Longitudinal Evaluation of Neuropsychiatric Symptoms in Huntington's Disease
Abstract
A group of 111 patients with Huntington's disease (HD) underwent a minimum of three annual neuropsychiatric assessments, using the Problem Behaviors Assessment for Huntington's Disease (PBA-HD). Longitudinal prevalence of neuropsychiatric symptoms was notably higher than baseline prevalence, suggesting that previous studies may have underestimated the extent of this clinical problem. Moreover, apathy, irritability, and depression were each associated with distinct longitudinal profiles. Apathy progressed over time and across disease stages. Irritability also increased significantly, but only in early stages of HD. Depression did not increase significantly at any stage of disease. The neuropsychiatric syndrome of apathy appears to be intrinsic to the evolution and progression of HD.
Neuropsychiatric disturbances are a common feature of Huntington's disease (HD), recognized since the earliest descriptions of this condition.1 A wide range of neuropsychiatric symptoms have been reported, including depression, anxiety, irritability, apathy, perseveration, and psychosis.2–4 Such symptoms are often a source of considerable distress to affected individuals and their families5 and have been shown to exert a greater impact on levels of functional disability6 and quality of life7 than either motor or cognitive symptoms. With recent advances in understanding the pathogenesis of HD and clinical trials of rational neuroprotective agents finally on the horizon,8 it is essential that any future treatment should have a beneficial effect on neuropsychiatric symptoms in addition to motor and cognitive aspects of the disease.
Despite their clinical importance, the natural history of neuropsychiatric symptoms and their relationship to the underlying disease-process in HD remain poorly understood. In the past, it was assumed that, unlike motor and cognitive symptoms, neuropsychiatric disturbances are “heterogeneous, episodic, and without clear temporal progression.”9 More recent studies suggest that HD is characterized by specific clusters of neuropsychiatric symptoms2,10,11 that may differ in their underlying basis. Research using the Problem Behaviors Assessment for Huntington's Disease (PBA-HD)2 has demonstrated three symptom clusters, derived from principal-component analysis, reflecting apathy, irritability, and depression, respectively.2,10 Moreover, cross-sectional analysis demonstrated that the extent of apathy was closely related to duration of illness,2 and highly correlated with motor, functional, and cognitive indices of disease severity,12 whereas irritability and depression did not have a clear relationship with these markers of disease progression.
It was proposed that the clinical syndrome of apathy is fundamental to the evolution and progression of HD, arising as a direct consequence of damage to striato-frontal circuits and closely related to the dysexecutive syndrome observed in HD.12 In contrast, irritability and depression—common, but apparently not inevitable manifestations of HD—appear to have a more complex relationship to the underlying disease-process. This supposition, however, was based on cross-sectional data, which could potentially present a misleading picture. Longitudinal data are required, not only to gain a better understanding of the natural history of neuropsychiatric symptoms, but also to allow a more accurate estimation of their prevalence over the course of the disease. This knowledge has the potential to inform selection of end-points in clinical trials of neuroprotective agents and may also provide a rationale for symptomatic treatment trials of medication for the alleviation of neuropsychiatric symptoms.
We evaluated 1) the overall prevalence of neuropsychiatric symptoms at baseline and longitudinally; 2) their pattern of progression over time; and 3) variation in longitudinal changes in relation to disease stage at initial assessment. We anticipated that longitudinal prevalence would be higher than baseline point prevalence. On the basis of previous findings, we hypothesized that apathy would show a clear progression over time. By studying depression and irritability longitudinally, we aimed to shed light on the etiology and natural history of these symptoms.
METHOD
Participants
The study included 111 patients (43 men, 68 women) with clinically-diagnosed and genetically-confirmed HD, who attended a multidisciplinary HD research clinic and had undergone a minimum of three annual neuropsychiatric assessments. At first assessment, patients' mean age was 48 years (standard deviation [SD]: 12.6), and mean duration of illness was 5.5 (4.7) years. The mean number of assessments was 5 (2.6), with a mean inter-assessment duration of 1.3 (0.9) years.
Functional Assessment
Patients' level of functional impairment was clinically assessed with the HD Functional Capacity Scale,13 which yields a Total Functional Capacity (TFC) score of 0–13, with lower scores indicating greater functional impairment. This score can be used to further classify patients into one of five disease stages: Stage I (TFC: 11–13), Stage II (TFC: 7–10), Stage III (TFC: 3–6), Stage IV (TFC: 1–2), Stage V (TFC: 0). Mean TFC score at baseline was 7.8 (3.6).
Neuropsychiatric Assessment
The Problem Behaviors Assessment for Huntington's disease (PBA-HD)2 is a semistructured interview specifically developed to address the range of neuropsychiatric symptoms reported in HD. Individual items are rated for severity and frequency on a 5-point scale (0–4) according to detailed scoring guidelines, and a “PBA score” is generated for each symptom by multiplying the severity and frequency scores. The PBA-HD includes three subscales, which are calculated by taking the mean PBA score of the constituent items. The Apathy subscale comprises reduced activity and initiative, poor perseverance and quality of work, impaired judgment, personal neglect, and blunting of affect. The Irritability subscale includes poor temper control, verbal and physical aggression, behavioral inflexibility, and perseverative preoccupations. The Depression subscale consists of depressed mood, anxiety, depressive cognition, and suicidal ideation. The instrument has been shown to have good interrater reliability,2 and the Apathy and Depression subscales show significant correlation with the Marin Apathy Evaluation Scale and the Hamilton Rating Scale for Depression, respectively,14 supporting the validity of the instrument. Interviews were carried out by experienced raters. Patients were interviewed together with a companion (normally a partner or close relative), who was also interviewed separately; this was considered essential in view of the potential discrepancy between patient and companion report.15 Indeed, some symptoms, such as feelings of depression or suicidal ideation, may be more accurately reported by patients; whereas others, such as aggressive or demanding behavior, may be more objectively reported by companions. Furthermore, patients may lack insight into certain symptoms, such as blunting of affect or impaired judgment. For each symptom included in the PBA-HD, interviewers were required to make an overall judgment on the basis of information provided by the patient and caregiver, together with their own clinical judgment regarding the behavior and mental state of the patient.
Statistical Analysis
To assess the overall prevalence of neuropsychiatric symptoms at baseline and across the entire longitudinal follow-up period, the percentage of patients who endorsed individual symptoms 1) at baseline; and 2) at any assessment during the study period was calculated. Symptom presence was operationally defined as a PBA-HD Severity score of ≥2.
Longitudinal analysis of the three PBA-HD subscales and their constituent items were carried out with mixed-effect regression models. Mixed-effect regression models offer a number of advantages for modeling change over time in clinical populations. The inclusion of random subject effects accounts for the within-individual correlation of repeated observations. Furthermore, subjects are not assumed to have the same number of observations, or to have been assessed at the same time-points or intervals. Regression models were set up according to the method described by Hedeker and Gibbons,16 with PBA subscale score as the dependent variable, fixed and random slope and intercept effects for time (in years, from baseline assessment), and baseline stage of disease as a fixed covariate.
In order to characterize potential variation in longitudinal changes across different stages of disease, the longitudinal-analysis “window” was restricted to progression through two disease stages only: Stage I–II, Stage II–III, and so on. Thus, if a participant was at Stage II at baseline assessment and was followed up all the way through to disease Stage V, data-points from Stages II and III-only were included in the analysis. Separate mixed-effect regression models were carried out for each baseline disease-stage group, with PBA subscale score as the dependent variable and both fixed and random effects for time.
RESULTS
Prevalence of Individual Neuropsychiatric Symptoms Over the Follow-Up Period
The percentage of patients endorsing individual symptoms 1) at baseline; and 2) at any assessment, is shown in Figure 1. A consistent finding was that longitudinal prevalence was higher than baseline prevalence. Neuropsychiatric symptoms on the Apathy subscale were the most common, with longitudinal prevalence of individual symptoms varying between 84% and 99%. Irritability symptoms were also common, with overall prevalence rates ranging from 49% to 83%. Least prevalent were Depression symptoms, with longitudinal prevalence rates from 18% to 71%.
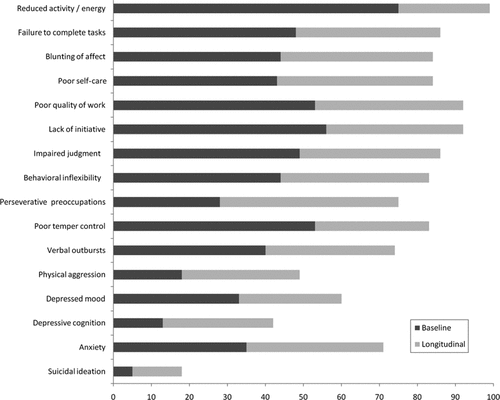
Longitudinal Analysis of PBA Subscales and Constituent Symptoms
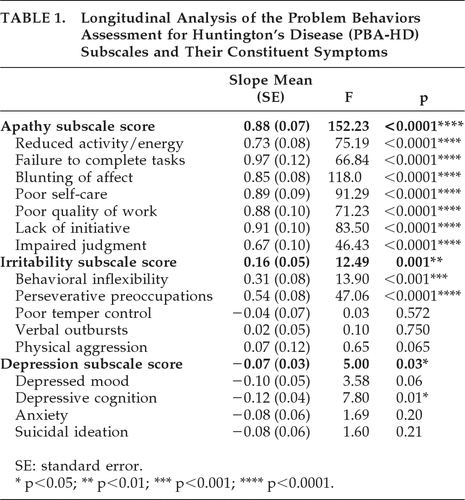
Table 1 shows estimated slope effects, F values, and statistical significance for each of the PBA-HD subscales and constituent symptoms. The F values and related statistical significance levels are a measure of linearity of the regression model, with higher values of F denoting a greater linear trend. The Apathy subscale and all seven constituent symptoms showed highly significant linear effects. The Irritability subscale also showed a significant linear effect, as did two of five constituent symptoms: behavioral inflexibility and perseverative preoccupations. The Depression subscale showed a marginally-significant longitudinal change, but this was significant in only one of four constituent symptoms: depressive cognition. Interestingly, however, whereas the regression slopes for the Apathy and Irritability symptoms showed increased severity over time (with the exception of poor temper control), the slopes for the Depression subscale and constituent symptoms had a negative value, indicating a reduction over time.
 |
Longitudinal Analysis of PBA Subscales Across Disease Stages
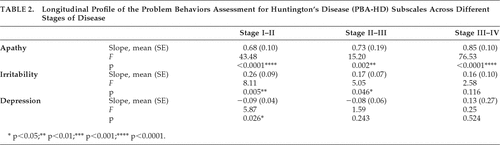
Restriction of regression analysis to a “window” of two disease stages and stratification of the group by disease stage at study entry yielded N of the following patient groups: Stage I: 26; Stage II: 21; Stage III: 39; Stage IV: 9 (not all patients had at least three suitable data-points within a two-stage window). Given the small number of participants entering the study at Stage IV, this group was excluded from subsequent analysis.
Estimated slope effects, F values, and their related statistical significance for the three PBA-HD subscales, stratified by disease stage at entry, are shown in Table 2. For the Apathy subscale, significant linear effects were consistently observed, irrespective of disease stage at study entry. For the Irritability subscale, there was a significant linear effect in the Stage I–II group, a marginally-significant linear effect in the Stage II–III group, and no effect in the Stage III–IV group. For the Depression subscale, there was a marginally significant linear effect for participants entering the study at Stage I; however, this was associated with a negative regression slope, indicating a reduction over time.
 |
DISCUSSION
The prevalence of neuropsychiatric symptoms was notably higher when consecutive longitudinal assessments were taken into account, as compared with a single baseline assessment. This is an important observation because it suggests that previous cross-sectional studies may have underestimated the prevalence of neuropsychiatric symptoms in HD. Overall, neuropsychiatric symptoms were extremely common in our sample, with some symptoms occurring in 99% of patients and no single patient remaining completely free from such symptoms over the follow-up period.
In keeping with our hypothesis, scores on the Apathy subscale and its constituent symptoms showed a progressive worsening over time, a pattern that was evident across all stages of disease. This reinforces our previous cross-sectional findings that apathy was closely related to duration of illness2 and highly correlated with cognitive, motor, and functional indices of disease severity.12 Our findings support and extend other cross-sectional observations of greater apathy with increased disease severity.10–14 Notably, we found apathy symptoms to be present, at least to a mild degree, in almost all patients studied. The inference is that apathy is intrinsic to the evolution and progression of HD, showing a progressive worsening over the course of the disease.
It is useful to view our findings in relation to models of the neural substrate of goal-directed behavior. Levy and Dubois17 define apathy as “a quantitative reduction in self-generated voluntary and purposeful behaviors” and outline three subtypes: “Emotional-Affective,” “Cognitive,” and “Auto-Activation,” each of which are associated with damage to different parts of the prefrontal cortex (PFC) and basal-ganglia circuits. Within this framework, Emotional-Affective apathy gives rise to emotional blunting and loss of interest in previously-motivating activities or stimuli; it is associated with damage to the orbitofrontal PFC and ventral striatum. Cognitive apathy reflects impaired frontal-executive functioning, giving rise to poor planning, organization, and set-shifting, and is characteristic of damage to the lateral PFC and dorsal caudate. Auto-Activation apathy refers to a reduction in self-generated, spontaneous action and thought, and is associated primarily with damage to the dorsomedial PFC and internal segment of the globus pallidus. In HD, the primary site of pathology is thought to be the striatum,18 which is richly connected to the PFC via a series of parallel cortico-striatal circuits.19 According to the framework proposed by Levy and Dubois, apathy occurs after basal-ganglia damage because of disruption of the basal-ganglia–PFC circuit, resulting in reductions in both overall activation and temporo-spatial focalization in activation of the PFC. The PBA-HD Apathy subscale used in the present study contained items pertaining to each of the proposed apathy subtypes: emotional blunting (Emotional-Affective), impaired judgment and reduced quality of work (Cognitive), and reduced activity and initiative (Auto-Activation). Progressive and marked impairments were present in all three domains, indicating a fundamental and pervasive deficit of apathy in HD, secondary to striatal-frontal dysfunction.
In keeping with previous cross-sectional studies,2,3 irritability was very common, with over 80% of patients showing poor temper control and almost 50% reporting some level of physical aggression during the follow-up period. Longitudinal analysis revealed an overall increase in irritability with time. However, this increase did not occur across all stages of disease. Rather, scores on the PBA-HD Irritability subscale showed a significant increase among patients entering the study at Stage I, and a marginally significant linear trend in patients entering the study at Stage II, suggesting that irritability develops in the early stages of HD, reaching a peak (or plateau) by Stage III. Interestingly, this observation is consistent with the finding of a longitudinal increase in irritability over a 4-year period in pre-manifest HD gene-carriers, as compared with non-carriers.20
The most commonly reported irritability symptoms were poor temper control, verbal outbursts, and behavioral inflexibility. It is reasonable to relate these symptoms to the established deficits in frontal-executive functioning in HD. Poor response-inhibition21 may contribute directly to poor temper control and verbal/physical aggression. Reduced cognitive flexibility22 may result in patients' becoming increasingly inflexible behaviorally. It is interesting, then, that a longitudinal progression of such symptoms could only be detected in the early stages of HD. One possibility is that irritability is partially controlled by psychiatric medication. Indeed, the study patients were attending a multidisciplinary research and clinical-management clinic, with psychiatric input, and may, therefore, have been more likely to be prescribed psychiatric medication than HD patients in other settings. Another possibility is that there is an interaction between irritability and apathy; that is, irritability and aggression may plateau as apathy and abulia increase.
Depressive symptoms were common, particularly when longitudinal rather than baseline prevalence was examined, with 60% of patients experiencing low mood and 71% experiencing anxiety over the course of the study. However, in contrast to the Apathy and Irritability subscales, scores on the PBA-HD Depression subscale did not show any significant increase over time. Rather, there was a significant reduction in depressive symptomatology in patients entering the study at Stage I and a nonsignificant decline in those entering the study at Stage II. It is necessary to exercise caution in interpreting this observation since, in common with irritability, it is likely that depressive symptoms were at least partially alleviated by antidepressant medication. Nevertheless, the notion that depression is more common in the mild-to-moderate stages of disease is in keeping with the findings of Paulsen et al.,23 who reported the highest rate of depression in HD patients who were in Stage II of disease. Others,23 as well as ourselves,5,12 have previously suggested that rates of depression and anxiety may subside in later stages of disease as emotional blunting increases and insight lessens.
The etiology of depression in HD is intriguing. It would be reasonable to attribute depression to reactive factors in individuals affected by a progressive and fatal neurodegenerative disease that has likely already claimed a parent. However, the observation of higher rates of depression in double-blind studies of at-risk gene-carriers, as compared with non-carriers,24 suggests that HD increases susceptibility to depression, even in the pre-manifest phase of disease. Indeed, greater frontal hypometabolism has been observed in depressed, versus non-depressed HD patients.25,26 It is interesting, then, that rates of depressive symptoms, although common, were not the ubiquitous sign that apathy appears to be, nor as common as irritability and related symptoms. Our data would seem to suggest that not all patients with HD experience low mood. Further research is required to attempt to validate this finding and to illuminate factors that influence depression in HD, be they genetic, organic, or environmental.
Longitudinal studies of HD have generally focused on motor and cognitive aspects of the condition, with very few addressing specific neuropsychiatric changes. One study reported no significant change on the UHDRS Behavioral Scale over a 1-year interval.27 A more recent publication of the 12-month longitudinal analysis from the TRACK-HD Study28 failed to find a significant longitudinal change in apathy, irritability or affect in early HD by use of a shortened form of the PBA-HD. The apparent discrepancy between the present study and previous reports is likely to reflect the longer follow-up period, our use of a more detailed neuropsychiatric interview, and the separation of symptoms into clinically-relevant clusters.
The finding of distinct longitudinal profiles in the three PBA-HD subtypes supports the separation of neuropsychiatric symptoms in HD into distinct clusters. We examined three clusters, based on our previous principal-components analysis of the PBA-HD.2 A recent study of 1,690 HD patients enrolled in an international multicenter study29 distinguished four symptom-clusters by principal-components analysis, three of which were similar to the PBA-HD subscales (Depression, Drive/Executive Function, Irritability/Aggression) and a fourth, “Psychosis” cluster, which included hallucinations and delusions.11 Hallucinations and delusions were rare in the present study, occurring in only 3% and 10% of patients, respectively, across the entire study period. However, it would be useful to further characterize psychotic symptoms in HD in a larger sample than in the present study, in terms of their overall prevalence, association with disease stage, and possible risk factors.
It is important to address potential limitations in the study. In the PBA-HD, the rater is required to score symptoms over the preceding 4-week period. The rationale for this is to increase accuracy in both patients' recall of feelings and events and the reliability of interviewers' rating of symptoms. We acknowledge, however, that this method has the potential to miss symptoms that have occurred between assessments but outside the 4-week period before the interview. A recently revised version of the PBA-HD overcomes this issue by documenting 1) the severity and frequency of symptoms within the preceding 4-week period; and 2) the severity at its worst since the last assessment. Nevertheless, the longitudinal prevalence of neuropsychiatric symptoms was consistently higher than baseline-point prevalence, suggesting that our data provide a more accurate picture of neuropsychiatric problems in HD than previous, cross-sectional reports. The issue of psychiatric medication also warrants consideration. As previously noted, the patients were attending a multidisciplinary clinic with specialist psychiatric input, where the instigation of psychiatric medication, when indicated, was routine. It is, therefore, likely that medication could have influenced the severity of affective symptoms such as depression and irritability. We cannot say for certain whether the overall pattern of symptom prevalence and progression would be the same in an entirely unmedicated cohort, nor would such a study be ethically defensible. Despite the high prevalence of neuropsychiatric symptoms in HD, there have been very few symptom-treatment trials, with most reports in the literature being limited to single cases or small case-series.30 Our findings highlight the need for symptomatic trials of psychiatric medication in HD in order to provide an evidence-base for the potential alleviation of distressing and disruptive symptoms in this condition.
To our knowledge, this observation represents the most comprehensive and lengthy longitudinal study of neuropsychiatric symptoms in HD. We have demonstrated that the longitudinal prevalence of neuropsychiatric symptoms is markedly higher than shown in cross-sectional studies of prevalence, suggesting that previous studies may have underestimated the extent of this clinical problem. The three main neuropsychiatric syndromes in HD—namely, apathy, irritability, and depression—were each associated with a distinct longitudinal profile, suggesting a different neurobiological substrate for each. Our data strongly support the notion that apathy, as a neuropsychiatric syndrome, is intrinsic to the evolution and progression of HD. Moreover, the PBA-HD Apathy subscale provides a sensitive measure of apathy that has the potential to be used as an end-point in clinical trials.
1. : On chorea. The Medical and Surgical Reporter 1872; 26:317–321Google Scholar
2. : Behavioural changes in Huntington's disease. Neuropsychiatry Neuropsychol Behav Neurol 2001; 14:219–226Medline, Google Scholar
3. : Neuropsychiatric aspects of Huntington's disease. J Neurol Neurosurg Psychiatry 2001; 71:310–314Crossref, Medline, Google Scholar
4. : Psychopathology in verified Huntington's disease gene-carriers. J Neuropsychiatry Clin Neurosci 2007; 19:441–448Link, Google Scholar
5. . Neuropsychological and neuropsychiatric aspects of Huntington's disease, in Huntington's Disease. 3rd Edition. Edited by Harper PBates GJones L. Oxford, England, Oxford University Press, 2002, pp 62–94Google Scholar
6. : Behavioural abnormalities contribute to functional decline in Huntington's disease. J Neurol Neurosurg Psychiatry 2003; 74:120–122Crossref, Medline, Google Scholar
7. : Health-related quality of life in Huntington's disease: which factors matter most? Mov Disord 2009; 24:574–578Crossref, Medline, Google Scholar
8. : Huntington's disease: from molecular pathogenesis to clinical treatment. Lancet Neurol 2011; 10:83–98Crossref, Medline, Google Scholar
9.
10. : Behavioural problems in Huntington's disease using the Problem Behaviors Assessment. Gen Hosp Psychiatry 2008; 30:155–161Crossref, Medline, Google Scholar
11. . Factor analysis of behavioural symptoms in Huntington's disease. J Neurol Neurosurg Psychiatry 2011; 82:411–412Crossref, Medline, Google Scholar
12. : Behavior in Huntington's disease: dissociating cognition-based and mood-based changes. J Neuropsychiatry Clin Neurosci 2002; 14:37–43Link, Google Scholar
13. : Huntington disease: clinical care and evaluation. Neurology 1979; 29:1–3Crossref, Medline, Google Scholar
14. : Apathy is not depression in Huntington's disease. J Neuropsychiatry Clin Neurosci 2009; 21:266–270Link, Google Scholar
15. : A comparison of self-report and caregiver assessment of depression, apathy, and irritability in Huntington's disease. J Neuropsychiatry Clin Neurosci 2005; 17:378–383Link, Google Scholar
16. : Mixed-effects regression models for continuous outcomes, in Longitudinal Data Analysis for Biomedical and Behavioral Sciences. Hoboken, NJ, Wiley, 2006, pp 47–80Google Scholar
17. : Apathy and the functional anatomy of the prefrontal cortex–basal ganglia circuits. Cereb Cortex 2006; 16:916–928Crossref, Medline, Google Scholar
18. : Neuropathological classification of Huntington's disease. J Neuropathol Exp Neurol 1985; 44:559–577Crossref, Medline, Google Scholar
19. : Basal ganglia–thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal,” and “limbic” functions. Prog Brain Res 1990; 85:119–146Crossref, Medline, Google Scholar
20. : Longitudinal personality changes among pre-symptomatic Huntington disease gene-carriers. Neuropsychiatry Neuropsychol Behav Neurol 2002; 15:192–197Medline, Google Scholar
21. : Inhibition of subliminally-primed responses is mediated by the caudate and thalamus: evidence from functional MRI and Huntington's disease. Brain 2003; 126(Pt 3):713–723Crossref, Medline, Google Scholar
22. : Executive and mnemonic functions in early Huntington's disease. Brain 1996; 119( Pt 5):1633–1645Crossref, Medline, Google Scholar
23. : Depression and stages of Huntington's disease. J Neuropsychiatry Clin Neurosci 2005; 17:496–502Link, Google Scholar
24. : Psychiatric disorders in preclinical Huntington's disease. J Neurol Neurosurg Psychiatry 2007; 78:939–943Crossref, Medline, Google Scholar
25. : Cerebral glucose consumption measured by PET in patients with and without psychiatric symptoms of Huntington's disease. Psychiatry Res 1989; 29:361–362Crossref, Medline, Google Scholar
26. : Paralimbic frontal lobe hypometabolism in depression associated with Huntington's disease. Neurology 1992; 42:1791–177Crossref, Medline, Google Scholar
27. : Unified Huntington's Disease Rating Scale: a follow-up. Mov Disord 1998; 13:915–919Crossref, Medline, Google Scholar
28. : Biological and clinical changes in pre-manifest and early-stage Huntington's disease in the Track-HD Study: the 12-month longitudinal analysis. Lancet Neurol 2011; 10:31–42Crossref, Medline, Google Scholar
29. . Observing Huntington's disease: the European Huntington's Disease Network's REGISTRY. PLoS Currents: Huntington's Disease 2010; Sept. 28: PMC2947793Google Scholar
30. : An overview of psychiatric symptoms in Huntington's disease. Curr Psychiatry Rep 2001; 3:379–388Crossref, Medline, Google Scholar



