Apathy Is Not Depression in Huntington’s Disease
METHODS
Participants and Procedures
All participants were still living at home and visiting the outpatient clinic of the department of neurology of the Radboud University Nijmegen Medical Centre, The Netherlands. They were assessed by a multidisciplinary team specializing in Huntington’s disease. Consecutive patients were asked to give their informed consent to participate in a study on behavioral and mood disturbances in Huntington’s disease. All participants had received a genetically confirmed diagnosis of Huntington’s disease, with available data on CAG repeat length, and all showed at least some specific mild motor signs of the illness. We recorded both demographic and medication data as provided by the patients themselves and their caregivers. Neurological signs and symptoms were assessed using the Unified Huntington’s Disease Rating Scale (UHDRS). 5 Cognitive function was assessed with the Mini Mental State Examination. We assessed activity of daily living (ADL) and instrumental ADL functioning to estimate disease severity using Shoulson and Fahn’s total functional capacity scale, also part of the UHDRS. 6
Psychiatric Evaluation
All participants were interviewed in the afternoon to avoid influence of diurnal variation. Psychiatric interviewing was guided by a semistructured interview using the Comprehensive Psychopathology Rating Scale (CPRS). 7 Syndromal depression was diagnosed according to the DSM-IV criteria with help of the Mini-International Neuropsychiatric Interview (MINI). In addition, we calculated total numbers for DSM-IV depressive symptoms. Severity of depressive symptoms was measured with the Hamilton Rating Scale for Depression (HAM-D). A DSM-IV diagnosis of major depression in combination with a score on the HAM-D above 18 was classified as severe depression. Apathy was separately rated in a dimensional way with the Marin Apathy Evaluation Scale (AES). 8 The total score on the AES and also the scores on apathy subsyndromes were determined. Following Marin, we distinguished three subcomponents of apathy: the cognitive, motor, and emotional components. 9 A score on the AES of 40 or higher was considered severe apathy symptomatology. 8
Finally, we presented the Problem Behavior Assessment for Huntington’s disease (PBA-HD) to a close relative or caregiver. 3 The PBA-HD is an instrument for (dimensionally) rating the presence, severity, and frequency of behavioral abnormalities in Huntington’s disease patients. Items can be classified in three factors of (problem) behavior: apathy, irritability, and depression. 3 Like Craufurd et al. 3 we counted symptoms as present if the severity rating was 2 or higher. For this study, we were only interested in the apathy and depression factors.
Statistical Analysis
For statistical analysis, we used the SPSS for Windows, version 12.0. Pearson correlation coefficients were calculated to assess associations between the various scales.
RESULTS
General Characteristics of the Study Group
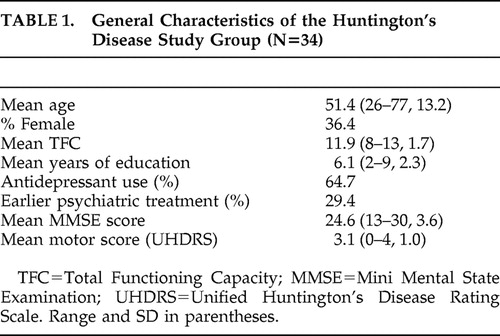
Table 1 summarizes the general characteristics of the study group. In the study group of 34 participants, 10 fulfilled the criteria for major depressive disorder (29%). Table 2 shows the results of the various depression, apathy, and behavioral assessments. Depressed patients showed no difference in age, sex, mean total functional capacity, or mean MMSE score compared to those without depression. There was no difference in use of antidepressants between depressed and nondepressed patients. As expected, depressed patients did differ in number of depressive symptoms and mean HAM-D score from the nondepressed patients, but there was no difference in AES score between these groups. Severe depression (HAM-D score >18) was present in four patients (12%) and severe apathy (AES >40) in 18 patients (52%). Only two of four patients with severe depressive symptomatology also showed severe apathy. Of the 18 patients with severe apathy, only two also showed severe depressive symptomatology.
 |
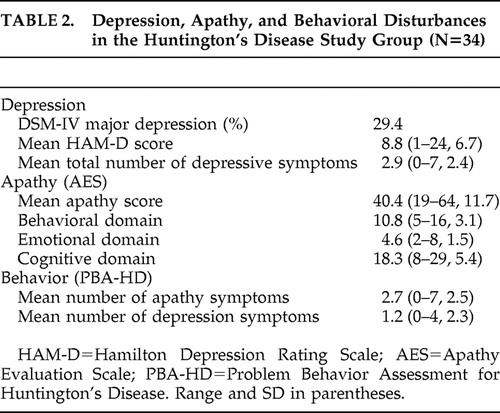 |
The most prominent symptom reported with the PBA-HD was loss of energy (55%). Depressed mood was reported on this instrument in 43% of participants. Clustering of symptoms as proposed by Craufurd et al. 3 resulted in a depression syndrome in 13 patients (39%) and an apathy syndrome in 18 patients (52%).
Coherence of Various Approaches to Depression and Apathy
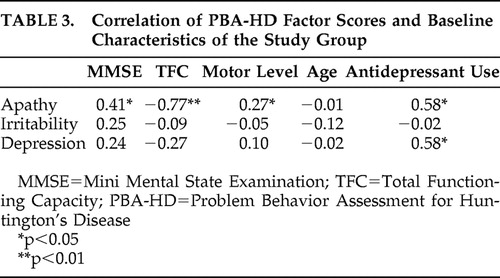
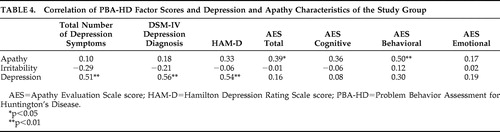
Table 3 shows the correlation between the factor scores on the PBA-HD and various baseline characteristics of the study group, and Table 4 shows the correlation of these factors with various scores on the other instruments. The depression factor of the Problem Behavior Assessment showed a significant correlation with the HAM-D score, DSM-IV diagnosis of depression, and total number of depression symptoms. There was no correlation with any of the apathy subscales, with any illness characteristic of Huntington’s disease, or with age. The apathy factor of the Problem Behavior Assessment correlated significantly with the score on the AES and, more specifically, with the behavioral subscale of the AES, with the motor level of Huntington’s disease and also with the MMSE and the total functional capacity. Both the apathy and depression factors of the Problem Behavior Assessment correlated with the use of antidepressant medication.
 |
 |
DISCUSSION
This study is one of the few focusing on the relationship between depression and apathy in Huntington’s disease. The main finding is that apathy is far more common than depression in Huntington’s disease. Moreover, apathy and depression are not related but are largely distinct dimensions, and we found that apathy was linked to other clinical characteristics such as cognitive deterioration and functional decline, whereas depression was not. In other words, apathy was more frequent in more advanced stages of Huntington’s disease. The fact that depression is an early event in Huntington’s disease, and the possibility that older age and cognitive deficits are related to apathy, may explain the dissociation between apathy and depression. In our study there was no correlation between apathy and age.
Our findings are supported by the results of other studies. Hamilton et al. 10 found that a composite apathy/executive dysfunction behavioral index strongly correlated with decline in activity of daily living. Another study on apathy and depression in Huntington’s disease also showed an association of the presence of apathy with frontal, cognitive measures, with no such association for depression. 2 Although this study used another measure for apathy that makes comparisons difficult, the major findings of it are in accordance with ours. Our findings concur with those of Thompson et al. 11 Our PBA-HD results also confirmed the findings of the Craufurd et al. 3 study regarding the different domains of problem behavior.
Although we included participants with early stages of Huntington’s disease and only mild functional disability, the number of patients with apathy was quite high in our study. This was in line with the study of Baudic et al., 2 who studied a similar group of subjects and underlined the fact that apathy is a major problem even in early and middle stages of Huntington’s disease and that it co-occurs with cognitive decline as measured with the Mattis Dementia Rating Scale. The mean AES score in our group was 40.3, and more than half of our subjects scored over 40 on the AES. Although there are no reliable AES cutoff scores that separate apathetic from nonapathetic subjects, earlier reports suggested “normal” scores between 25–35. In his original publications Marin described “healthy” elderly having a mean AES score of 26 and “severely depressed subjects” having a mean score of 40.5. 8 Our participants showed only minimal to moderate levels of depression, but nonetheless could be considered to show symptoms of severe apathy.
Marin 4 and others have also observed dissociation between apathy and depression 12 in several groups of patients with other forms of cerebral pathology. Apathy is often observed in combination with advanced cerebral disease and cognitive dysfunction and may interfere with successful rehabilitation (e.g., in patients with traumatic brain injury). 13 In one study on apathy in various neurological disorders that included a group of 35 Huntington’s disease patients, Levy et al. 12 concluded that there was no clear correlation between apathy and depression measurements. As in our study, they found apathy to be highly prevalent in both the Huntington’s disease group and in other groups of subjects with various other cerebral diseases. Apathy was detected in 59% of Huntington’s disease patients (20/34), in 33% of patients with Parkinson’s disease (13/40), in 80% of patients with Alzheimer’s disease (24/30), and in 91% of progressive supranuclear palsy patients (20/22). They also found a correlation between the severity of apathy and the degree of cognitive decline in all of these diseases.
Both in patients with Alzheimer’s disease and in patients with vascular dementia, we found in previous studies that more advanced dementia was associated with the apathetic aspects of depression rather than the mood-related aspects. 14 , 15 Furthermore we found that these depressed patients with a symptom profile with predominant apathetic features also showed specific neuropsychological deficits, with a negative correlation between these symptoms and the results on frontal tasks. 16 , 17
Only a few studies observed a major overlap between depression and apathy in patients with neurological cerebral disease. Starkstein 18 assessed subjects with Alzheimer’s disease with the AES and found apathy in 37%, with 64% of these subjects also fulfilling criteria for depression. A similar assessment of depressed elderly subjects without Alzheimer’s disease showed that 32% of these also fulfilled criteria for apathy. They found a correlation between the severity of apathy and cognitive deterioration in the Alzheimer’s disease group. Kant et al. 19 studied apathy in subjects with personality changes and mood disorder after closed head injury. They found that apathy and depression occurred separately in about 10% of subjects and occurred in combination in 60%.
Contradictory findings regarding the relationship between apathy and depression are probably due to methodological issues. These include the clinical stage of Huntington’s disease, the different assessment methods used in diagnostic practice, and the lack of a “golden standard” definition of both apathy and depression.
One of the strengths of our study is that the broad and comprehensive psychiatric evaluation of the patients was not restricted to DSM-IV diagnosis, but was directed at a broader range of psychiatric and behavioral dimensions. Other limitations may be the selection of patients with early- to middle-stage Huntington’s disease and the fact that we could not control for age and use of medication. Nevertheless, our study contributes to the construct validity of the concept of apathy in Huntington’s disease; ratings of apathy with the Problem Behavior Assessment do correlate with those of the Apathy Evaluation Scale (convergent validity), while ratings of depression on the Problem Behavior Assessment do not (divergent validity).
In conclusion, our results indicate that apathy and depression are separable and independent behavioral dimensions in Huntington’s disease, with apathy in Huntington’s disease related to neurodegeneration and connected to cognitive dysfunction and functional decline. It is a prominent feature of early- and middle-stage Huntington’s disease and, due to its major effect on daily functioning and quality of life, could be an important target for therapeutic interventions in the near future. Based on current knowledge, depression in the early stages of Huntington’s disease should be treated like other forms of depression, with selective serotonin inhibitors as a first choice and subsequent steps based on the treatment guidelines. When apathy becomes a dominant feature, success of conventional antidepressant medication may be limited. 20 One conspicuous finding of our study was that many of the participants, whether depressed or not or apathetic or not, received antidepressants. In the future, other options such as dopaminergic antidepressants or psychostimulants as a specific medication targeted at apathy should be studied.
1. Naarding P, Kremer HP, Zitman FG: Huntington’s disease: a review of the literature on prevalence and treatment of neuropsychiatric phenomena. Eur Psychiatry 2001; 16:439–445Google Scholar
2. Baudic S, Maison P, Dolbeau G, et al: Cognitive impairment related to apathy in early Huntington’s disease. Dement Geriatr Cogn Disord 2006; 21:316–321Google Scholar
3. Craufurd D, Thompson JC, Snowden JS: Behavioral changes in Huntington disease. Neuropsychiatry Neuropsychol Behav Neurol 2001; 14:219–226Google Scholar
4. Marin RS, Firinciogullari S, Biedrzycki RC: The sources of convergence between measures of apathy and depression. J Affect Disord 1993; 28:7–14Google Scholar
5. The Huntington Study Group: Unified Huntington’s Disease Rating Scale: reliability and consistency. Mov Disord 1996; 11:136–142Google Scholar
6. Marder K, Zhao H, Myers RH, et al: Rate of functional decline in Huntington’s disease. Huntington Study Group. Neurology 2000; 54:452–458Google Scholar
7. Asberg M, Schalling D: Construction of a new psychiatric rating instrument, the Comprehensive Psychopathological Rating Scale (CPRS). Prog Neuropsychopharmacol 1979; 3:405–412Google Scholar
8. Marin RS, Biedrzycki RC, Firinciogullari S: Reliability and validity of the Apathy Evaluation Scale. Psychiatry Res 1991; 38:143–162Google Scholar
9. Marin RS: Apathy: concept, syndrome, neural mechanisms, and treatment. Semin Clin Neuropsychiatry 1996; 1:304–314Google Scholar
10. Hamilton JM, Salmon DP, Corey-Bloom J, et al: Behavioral abnormalities contribute to functional decline in Huntington’s disease. J Neurol Neurosurg Psychiatry 2003; 74:120–122Google Scholar
11. Thompson JC, Snowden JS, Craufurd D, et al: Behavior in Huntington’s disease: dissociating cognition-based and mood-based changes. J Neuropsychiatry Clin Neurosci 2002; 14:37–43Google Scholar
12. Levy ML, Cummings JL, Fairbanks LA, et al: Apathy is not depression. J Neuropsychiatry Clin Neurosci 1998; 10:314–319Google Scholar
13. Duffy J: Apathy in neurologic disorders. Curr Psychiatry Rep 2000; 2:434–439Google Scholar
14. Janzing JG, Hooijer C, van’t Hof MA, et al: Depression in subjects with and without dementia: a comparison using GMS-AGECAT. Int J Geriatr Psychiatry 2002; 17:1–5Google Scholar
15. Naarding P, de Koning I, van Kooten F, et al: Depression in vascular depression. Int J Geriatr Psychiatry 2003; 18:325–330Google Scholar
16. Janzing JGE, Naarding P, Eling PA: Depressive symptom quality and neuropsychological performance in dementia. Int J Geriatr Psychiatry 2005; 20:479–484Google Scholar
17. Naarding P, de Koning I, van Kooten F, et al: Poststroke dementia and depression: frontosubcortical dysfunction as missing link? Int J Geriatr Psychiatry 2007; 22:1–8Google Scholar
18. Starkstein SE, Petracca G, Chemerinski E, et al: Syndromic validity of apathy in Alzheimer’s disease. Am J Psychiatry 2001; 158:872–877Google Scholar
19. Kant R, Duffy JD, Pivovarnik A: Prevalence of apathy following head injury. Brain Inj 1998; 12:87–92Google Scholar
20. Alexopoulos GS, Meyers BS, Young RC, et al: Executive dysfunction and long-term outcomes of geriatric depression. Arch Gen Psychiatry 2000; 57:285–290Google Scholar



