.8, and the test-retest reliability was >0.9 for the whole scale. Depression cluster items showed good internal consistency (Cronbach’s alpha=0.81) (42). | Although the whole tool has established validity and reliability, single items are often used in isolation to assess only one symptom. Depression cluster items have established reliability data, but individual symptoms do not. |
| BDI-II (36) | Criterion validity: diagnosis of depression (compared with the study gold-standard Schedules for Clinical Assessment in Neuropsychiatry) at a score of 10 or 11 had a sensitivity of 1.0, specificity of 0.66, and area under the curve of 0.856 (51). | Internal consistency in outpatients was high (Cronbach’s alpha=0.92) (52). | Used to assess depressive symptoms only. |
| PBA-s (18) | Construct validity: factor analysis (after excluding paranoid thinking and hallucinations as a result of low incidence) revealed three factors (apathy, irritability, and affective) with eigenvalues >1, consistent with the factor structure in the PBA-HD. Face validity: used in several large clinical or observational trials. | Interrater reliability was considered decent for severity (0.74, adjusted=0.77) and frequency (0.76, adjusted=0.8). No reliability data for individual symptoms were available. | Although the whole tool has established validity and reliability, single items are often used in isolation to assess only one symptom. No individual symptom item has its own established reliability. |
| BDI (35) | Content validity: addresses most of DSM-III criteria for depression and intentionally excluded other criteria due to the frequency of their presence in nondepressed patients (53). Convergent validity: the “feel sad” item correlates with the UHDRS “depressed mood” item (r=0.834) (41). The mean correlation coefficient with HAM-D was 0.73 (53). | Internal consistency: the mean coefficient alpha for psychiatric patients was 0.86, and for nonpsychiatric patients it was 0.81 (53). Interrater agreement between patients with Huntington’s disease and their caregivers was moderate to good (54). | Used to assess depressive symptoms only. |
| BPRS (37) | Interrater reliability for depressive mood was 0.82. | In this review, it was used only to measure depressive symptoms. Depressive mood items have decent reliability data, but there is no real validity data for its use in Huntington’s disease. | |
| HADS-SIS (38) | Convergent validity: correlation between the modified HAM-D and depression items of the HADS-SIS was equal to 0.75 (p<0.05). | Internal consistency of the depression subscale Spearman-Brown coefficients ranged between 0.72 and 0.81. | Validity and reliability data are available for the depression subscale (the only one used in this review), but data are weaker compared with other scales. |
| GDS-short form (39) | Convergent validity with BDI in preoperative surgical patients (Spearman’s r=0.704, p<0.01) (55). | Internal consistency: Cronbach’s alpha was 0.749 (in adults >64 years old) (56). | No true validity data for Huntington’s disease are available, and because it was created for older adults, this makes validity in a younger Huntington’s disease population even more questionable. |
| FLOPS (57) | Construct validity: used in measuring frontal lobe symptoms in patients with frontal lobe lesions versus the same patients before they had lesions, as well as healthy control subjects (p<0.001). Factor analysis revealed that 83% of items were loaded on three factors (58). Face validity: patients with Huntington’s disease were included in the factor analysis (58). | Internal consistency: Cronbach’s alpha was 0.95. | Not clearly valid in Huntington’s disease, although patients with Huntington’s disease were included in the sample for factor analysis (58). |
TABLE 4. Reliability and validity of scales identified in this review to measure neuropsychiatric symptoms in Huntington’s diseasea
Neuropsychiatric scales that were specific to Huntington’s disease, from which individual depression items were used, include the Problem Behaviors Assessment (40), Problem Behaviors Assessment–short form (18), and UHDRS-b (20). The individual depression item on the UHDRS-b is shown to have convergent validity with the BDI-II and HAM-D (41), but reliability has not been established for the use of the individual depression item in any of these tools.
Most scales were used to assess depression in just one study, including the HAM-D (2), BDI (24), BPRS (24), HADS-SIS (28), and Geriatric Depression Scale–short form (33); the BDI-II was used to measure depression in two studies (23, 33). The single depression items from the Huntington’s-disease-specific scales were each used in one study (UHDRS-b [30], Problem Behaviors Assessment [26], Problem Behaviors Assessment–short form [33]).
Simple regression was most commonly used to examine this relationship, with a median effect size (r) of 0.48 (range 0.003–0.76). All studies reported an effect size supporting the relationship of increased depression with decreased functional capacity, although statistically significant results were reported in only five of the studies.
Suicidality.
The association between suicidality and functional capacity was examined in two studies (1, 31). These studies were cross-sectional and descriptive, with a median sample size of 939 participants. Single suicidality items from the Problem Behaviors Assessment and UHDRS-b were used to measure suicidality in these studies. There is no reliability or validity data for use of the single suicidality item with either of these scales. With the Problem Behaviors Assessment, the suicidality item is considered part of the depression cluster (40, 42). In terms of the UHDRS-b, different cutoffs for suicidal and nonsuicidal have been used in Huntington’s disease studies, and the lack of validated cutoff scores has been noted as a limitation in previous studies (43).
Binary logistic regression and hierarchical multiple regression were used to study the relationships between suicidality and functional capacity, and no statistically significant effects were reported. The average effect size was 0.1.
In one study, patients were separated into suicidal and nonsuicidal groups for analysis, although the original data were continuous in nature (32). Investigators determined a cutoff score for whether someone was suicidal or nonsuicidal on the basis of clinical experience and not through statistically valid methods. As noted above, this presents a problem with the validity of study methods, and results must be questioned accordingly.
Anxiety.
The relationship between anxiety and functional capacity in Huntington’s disease was examined in two studies (2, 26). Both studies were cross-sectional and descriptive, with sample sizes <100. Anxiety was measured using single items from the Problem Behaviors Assessment and UHDRS-b. There are no reliability data for the single anxiety item in either of these scales, and the anxiety item has been shown to be included in the depression cluster for both scales (19, 40, 42). Simple and multiple regressions were used to study the relationship between anxiety and functional capacity, and the mean correlation was 0.23. No statistically significant relationships were reported.
Irritability.
The relationship between irritability and functional capacity was examined in just one study (2). This cross-sectional, descriptive study (N=80) used the single irritability item from the UHDRS-b. As with all other individual symptoms, there are no reliability data to support the use of this single item measure from the UHDRS-b. Interestingly, the effect size reported (r=0.02) indicates an unexpected relationship, where worse irritability is associated with better functional capacity (2). However, definitive conclusions about the relationship between these variables cannot be reached with such a small effect size reported in just one study.
Aggression.
The relationship between aggression and functional capacity was examined in two studies (1, 2). Both studies were cross-sectional (N=80 and N=1,727, respectively) and used a single aggression item from the UHDRS-b. As mentioned, there are no reliability data for use of individual items on the UHDRS-b. Additionally, aggression and irritability are considered part of one symptom cluster (19), bringing into question the validity of the single-item aggression assessment. Simple and multiple regression were used to examine the relationship, and the average correlation coefficient was 0.11. As with anxiety and irritability, no statistically significant relationship has been shown between aggression as an individual symptom and functional capacity, and effect sizes were small.
Apathy.
The association between apathy and functional capacity was explored in five studies in this review (2, 3, 26, 29, 32). All of these studies were cross-sectional, with a median sample size of 80 (range 22–1,993). Individual apathy items from the UHDRS-b, Problem Behaviors Assessment, and Problem Behaviors Assessment-short form were used to measure apathy in four of these studies. Although an apathy cluster has been validated for these scales, the use of a single apathy item does not have validity or reliability data to support it. One study used the Frontal Lobe Personality Scale and combined the apathy and executive dysfunction subscales for regression analyses due to high intercorrelation of these variables (32). Statistical approaches to study the relationship between apathy and functional capacity in these studies included simple and multiple regression, one-way between-groups analysis of variance (ANOVA), and multivariate logistic regression. Three of the five studies found statistically significant evidence that increased apathy correlated with decreased functional capacity, and the median effect size (r) of all studies was 0.47.
Delusions.
The relationship between delusions and functional capacity was examined in one cross-sectional study (N=1,727) (1). The delusions item on UHDRS-b was used to assess this symptom, and as with most other single-items from the UHDRS-b, there is no reliability or validity data for use of this item to assess delusions. No statistically significant relationship between delusions and functional capacity was found (1). The reported effect size from hierarchical multiple regression was quite small (B=0.04) (1).
Hallucinations.
The relationship of hallucinations with functional capacity was also examined in the above study (1). The individual hallucination item of the UHDRS-b does not have its own validity or reliability data. As with delusions, the reported effect size was negligible (B=0.01), and no statistically significant relationship was found (1).
Obsessions, compulsions, perseveration.
None of the studies in this review measured obsessions, compulsions, or perseveration as individual symptoms in association with functional capacity, though an obsessive-compulsive symptom cluster was reported in two studies (see below). Perseveration has been identified as part of the irritability cluster on the Problem Behaviors Assessment–short form (18). On the UHDRS-b, “inflexibility” is used to describe irritability and is not rated as a separate item (20). Presumably because of this conflation of concepts, a perseveration item was not included in the studies in this review that used an irritability–aggression cluster. Relationships of these symptom clusters with functional capacity are summarized in the following section.
Relationship of Neuropsychiatric Symptom Clusters With Functional Capacity
Depression clusters.
As noted above, depression was measured and associated with functional capacity as a single symptom in seven studies. Two different depression clusters were also identified in this review. One depression cluster, including depression and anxiety items of the UHDRS-b, was used to measure depression in two studies (1, 27). Both studies were descriptive in nature, though one was cross-sectional and the other longitudinal (mean N=1,344). There is some argument for validity of this cluster; in factor analysis of the entire UHDRS, including motor and cognitive subscales, depression/anxiety was one of 15 factors accounting for 77% of variance on UHDRS (27). However, this cluster is not consistent with what has been identified in neuropsychiatric-specific scales such as the UHDRS-b and Problem Behaviors Assessment–short form. A mixed-effects model and hierarchical multiple regression were used for analysis, and the mean beta coefficient was small (0.04). One study found a statistically significant association of its depression cluster with functional capacity as measured by the Independence Scale (27). The direction of this effect is consistent with associations found for individual depression measures, with increased depression related to decreased functional capacity.
A second depression cluster, including depression, anxiety, low self-esteem, and suicidality items, was used in one cross-sectional study (N=1,993) (29). This cluster was identified in a factor analysis of the UHDRS-b (eigenvalue of 2.34) (19) and is similar to the depression cluster identified in the Problem Behaviors Assessment–short form (18). Therefore, this cluster appears to be the most valid cluster for measuring depression in Huntington’s disease. One-way between-groups ANOVA or nonparametric Kruskal-Wallis calculations revealed a statistically significant association matching the direction of previously reported associations, but the effect size was quite small (r=0.01) (29). Statistical separation of participants into groups with and without symptoms, rather than linear regression, may have decreased the precision of the measured effect.
Irritability–aggression cluster.
An irritability–aggression cluster was also used in the study just described (29). Irritability–aggression has been shown to be a separate factor on UHDRS-b (eigenvalue 1.58) (19) and on UHDRS (27). As with the depression cluster above, researchers reported a statistically significant association of this irritability–aggression cluster with functional capacity, though the effect size was negligible (r=0.01) (29).
Obsessive-compulsive cluster.
Three studies included an obsessive-compulsive cluster in their analysis (1, 25, 29). All studies analyzed cross-sectional data for their analysis (median N=1,642) and used the obsessions and compulsions items on the UHDRS-b. These items were identified as a unique cluster of the UHDRS (27) but were found to be part of the apathy cluster in factor analysis of the UHDRS-b (19). As with the depression clusters, the cluster identified through analysis of the UHDRS-b alone is likely the most valid for this phenomenon. t Tests, one-way between-group ANOVA, and hierarchical multiple regression were used to analyze the relationship between this cluster and functional capacity, and the median correlation coefficient was 0.08.
Two studies separated participants into groups for analysis representing those with and without obsessive-compulsive symptoms (25, 29). One of these also compared high versus low obsessive-compulsive symptom groups, and effect sizes were different depending on how groups were defined (25). Despite this, results for both analyses were clinically and statistically significant in that the group with more obsessive-compulsive symptoms had lower function than the group with fewer or no obsessive-compulsive symptoms (25). Although there is evidence that an obsessive-compulsive symptom cluster may be associated with decreased functional capacity in Huntington’s disease, these symptoms are included in the apathy symptom cluster in the literature (19), bringing into question the utility of these results for future studies.
Psychosis cluster.
A psychosis cluster, including delusion and hallucination items, was included in two cross-sectional studies (N=80 and N=1,993) (2, 29). These items have been established as a separate factor on the UHDRS-b (eigenvalue of 1.389) (19). Simple and multiple linear regression, along with one-way between-groups ANOVA, were used to examine the relationship of this psychosis cluster with functional capacity (mean r=0.14). Increased psychosis cluster score was statistically significantly associated with decreased functional capacity in one of two studies, and the average effect size, although small, was greater than what has been reported for several other symptom clusters. Hallucinations and delusions occur rarely in Huntington’s disease (18); thus, these statistically significant results may be less clinically significant than other, more pervasive symptoms.
Other.
One final article suggested a relationship between neuropsychiatric symptoms and functional capacity in Huntington’s disease (44), but it did not describe which symptoms were included in the clusters measured by the Revised Symptoms Checklist−90. As a result of a lack of specificity in describing the contents of the clusters, effect sizes reported in the study cannot be translated for use in a future study of symptom clusters and functional capacity. For this reason, this article was excluded from this synthesis.
Discussion
In the 14 studies identified for this review, measurement of functional capacity was varied. The Total Functional Capacity Scale was used commonly, but most authors seemed unaware that the Shoulson and Fahn and UHDRS versions of this scale are not identical. Psychometric data for one version of the Total Functional Capacity Scale should not be used to support use of the other version, particularly considering the brevity of item description for the UHDRS scale compared with the Shoulson and Fahn scale. Interestingly, though the HD-ADL scale has good validity and reliability for use in Huntington’s disease, it was used in only one study in this review. This could be related to its inclusion of an apathy item, which would introduce redundancy in association with neuropsychiatric symptoms. The Total Functional Capacity Scale does not have this redundancy issue and may therefore be most appropriate for use in a study considering both neuropsychiatric and functional capacity variables. However, investigators should be careful to consider which version of the scale they are using and include only the psychometric data applicable to that version.
Some have suggested that the Total Functional Capacity Scale measures function primarily in terms of motor ability, as scores drop when patients approach a motor-manifest diagnosis (45). If this is the case, it may not be the best tool to measure direct effects of neuropsychiatric symptoms; any effect noted between neuropsychiatric symptoms and Total Functional Capacity Scale could be mediated by effects of motor symptoms. Additionally, a ceiling effect has been found when using the Total Functional Capacity Scale in persons with prodromal Huntington’s disease (45), which may limit the ability to find significant associations with other variables in this subpopulation. More work with a strong theoretical basis is needed to define direct versus indirect effects of neuropsychiatric axis dysfunction on functional deficits. Qualitative work in this area could also help define such a model.
Functional capacity instruments were not the only tools for which psychometric data were questionable or confusing in this review. Neuropsychiatric symptom measures were frequently used in a manner inconsistent with established reliability and validity data. Most commonly, individual symptom items from composite neuropsychiatric symptom scales were analyzed independently or in a nonvalid cluster. Although some have questioned the validity of cluster scores created by adding individual symptom scores (46), there is a consensus in the field regarding the composition of neuropsychiatric symptom clusters (18, 19). Thus, it is notable that validated clusters or overall scores were rarely used in studies in this review.
For statistical analysis, univariate correlations were most frequently used, not accounting for the possible effects of other variables on the relationship. As previously mentioned, this limits the ability to determine true effects of neuropsychiatric symptoms in the setting of concurrent motor and cognitive dysfunction. A few studies compared group characteristics, but this was typically done by dichotomizing score values in an arbitrary or nonvalidated fashion. Other than Sheppard et al.’s (33) use of previously criterion-referenced cutoff scores of the BDI-II and Geriatric Depression Scale–short form, formation of groups for comparison on the basis of these continuous scales was speculative.
In terms of the relationship between variables, studies in this review found that increased neuropsychiatric symptoms relate to decreased functional capacity in Huntington’s disease. Depression and apathy as individual symptoms were examined in more studies than other individual symptoms or clusters; while all of these studies reported decreased functional capacity with greater depression and apathy, effect sizes were quite variable. Total neuropsychiatric symptoms, clusters, and other individual symptoms were each examined in three or fewer studies, but the direction of the effect for all these symptoms was consistent; worse neuropsychiatric symptoms were associated with worse functional capacity. Despite the methodological weaknesses identified above, the general trend in this review reveals a compelling relationship between neuropsychiatric symptoms and functional capacity. Further examination is warranted with thoughtful consideration of an underlying theoretical model, instrument use, and analysis.
Conclusions
There is general evidence that neuropsychiatric symptoms are associated with decreased functional capacity in Huntington’s disease. Relationships with depression and apathy have been most commonly reported. The relationship of functional capacity with other individual symptoms, validated symptom clusters, and overall neuropsychiatric scores remains unclear, as nonvalidated tools and small number of studies limit meaningful synthesis. It is important to examine these relationships because interventional studies are needed to address neuropsychiatric symptoms in Huntington’s disease, and functional capacity is a commonly used outcome.
This review highlights several limitations of current research in this area. First, most studies lack a guiding theoretical framework or at least fail to include this in their publication. We successfully applied the theory of unpleasant symptoms to conceptualize the relationship between neuropsychiatric symptoms and functional capacity in Huntington’s disease. Future studies should use a prospective, longitudinal design to confirm the direction of the effects depicted in the model. Use of this framework in future research may encourage further inquiry into the effects of bothersome symptoms in Huntington’s disease and related diseases.
Although this review does not definitively confirm the strength of the relationships between variables, it does reveal an overall negative effect of neuropsychiatric symptoms on functional capacity. This supports the argument that more research is needed regarding treatment of neuropsychiatric symptoms, as interventional research in Huntington’s disease has historically focused on motor symptoms. Huntington’s disease is not the only neurodegenerative disease for which treatment of motor symptoms is given precedence over other bothersome symptoms in research and clinical settings. For example, psychosis can be quite problematic in Parkinson’s disease, but until recently, no medications were approved to treat this symptom (47). We hope that this review encourages further dialogue about the important effects of neuropsychiatric symptoms in neurodegenerative disease.
Finally, many instruments identified in this review were used in a way that generates serious questions about the validity of study results. In future studies of Huntington’s disease and otherwise, investigators should carefully consider what an instrument was validated to measure and ensure that administration and analysis is consistent with existing psychometric data. In the case of functional capacity, in which evidence for reliability or validity of the existing tools is underwhelming, perhaps a new instrument could be created. Such a tool could also attempt to capture domains of function (i.e., social) not currently reflected in the Total Functional Capacity Scale, which might detect functional changes currently missed in prodromal patients and patients with early stages of disease. Future studies in this area should use a prospective design and comprehensively address the identified measurement concerns for better understanding of this phenomenon.
1 : Cognitive and psychiatric aspects of Huntington disease contribute to functional capacity. J Nerv Ment Dis 2004; 192:72–74Crossref, Medline, Google Scholar
2 : Huntington’s disease from the patient, caregiver and physician’s perspectives: three sides of the same coin? J Neural Transm (Vienna) 2012; 119:1361–1365Crossref, Medline, Google Scholar
3 : Relationships among apathy, health-related quality of life, and function in Huntington’s disease. J Neuropsychiatry Clin Neurosci 2018; 30:194–201Link, Google Scholar
4 : Health-related quality of life in Huntington’s disease: which factors matter most? Mov Disord 2009; 24:574–578Crossref, Medline, Google Scholar
5 : Quality of life in Huntington’s disease: a comparative study investigating the impact for those with pre-manifest and early manifest disease, and their partners. J Huntingtons Dis 2013; 2:159–175Crossref, Medline, Google Scholar
6 : Caregiver burden in Huntington’s disease. Rehabil Psychol 2007; 53:311–318Crossref, Google Scholar
7 What is Huntington’s Disease? 2018. http://hdsa.org/what-is-hd.Google Scholar
8 : Longitudinal evaluation of neuropsychiatric symptoms in Huntington’s disease. J Neuropsychiatry Clin Neurosci 2012; 24:53–60Link, Google Scholar
9 : The Huntington Disease Health Index Study (HD-HI). Houston, Tex, Huntington Study Group Annual Meeting, 2018Google Scholar
10 : Functional capacity assessment: a test battery and its use in rehabilitation; in Advances in Industrial Ergonomics & Safety, I. Edited by MitalA. New York, Taylor & Francis, 1992, pp 1171–1177Google Scholar
11 : Huntington disease: clinical care and evaluation. Neurology 1979; 29:1–3Crossref, Medline, Google Scholar
12 : Huntington disease: functional capacities in patients treated with neuroleptic and antidepressant drugs. Neurology 1981; 31:1333–1335Crossref, Medline, Google Scholar
13 NINDS Common Data Elements: Huntington’s Disease, 2018. https://www.commondataelements.ninds.nih.gov/HD.aspx#tab=Data_StandardsexGoogle Scholar
14 : The middle-range theory of unpleasant symptoms: an update. ANS Adv Nurs Sci 1997; 19:14–27Crossref, Medline, Google Scholar
15 . Analysis of University of California in San Francisco (UCSF) symptom management theory and theory implication for persons with neurological disorders/diseases. J Neurosurg Neurol Nurs 2017; 6(2):55–65Crossref, Google Scholar
16 : Paralimbic frontal lobe hypometabolism in depression associated with Huntington’s disease. Neurology 1992; 42:1791–1797Crossref, Medline, Google Scholar
17 : Striosomes and mood dysfunction in Huntington’s disease. Brain 2007; 130:206–221Crossref, Medline, Google Scholar
18 : Reliability and factor structure of the Short Problem Behaviors Assessment for Huntington’s disease (PBA-s) in the TRACK-HD and REGISTRY studies. J Neuropsychiatry Clin Neurosci 2015; 27:59–64Link, Google Scholar
19 : Factor analysis of behavioural symptoms in Huntington’s disease. J Neurol Neurosurg Psychiatry 2011; 82:411–412Crossref, Medline, Google Scholar
20 : Unified Huntington’s Disease Rating Scale: reliability and consistency. Mov Disord 1996; 11:136–142Crossref, Medline, Google Scholar
21 : Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969; 9:179–186Crossref, Medline, Google Scholar
22 : Clinical correlates of dementia and disability in Huntington’s disease. J Clin Neuropsychol 1984; 6:401–412Crossref, Medline, Google Scholar
23 : Characterization of depression in prodromal Huntington disease in the neurobiological predictors of HD (PREDICT-HD) study. J Psychiatr Res 2013; 47:1423–1431Crossref, Medline, Google Scholar
24 : Correlates of early disability in Huntington’s disease. Ann Neurol 1986; 20:727–731Crossref, Medline, Google Scholar
25 : Comorbidities of obsessive and compulsive symptoms in Huntington’s disease. J Nerv Ment Dis 2010; 198:334–338Crossref, Medline, Google Scholar
26 : Cognitive deficits predict poorer functional capacity in Huntington’s disease: but what is being measured? Neuropsychology 2015; 29:268–273Crossref, Medline, Google Scholar
27 : Rate of functional decline in Huntington’s disease. Neurology 2000; 54:452–458Crossref, Medline, Google Scholar
28 : The neuroanatomy of subthreshold depressive symptoms in Huntington’s disease: a combined diffusion tensor imaging (DTI) and voxel-based morphometry (VBM) study. Psychol Med 2014; 44:1867–1878Crossref, Medline, Google Scholar
29 : Neuropsychiatric symptoms in a European Huntington’s disease cohort (REGISTRY). J Neurol Neurosurg Psychiatry 2014; 85:1411–1418Crossref, Medline, Google Scholar
30 : Earliest functional declines in Huntington disease. Psychiatry Res 2010; 178:414–418Crossref, Medline, Google Scholar
31 : Suicidality in Huntington’s disease. J Affect Disord 2012; 136:550–557Crossref, Medline, Google Scholar
32 : Behavioural abnormalities contribute to functional decline in Huntington’s disease. J Neurol Neurosurg Psychiatry 2003; 74:120–122Crossref, Medline, Google Scholar
33 : Everyday functioning in Huntington’s disease: a laboratory-based study of financial management capacity. Appl Neuropsychol Adult 2017; 24:176–182Crossref, Medline, Google Scholar
34 : A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62Crossref, Medline, Google Scholar
35 : An inventory for measuring depression. Arch Gen Psychiatry 1961; 4:561–571Crossref, Medline, Google Scholar
36 : Beck Depression Inventory-II. San Antonio, Tex, Psychological Corporation, 1996Google Scholar
37 : The Brief Psychiatric Rating Scale. Psychol Rep 1962; 10:799–812Crossref, Google Scholar
38 : A clinical scale for the self-assessment of irritability. Br J Psychiatry 1978; 132:164–171Crossref, Medline, Google Scholar
39 . Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol 1986; 5:165–173Crossref, Google Scholar
40 : Behavioral changes in Huntington Disease. Neuropsychiatry Neuropsychol Behav Neurol 2001; 14:219–226Medline, Google Scholar
41 : Discriminant analysis of Beck Depression Inventory and Hamilton Rating Scale for Depression in Huntington’s disease. J Neuropsychiatry Clin Neurosci 2011; 23:399–402Link, Google Scholar
42 : Behavioural problems in Huntington’s disease using the Problem Behaviours Assessment. Gen Hosp Psychiatry 2008; 30:155–161Crossref, Medline, Google Scholar
43 : Suicidal ideation in Huntington disease: the role of comorbidity. Psychiatry Res 2011; 188:372–376Crossref, Medline, Google Scholar
44 : Psychiatric symptoms in Huntington’s disease before diagnosis: the predict-HD study. Biol Psychiatry 2007; 62:1341–1346Crossref, Medline, Google Scholar
45 : Challenges assessing clinical endpoints in early Huntington disease. Mov Disord 2010; 25:2595–2603Crossref, Medline, Google Scholar
46 : Exploring the validity of the short version of the Problem Behaviours Assessment (PBA-s) for Huntington’s disease: a Rasch analysis. J Huntingtons Dis 2015; 4:347–369Crossref, Medline, Google Scholar
47 : Pimavanserin for patients with Parkinson’s disease psychosis: a randomised, placebo-controlled phase 3 trial. Lancet 2014; 383:533–540Crossref, Medline, Google Scholar
48 : Assessment of functional capacity in neurodegenerative movement disorders: Huntington’s disease as a prototype; in Quantification of Neurologic Deficit. Edited by Munsat T. Boston, Butterworths, 1989, pp 271–283Google Scholar
49 : Predictors of phenotypic progression and disease onset in premanifest and early-stage Huntington’s disease in the TRACK-HD study: analysis of 36-month observational data. Lancet Neurol 2013; 12:637–649Crossref, Medline, Google Scholar
50 : Unified Huntington’s disease rating scale for advanced patients: validation and follow-up study. Mov Disord 2013; 28:1717–1723Crossref, Medline, Google Scholar
51 : Validation of self-report depression rating scales in Huntington’s disease. Mov Disord 2010; 25:91–96Crossref, Medline, Google Scholar
52 : Common and specific dimensions of self-reported anxiety and depression: the BDI-II versus the BDI-IA. Behav Res Ther 1999; 37:183–190Crossref, Medline, Google Scholar
53 : Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clin Psychol Rev 1988; 8:77–100Crossref, Google Scholar
54 : A comparison of self-report and caregiver assessment of depression, apathy, and irritability in Huntington’s disease. J Neuropsychiatry Clin Neurosci 2005; 17:378–383Link, Google Scholar
55 : An efficient screening tool for preoperative depression: the Geriatric Depression Scale-Short Form. Anesth Analg 2008; 106:805–809Crossref, Medline, Google Scholar
56 : Psychometric properties of the 15-item Geriatric Depression Scale in functionally impaired, cognitively intact, community-dwelling elderly primary care patients. J Am Geriatr Soc 2005; 53:1570–1576Crossref, Medline, Google Scholar
57 : Assessing frontal lobe behavioral syndromes with the frontal lobe personality scale. Assessment 1999; 6:269–284Crossref, Medline, Google Scholar
58 : Factor analysis of the frontal systems behavior scale (FrSBe). Assessment 2003; 10:79–85Crossref, Medline, Google Scholar



