Frequency and Pathophysiology of Apathy in Huntington Disease: A Systematic Review and Meta-Analysis
Abstract
Objective:
Apathy is a common behavioral symptom of Huntington disease (HD). This systematic review describes current evidence on the pathophysiology, assessment, and frequency of apathy in HD.
Methods:
This systematic review was conducted in accordance with PRISMA guidelines. Using a comprehensive search strategy, the investigators searched the MEDLINE, Embase, and PsycINFO databases. All studies that evaluated apathy in HD patients with a valid scale and reported apathy frequency or scores were included. Apathy scores were analyzed by mean or standardized mean differences in accordance with Cochrane guidelines.
Results:
A total of 1,085 records were screened and 80 studies were ultimately included. The Problem Behaviors Assessment—Short was the most frequently used apathy assessment tool. Apathy frequency generally ranged from 10%–33% in premanifest HD to 24%–76% in manifest HD. A meta-analysis of 5,311 records of patients with premanifest HD showed significantly higher apathy scores, with a standardized mean difference of 0.41 (CI=0.29–0.52; p<0.001). A comparison of 1,247 patients showed significantly higher apathy scores in manifest than premanifest HD, with a mean difference of 1.87 (CI=1.48–2.26; p<0.001). There was evidence of involvement of various cortical and subcortical brain regions in HD patients with apathy.
Conclusions:
Apathy was more frequent among individuals with premanifest HD compared with those in a control group and among individuals with manifest HD compared with those with premanifest HD. Considering the complexity and unique pattern of development in neurodegenerative disease, further studies are required to explore the pathophysiology of apathy in HD.
Apathy is a common behavioral symptom of neurodegenerative diseases. The term is derived from the Greek word “apatheia,” meaning without passion. In the 19th century, apathy entered the medical lexicon to describe indifference or inability to feel emotions (1). Other definitions such as loss of motivation, reduced goal-directed behavior, or a decrease in self-initiation of actions were proposed and subsequently added to the definition (2). The concept of apathy as a neuropsychiatric syndrome was first proposed by Marin in 1990 in an attempt to differentiate apathy from other clinical disorders. He suggested defining apathy as a separate clinical condition marked by “diminished motivation not attributable to the level of consciousness, cognitive impairment, or emotional stress” (3). In 1998, Levy et al. (4) showed that apathy did not necessarily correlate with depression and can be a separate clinical entity across different dementia groups. In the 2000s, research groups attempted to establish a consensus definition of apathy. Starkstein et al. (5) proposed one of the first diagnostic criteria for apathy, which was later revised by Robert et al. (6, 7).
Apathy is one of the most common neurobehavioral symptoms of Huntington disease (HD), which is an inherited neurodegenerative disease characterized by progressive movement disorders, cognitive impairment, and behavioral changes (8). HD is caused by an expanded cytosine-adenine-guanine trinucleotide repeat in the huntingtin gene (HTT) located on chromosome 4 (9). A recent analysis of the Enroll-HD database found apathy to be the most influential factor in working capacity among those with premanifest HD (10). Because patients with HD can be unaware of their symptoms (anosognosia), apathy may be underreported yet impose a great burden on caregivers (11, 12). Moreover, apathy has been strongly associated with the progression and prediction of functional decline in those with HD (13). Despite being a common and debilitating symptom, the pathophysiology of apathy is still not fully understood, and to date, no medication has been approved for this condition (14).
In this systematic review and meta-analysis, we aimed to provide a broad perspective on the available evidence regarding apathy in HD, including its frequency in patients with manifest or premanifest HD, its pathophysiology, the clinical instruments used for its assessment, and its association with other clinical domains of HD.
Methods
Design
This systematic review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines (15). A protocol of the study was registered in the international prospective register of systematic reviews (PROSPERO; CRD42022296392).
Search Strategy and Information Sources
A search strategy was developed using the keywords “Huntington disease” and “apathy” and related keywords such as “motivation,” “interest,” “self-activation,” “psychic akinesia,” “athymia,” and “abulia.” A detailed search strategy can be found in appendix 1 of the online supplement to this article. The MEDLINE (via PubMed and OVID), Embase, and PsycINFO databases were searched in November 2021. To include all relevant data, we placed no limitations on the search, including date or language.
Eligibility Criteria and Selection Process
Titles and abstracts were screened by independent reviewers (S.A.Z., H.M.C., and K.S.R.), and the full texts of relevant articles were reviewed for eligibility. Animal studies, case reports, and conference abstracts were excluded during title and abstract screening. Disagreements were reconciled through discussion or by using the expert view of a third reviewer (E.F.S. or A.L.T.).
We included all studies that evaluated apathy in HD patients. The exclusion criteria were studies that did not use a valid scale for scoring apathy or that used a scale not specific for apathy, such as the Beck Depression Inventory (BDI), which evaluates loss of interest related to depression; studies that did not report the number of patients with apathy or the apathy scores for each group; and therapeutic or interventional studies.
Data Collection and Data Items
A predefined data extraction sheet was used to collect information such as sample size, staging of disease, type of assessment, apathy scoring, and number of cases. The primary outcomes were the apathy scores and the frequency of apathy. The secondary outcomes were the pathophysiology of apathy in HD and the association of apathy with other HD symptoms.
Risk of Bias Assessment
Given the heterogeneity of the included studies and the lack of a unified standardized risk of bias (ROB) assessment tool for various study designs, we designed a tool tailored for this study. We partially used the Newcastle-Ottawa Scale and a quality assessment tool developed by Collins et al. (16, 17). Our modified criteria contain three domains (selection, outcome, and comparability) and nine questions, with a total score ranging from 0 to 14 (see appendix 2 of the online supplement).
Data Synthesis
The meta-analyses were performed with Review Manager, version 5.4, in accordance with the guidelines of the Cochrane Collaboration (18). To extract the data from plots, we used WebPlotDigitizer, version 4.5 (19). Mean differences and standardized mean differences (SMDs) were calculated when the same or different apathy scales, respectively, were used. To compare different subgroups of HD patients, we synthesized a combined group using the means and standard deviations of each subgroup. Heterogeneity was assessed with Cochrane’s Q test and was defined as an I2 value >50% or a p value <0.05. We performed each meta-analysis with both fixed- and random-effects models and presented the most appropriate model based on heterogeneity and funnel plot asymmetry.
Results
Study Selection and ROB Assessment
The initial search yielded 1,085 records (Figure 1). After the removal of duplicates, the remaining 621 records were screened, and 94 studies were selected for full-text review. Twenty-two studies were excluded based on the exclusion criteria (see appendix 3 of the online supplement), and 69 studies were included. Eleven studies were added by screening the reference lists of relevant articles. Ultimately, a total of 80 studies were included. A detailed description of included studies and the result of the ROB assessment are available in appendixes 4 and 5 of the online supplement, respectively.
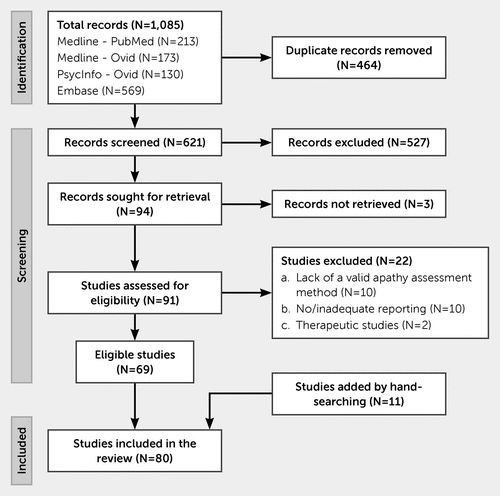
FIGURE 1. Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram for the inclusion and exclusion of studies
Apathy Assessment Tools in HD
Several tools have been used for apathy assessment. The scales are listed in order of historical development.
Irritability-Apathy Scale (IAS).
The IAS is one of the oldest assessment tools for apathy (20). In addition to the original study, we found another study that used the IAS for the assessment of apathy in those with HD (21). The IAS is a semistructured interview performed by a clinician with an informed companion of a patient (20). It has two subscales: irritability (five items; total score range: 5–17) and apathy (five items; total score range: 5–25). Apathy is considered to be present when three or more items out of five are endorsed (20).
Apathy Evaluation Scale (AES) and Apathy Scale (AS).
Seven studies used the AES (2, 22–27), and 11 used the AS (28–38). The AES was designed by Marin et al. with three versions, namely clinician (AES-C), informant (AES-I), and self-rated (AES-S), based on the source of information (39). The AES has 18 items with a 4-point scoring system (score range: 18–72) and is used to evaluate symptoms in the 4 weeks prior to the assessment. Despite some debate, a score of 40 or 41 has been used as the cutoff point for apathy in HD patients (2, 22, 26, 27). The AS is a shorter version of the AES that contains 14 items, and each item is rated from 0 to 3 points. A total score equal to or higher than 14 defines apathy (40). The AS was also used for the development of the Baltimore Apathy and Irritability Scale (BAIS) (37).
Frontal System Behavioral Scale (FrSBe).
This scale was originally developed as the Frontal Lobe Personality Scale (FLOPS) to assess behavioral changes in those with frontal dysfunction (41, 42). Eleven studies used the FrSBe for the assessment of apathy in HD patients (11, 41, 43–51). The FrSBe has three subscales, namely, apathy (14 items), disinhibition (15 items), and executive dysfunction (17 items), with each item rated from 1 to 5. For each of the three subscales, raw scores are converted to T-scores, which are corrected for age, education, and gender. A T-score of 65 or more is considered abnormal (42).
Neuropsychiatric Inventory (NPI).
Four studies used the NPI (4, 52–54). This informant-based interview was designed to evaluate 10 neuropsychiatric symptoms over the previous 4 weeks by scoring the severity (1–3 points) and frequency (1–4 points) of each symptom (55). The magnitude of each behavior is calculated by multiplying the severity by its frequency. Caregiver distress is also rated from 0 (not distressing at all) to 5 (extremely distressing) for each positive symptom. The Neuropsychiatric Inventory Questionnaire (NPI-Q) was subsequently developed by adding two more items and removing the frequency assessment (56).
Unified Huntington’s Disease Rating Scale Behavioral Scale (UHDRS-b).
Seven studies used the UHDRS-b for apathy assessment in HD patients (57–63). The UHDRS evaluates four main domains, including motor, cognitive, behavioral, and functional independence (64). The behavioral domain originally comprised 10 items, and apathy appeared in the mood subdomain. In later versions, apathy became an independent item. Each item is rated on a scale from 0 to 4 for frequency and severity during the previous month. A score of 2 or more is considered an indication of the presence of a symptom (64).
Problem Behavior Assessment for Huntington Disease (PBA-HD).
The PBA-HD was used in eight studies (8, 27, 65–70). Based on the UHDRS-b, Craufurd et al. (8) adapted a more extensive 40-item tool with a similar 0–4 scale for severity and frequency. To maintain compatibility with the UHDRS-b, they defined the presence of a symptom by a score of 2 or more and limited the inquiry to the 4 weeks before the interview.
Problem Behavior Assessment–Short (PBA-s).
The PBA-s was the most frequently used assessment for apathy in HD patients (32 studies) (10, 12, 13, 23, 29, 43, 63, 71–95). It is a short version of the PBA-HD with 11 items and has shown high reliability. It is the recommended apathy assessment instrument for most HD clinical trials (96).
Dimensional Apathy Scale (DAS).
The DAS is a relatively new scale and was used in two studies (2, 22). The DAS was designed to minimize the impact of physical disability and to provide a multidimensional approach to apathy assessment (97). This scale has 24 items and three subscales: executive, emotional, and initiation apathy. Each item is scored on a 4-point Likert scale (range: 0–3). The recommended cutoff points for abnormal values in HD patients are ≥13 points on the executive subscale, ≥15 points on the emotional subscale, ≥16 points on the initiation subscale, and ≥38 points for the total score (2). Self- and observer-rated versions of the DAS, as well as a brief version (b-DAS) with nine items, are currently available (98).
Lille Apathy Rating Scale (LARS).
This scale includes 33 items divided into nine domains, all of which address different manifestations of apathy (99). The first three questions are rated −2 to 2, and the remaining questions are rated −1 to 1. The total score ranges between −36 and 36, with a higher score indicating more severe apathy. A short version (LARS-s) was also developed with a range of scores between −15 and 15 (100). The proposed cutoff scores for clinically relevant apathy are >−21 for the LARS and >−7 for the LARS-s (100). Only one study used the LARS-s with HD patients (80).
Effort-based decision-making tasks.
Effort-based decision-making tasks measure the level of effort an individual dedicates to a specific reward. Atkins et al. (22) and McLauchlan et al. (23) applied effort-based tasks to assess apathy in HD patients. The patients showed impaired instrumental learning and blunted responses to loss but showed no alterations in reward-related effort (23). Patients with premanifest HD exhibited reduced cognitive effort, whereas physical effort was normal (22).
Questionnaires.
Three studies reported apathy with results from the Non-Motor Symptoms Questionnaire (NMSQuest) (101) or HD Clinical Characteristics (HDCC) questionnaire (102, 103). The NMSQuest is a self-administered 30-item questionnaire that evaluates various nonmotor symptoms (104). The HDCC provides a qualitative assessment of the frequency and timing of HD symptoms and signs and is currently a core assessment instrument in the REGISTRY and Enroll-HD studies (105). Both questionnaires collect yes or no responses and do not assess the severity of apathy.
Apathy Frequency and Scores in Patients With Premanifest and Manifest HD
The clinical diagnosis of HD has been historically defined by the presence of motor signs consistent with HD. A diagnostic confidence level of 4 on the UHDRS (i.e., ≥99% confidence that the motor abnormalities are unequivocal signs of HD) has been consistent with manifest HD. Some studies have categorized the premanifest stage into PreHD-A and PreHD-B based on the estimated time since disease onset. PreHD-A is associated with an estimated disease onset of more than 10.8 years, whereas in PreHD-B, the estimated disease onset is less than 10.8 years (95). The total functional capacity (TFC) scale has been frequently used to categorize HD patients based on the severity of the functional decline. It assesses functional independence in five domains: occupation, finances, activities of daily living, domestic chores, and care level (106).
The frequency of apathy among patients with premanifest HD ranged from 10% to 33% (2, 45, 80, 82, 83, 87, 88), but one study reported apathy in 64% of PreHD-B patients (88). Among patients with manifest HD, apathy frequency ranged from 24% to 76% (2, 4, 8, 21, 27, 35, 36, 52–54, 76, 80, 82, 83, 86–88, 102).
To compare patients with premanifest HD with healthy control individuals, we included 5,311 records from 12 studies (2, 12, 22, 29, 30, 44, 45, 65, 69, 88, 91, 95). Compared to individuals in a control group, patients with premanifest HD had a significantly higher apathy score with an SMD of 0.41 (CI=0.29–0.52; p<0.001). However, there was a significant level of heterogeneity among the studies (I2: 63%, p<0.001; Figure 2).
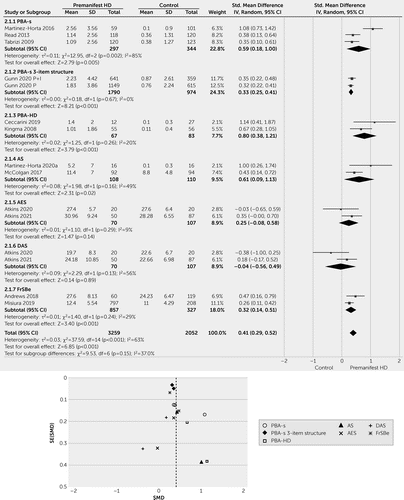
FIGURE 2. Apathy scores in patients with premanifest Huntington disease versus control individualsa
aAES=Apathy Evaluation Scale; AS=Apathy Scale; DAS=Dimensional Apathy Scale; FrSBe=Frontal Systems Behavior Scale; PBA-s=Problem Behaviors Assessment–Short.
The comparison of PBA-s scores between patients with manifest and premanifest HD based on the data from seven studies comprising 1,247 patients (74, 82, 84, 88, 89, 91, 95) showed significantly higher apathy scores in the manifest group with a mean difference of 1.87 (CI=1.48–2.26; p<0.001) and an SMD of 0.52 (CI: 0.41–0.64; p<0.001). This analysis showed homogeneity among the studies (I2=0, p=0.71; Figure 3).
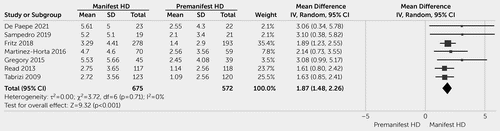
FIGURE 3. Apathy scores in patients with premanifest Huntington disease (HD) versus patients with manifest HD
Dimensions of Apathy in Premanifest and Manifest HD
Multidimensional assessments of apathy were performed in three studies (2, 22, 80). De Paepe et al. (80) combined items of the LARS-s in three domains: cognitive, emotional, and autoactivation. There were significantly higher apathy levels in the autoactivation domain in both premanifest and manifest HD groups than in the control group. The cognitive subscale was higher only in the manifest HD group, and the emotional subscale showed no significant difference among groups. Atkins et al. (2, 22) used the DAS to investigate dimensions of apathy, including executive, emotional, and initiation apathy. There were no significant differences between patients with premanifest HD and control individuals in any of the dimensions. However, the effort-based decision-making task showed lower cognitive motivation in patients with premanifest HD versus control individuals (22). Patients with manifest HD had higher DAS scores than those with premanifest HD and control individuals. Furthermore, by setting the DAS cutoff score at ≥38 points, the number of individuals with apathy was higher in the premanifest HD group than in the control group (2).
Correlations Between Apathy and Other HD Symptoms
Cognitive functioning.
Several studies have reported negative correlations between apathy and cognitive function (21, 25, 26, 34, 36, 41, 43, 44, 70, 74, 75, 77, 79, 86, 103). In a recent study, Andrews et al. (77) used 12 primary cognitive outcome variables from nine cognitive tasks and showed that apathy was a predictor of cognitive decline in patients with premanifest HD. They found a similar correlation in patients with manifest HD, but apathy was a weaker predictor of cognitive decline compared to UHDRS motor scores (77). Migliore et al. (75) performed a repeated-measures analysis of UHDRS cognitive domain scores in patients with manifest HD for up to 2 years of follow-up. There was a significant correlation between PBA-s apathy scores and the severity of cognitive decline (75). Van Duijn et al. (36) reported lower global cognitive function, as evaluated by the Mini-Mental State Examination (MMSE), and lower executive cognitive function in HD patients with apathy than in those without apathy. Reedeker et al. (34) showed that MMSE scores were the main predictor of apathy. Martinez-Horta and colleagues found strong correlations between apathy and all measures of the UHDRS cognitive score (86), and a moderate correlation between apathy and MMSE scores (79). In a study by Fritz et al. (84), although there was no association with clinician-rated cognition scores (Stroop test, Symbol Digit Modalities Test, and verbal fluency), apathy was significantly correlated with self-rated cognition measures, suggesting that apathy has a more severe effect on functional capacity and independence than objective cognition measures. In contrast, McAllister et al. (103) found that Symbol Digit Modalities Test and Stroop test scores were significantly correlated with apathy. In a study by Baudic et al. (21), HD patients with apathy showed significant deficits in global cognition (assessed by the Mattis Dementia Rating Scale), attention and executive function, and episodic memory, but they showed no significant differences in language and visuospatial task performance.
Social cognition.
In a study with patients with premanifest HD and apathy, Martinez-Horta et al. (29) demonstrated a significant decrease in the N170 component, which is a face-sensitive event-related brain potential. Interestingly, this deficit can be present for more than 15 years before the estimated time of disease onset (29). Using the awareness of social inference test, Osborne-Crowley et al. (81) and Kempnich et al. (46) showed decreased facial expression recognition among HD patients with apathy. In summary, there is evidence of decreased facial expression recognition among HD patients with apathy.
Irritability.
Bouwens et al. (32) reported a correlation between apathy and irritability over a 2-year follow-up period. However, Burns et al. (20) reported no correlation between measures of apathy, irritability, and aggression in HD patients. Similarly, Martinez-Horta et al. (73) did not find significant difference in apathy frequency when comparing two groups of HD patients with and without irritability or aggression.
Depression.
Several studies have shown an overlap between apathy and depression (21, 26, 36, 44, 45, 59, 77, 88). The seminal study by Levy et al. (4) involving patients with different neurodegenerative diseases showed that patients can have apathy without depression and vice versa. Similarly, Naarding et al. (27) found no association between apathy and depression in a small series of HD patients. De Paepe et al. (74) examined gray matter volume changes in HD patients to map apathy circuits and showed that UHDRS cognition scores were associated with apathy but not depression. Isaacs et al. (43) evaluated the performance of apathy scales (PBA-s and FrSBe) versus formal psychiatric assessment by a psychiatrist with HD experience. The PBA-s and psychiatric assessments were comparable in detection of depression but not apathy. On the other hand, the FrSBe detected apathy in accordance with psychiatric assessment (43).
Motor functioning.
Higher apathy scores were related to worse motor function, as generally assessed by the UHDRS total motor score (TMS) (25, 26, 28, 36, 46, 47, 59, 70, 77, 81, 86). Thompson et al. (70) evaluated three behavioral changes in HD patients (apathy, depression, and irritability) and found that motor symptoms were correlated only with apathy. Sousa et al. (25) reported that the TMS was the only factor that could independently predict apathy. Andrews et al. (77) reported that apathy scores were correlated with TMS in early HD patients. Conversely, a few studies did not find any correlation between apathy and motor scores (67, 80, 103). Van Duijn et al. (67) did not detect any significant change in PBA apathy scores or any correlation between apathy and the TMS. McAllister et al. (103) found no significant correlation between apathy and the TMS after reviewing the records of 6,316 individuals from the REGISTRY data.
Functional abilities.
Functional capacity and related independence scales are global measures of disability in activities of daily living. These measures indirectly assess related functions, such as overall motor disability and cognitive impairment (70). The functional domain of the UHDRS includes three components: TFC scale, functional assessment scale, and independence scale (IS) (64). The TFC scale has been widely used as a measure of clinical severity in HD (106). Several studies have reported a strong inverse association between TFC and apathy (2, 12, 27, 34, 36, 43, 50, 59, 70, 80, 86–88, 103). In a 36-month study, Tabrizi et al. (13) found this correlation only in patients with early manifest HD, not in patients with premanifest or late manifest HD. Two studies investigated employment as a measure of functional capacity. Jacobs et al. (85) identified cognitive impairment and apathy as two independent predictors of unemployment in a mixed population of patients with manifest and premanifest HD. Van der Zwaan et al. (10) performed an analysis on a sample of 2,791 individuals included in the Enroll-HD database and found that apathy was the most important factor of working capacity reduction among patients with premanifest HD. However, in patients with manifest HD, executive and motor dysfunction had a greater influence on the reduction in working capacity (10).
Pathophysiology of Apathy in HD Patients
The pathophysiology of HD has been classically linked to basal ganglia dysfunction. The striatum is one of the first brain regions affected by the HD-related neurodegenerative process. With disease progression, gray matter loss becomes evident beyond the striatum, affecting various cortical regions. White matter loss has also been reported in early stages with loss of functional connectivity in patients with premanifest HD (95).
Fourteen studies investigated the pathophysiology of apathy in HD patients (28–30, 44, 65, 74, 80, 82, 83, 86, 89, 90, 92, 95). Here, we discuss the most prominent findings.
Cingulate cortex.
The cingulate cortex has three main parts: anterior cingulate cortex (ACC), midcingulate cortex (MCC), and posterior cingulate cortex (PCC). The ACC is a processing center for autonomic function and emotional responses. The MCC is responsible for various aspects of cognitive control such as response selection, attention-related processing, and error detection. The PCC is a functionally heterogeneous region with high metabolic activity and dense connections to other brain regions (107–109).
Studies on normal motivated behavior have shown a strong correlation between aspects of the ACC and apathy (110). Decreased metabolic activity, as assessed by fluorodeoxyglucose positron emission tomography (PET), has been detected in the dorsal ACC in HD patients with apathy (86). De Paepe et al. (74) found atrophy of the MCC, not the ACC, on MRI in HD patients with apathy.
Other cortical regions.
Findings of PET studies in HD patients have shown significant decreases in metabolism in the prefrontal cortex (PFC) and its targets, including the ACC (86). In addition, the involvement of other cortical areas such as frontotemporal, parietal, insular, and occipital cortices have also been observed (82, 86). Martinez-Horta et al. (29) showed that visuoperceptual deficits (i.e., disruption of face-like object recognition) were associated with the severity of apathy in patients with premanifest HD, possibly resulting from impairments in the fusiform gyrus.
Basal ganglia, thalamus, and limbic system.
Degeneration of the striatum is a prominent feature of HD. Martinez-Horta et al. (86) provided evidence in favor of the hypothesis of a role for basal ganglia degeneration in apathy development by demonstrating atrophy of various gray matter regions, including the striatum, in HD patients with apathy. They also showed that a complex cortico-subcortical emotion-related network, which includes the hippocampus and amygdala, is affected in those with apathy (86). Misiura et al. (44) reported significant relationships between apathy and atrophy of the putamen and caudate but not the thalamus. In contrast, Baake et al. (83) showed a correlation between apathy and atrophy of the thalamus at baseline but no significant relationship between apathy and volume change of subcortical structures over a 2-year follow-up.
White matter.
MRI with diffusion tensor imaging (DTI) was used in four studies to detect white matter correlates of apathy in HD patients, and the results were inconsistent (30, 80, 89, 90). One study reported changes in the rectus gyrus, and two other studies found no significant correlation between apathy and white matter change (30, 89, 90). In an attempt to reduce the effect of heterogeneity on DTI measures, De Paepe et al. (80) considered apathy subtypes (cognitive, emotional, and autoactivation) in their analysis. They found correlations between different apathy profiles and white matter tracts, including the frontostriatal tract, which connects the presupplementary motor areas to the caudate nucleus (cognitive subtype); uncinate fasciculus, which connects the anterior temporal lobe to the amygdala and orbitofrontal cortex (autoactivation subtype); and dorsolateral PFC to caudate nucleus tract (cognitive subtype) (80).
A few studies also evaluated functional connectivity (28, 30). Nair et al. (28) used resting-state functional MRI data to model dysfunction of the direct and indirect pathways in patients with premanifest HD. Apathy was associated with dysfunction of the striatothalamic (direct pathway) connectivity (28).
Blood Markers Correlated With Apathy in HD Patients
The potential association of blood markers with apathy in HD was investigated in two studies by Bouwens et al. (31, 66). In the first study (66), they examined concentrations of C-reactive protein (CRP) and albumin and reported significant associations between CRP and several factors, including apathy, TFC, and cognitive impairment. However, the association disappeared after adjusting the multilevel regression model for antipsychotic use (66). Their second study (31) evaluated plasma levels of cytokines, including TNF-α, interleukin (IL)-1ra, IL-1, IL-5, IL-6, IL-8, and IL-10. Only cognitive dysfunction was weakly associated with IL-1ra and IL-6. Other neuropsychiatric symptoms, including apathy, showed no associations with cytokine levels (31).
Discussion
Apathy is a common neuropsychiatric symptom of HD and is associated with disease progression. Our meta-analysis revealed higher apathy scores in patients with manifest HD than in patients with premanifest HD. The heterogeneous data showed that patients with premanifest HD had higher apathy scores than healthy control individuals. The frequency of apathy generally ranged from 10% to 33% among patients with premanifest HD and from 24% to 76% among patients with manifest HD. The numbers varied significantly among studies, reflecting methodological differences (e.g., case definition, assessment tools, source of information, definition of premanifest and manifest HD subcategories) and characteristics of the sample studied, with more advanced HD leading to higher frequency and severity of apathy.
Several instruments have been used to investigate apathy in HD patients. The PBA-s was the most commonly used instrument due to its specific validation in HD and sensitivity to change over the course of the disease (96). Other apathy scoring tools, such as the AES, AS, and FrSBe (apathy subscale), have not undergone proper validation, specifically within HD patient populations. In the best-case scenario, the validity was assessed in a patient population with a variety of neurodegenerative diseases (111). Furthermore, differences in the suggested cutoff scores hampered the process of defining a more reliable picture of HD-related apathy. For the PBA-s, for instance, the cutoff scores were defined as severity score of ≥1, ≥2, or >2 in different studies. The reporting of apathy severity was also inconsistent among the studies. PBA and PBA-s scores have been reported in different ways; for example, studies have reported PBA-s apathy severity scores only (range: 0–4), the product of apathy severity and frequency scores (range: 0–16), the PBA-s three-item structure (apathy, perseveration, and disorientation; range: 0–48), the PBA-HD four-item factor (lack of perseverance, poor quality of work, lack of initiative, and poor self-care; range: 0–64), or the original PBA-HD factor with seven items (range: 0–16).
To overcome these shortcomings, consensus-based diagnostic criteria for apathy were proposed (7). Although these criteria do not measure apathy severity, they provide a diagnostic structure and thus a more reliable case definition. Four criteria should be met for a diagnosis of apathy: a quantitative reduction in goal-directed activity in comparison to the patient’s previous levels of functioning, symptoms and duration, exclusionary criteria, and severity. Three dimensions were defined for symptoms: behavior and cognition, emotion, and social interaction. The patient should have at least one symptom in at least two dimensions, and the symptoms should be persistent or frequently recur over at least 4 weeks (6, 7). The applicability of these diagnostic criteria in HD needs to be investigated as well as their interaction with clinical tools. Illustrating the relevance of this latter point, the PBA-s and FrSBe had divergent performances compared to formal psychiatric assessments to identify cases of apathy in HD (43). Therefore, additional studies are needed to establish gold-standard criteria and scales for the assessment of apathy in HD patients.
In this review, we excluded studies that did not use a dedicated apathy scale or subscale. For instance, the BDI assesses loss of interest as a part of depression and does not assess apathy as a discrete entity. While disorders of motivation, including apathy and anhedonia, are relevant elements of depression, they are neither necessary nor sufficient to define the depressive syndrome. Conversely, it is recognized that there is a clinical overlap between apathy and depression (7, 27). Since depressed mood is one of the early symptoms of HD and given the therapeutic implications, differentiating depressed mood from apathy is clinically relevant (7, 67). A better understanding of the overlapping versus divergent trajectories of these two neurobehavioral syndromes is definitely warranted in HD.
Apathy was associated with cognitive decline, affecting both global cognition and executive function. Of note, an inability to program and execute plans—core features of executive function—can result in reduced goal-directed behaviors, a subdomain of apathy, indicating a close link between these two constructs (21). Associations between apathy and cognitive decline have also been shown in other neurodegenerative diseases, such as Alzheimer’s disease (AD) and Parkinson’s disease (PD) (112, 113). Even among cognitively normal individuals, apathy was associated with a twofold increase in the risk of conversion to mild cognitive impairment (114). Taken together, these findings underscore the importance of apathy as a proxy for cognitive decline.
The association of apathy with irritability in HD can be explained by the involvement of relevant subcortical and frontal circuits. Only one study provided evidence in support of the connection between apathy and irritability (32). It has been suggested that apathy may mask irritability, resulting in the lack of overt external expression of anger (32). As anosognosia frequently occurs in HD patients, detection of irritability can be challenging in HD patients with apathy and requires a detailed interview with the patient and caregiver.
A role for a number of brain structures has been consistently reported across different disorders associated with apathy, especially PD and AD; these structures include the ventral tegmental area of the midbrain, ventral striatum, and various parts of the PFC, including the ACC (110, 115). There are fewer neuroimaging studies evaluating apathy in neurodegenerative diseases such as frontotemporal dementia, progressive supranuclear palsy, and HD than in PD and AD (115). Although the involvement of structures related to effort-based decision making seems to be a common feature of apathy across all these diseases, further studies are required to define the neural basis and pathophysiology of apathy in HD patients.
Our findings should be interpreted in the context of three main limitations. First, the data involved in the comparisons between patients with premanifest HD and control individuals were very heterogeneous. Second, due to the lack of consensus cutoff scores for apathy scales, we used only apathy scores, not the number of patients with apathy, for the meta-analysis. Third, investigating the potential associations between apathy and other clinical domains (e.g., cognition and social functioning) can be affected by confounding factors that were not well controlled in several studies during their design (e.g., randomization) and analysis (e.g., multivariate strategies).
Conclusions
In our review, apathy was more frequently observed in individuals with premanifest HD than those in a control group and in manifest versus premanifest HD. Despite the heterogeneity of the data, apathy appears to have a progressive nature in HD. The correlation between apathy and cognitive decline highlights the importance of apathy as a neurobehavioral symptom. Thus, apathy should be closely monitored throughout the disease course and may be considered a clinical surrogate biomarker for disease progression. Considering the complexity of apathy and its unique pattern of development in neurodegenerative diseases, further studies are required to explore its pathophysiology in HD.
1. : Historical crossroads in the conceptual delineation of apathy in Parkinson’s disease. Brain 2018; 141:613–619Crossref, Medline, Google Scholar
2. : Multidimensional apathy: the utility of the dimensional apathy scale in Huntington’s Disease. Mov Disord Clin Pract 2021; 8:361–370Crossref, Medline, Google Scholar
3. : Differential diagnosis and classification of apathy. Am J Psychiatry 1990; 147:22–30Crossref, Medline, Google Scholar
4. : Apathy is not depression. J Neuropsychiatry Clin Neurosci 1998; 10:314–319Link, Google Scholar
5. : Syndromic validity of apathy in Alzheimer’s disease. Am J Psychiatry 2001; 158:872–877Crossref, Medline, Google Scholar
6. : Is it time to revise the diagnostic criteria for apathy in brain disorders? The 2018 international consensus group. Eur Psychiatry 2018; 54:71–76Crossref, Medline, Google Scholar
7. : Diagnostic criteria for apathy in neurocognitive disorders. Alzheimers Dement 2021; 17:1892–1904Crossref, Medline, Google Scholar
8. : Behavioral changes in Huntington disease. Neuropsychiatry Neuropsychol Behav Neurol 2001; 14:219–226Medline, Google Scholar
9. : Huntington disease. Handb Clin Neurol 2018; 147:255–278Crossref, Medline, Google Scholar
10. : Predictors of working capacity changes related to Huntington’s disease: a longitudinal study. J Huntingtons Dis 2021; 10:269–276Crossref, Medline, Google Scholar
11. : Predictors of caregiver burden in Huntington’s disease. Arch Clin Neuropsychol 2021; 36:1426–1437Google Scholar
12. : Assessing mental health difficulties of persons with Huntington’s disease: does informant presence make a difference? J Neuropsychiatry Clin Neurosci 2020; 32:244–251Link, Google Scholar
13. : Predictors of phenotypic progression and disease onset in premanifest and early-stage Huntington’s disease in the TRACK-HD study: analysis of 36-month observational data. Lancet Neurol 2013; 12:637–649Crossref, Medline, Google Scholar
14. : Pharmacological management of apathy in dementia. CNS Drugs 2022; 36:143–165Google Scholar
15. : The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372:n71Crossref, Medline, Google Scholar
16. : A systematic review of the prevalence of depression, anxiety, and apathy in frontotemporal dementia, atypical and young-onset Alzheimer’s disease, and inherited dementia. Int Psychogeriatr (Epub Jul 20, 2020). doi:
17. : The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Ottawa Health Research Institute. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed Dec 16, 2021Google Scholar
18. : Cochrane Handbook for Systematic Reviews of Interventions version 6.2. Cochrane, 2021. http://www.training.cochrane.org/handbook. Accessed Dec 20, 2021Google Scholar
19. : WebPlotDigitizer, version 4.5, 2021. https://automeris.io/WebPlotDigitizer. Accessed Dec 20, 2021Google Scholar
20. : Clinical assessment of irritability, aggression, and apathy in Huntington and Alzheimer disease. J Nerv Ment Dis 1990; 178:20–26Crossref, Medline, Google Scholar
21. : Cognitive impairment related to apathy in early Huntington’s disease. Dement Geriatr Cogn Disord 2006; 21:316–321Crossref, Medline, Google Scholar
22. : Dissociable motivational deficits in pre-manifest Huntington’s disease. Cell Rep Med 2020; 1:100152Crossref, Medline, Google Scholar
23. : Insensitivity to loss predicts apathy in Huntington’s disease. Mov Disord 2019; 34:1381–1391Crossref, Medline, Google Scholar
24. : Huntington’s disease gene expansion carriers are aware of their degree of apathy. J Neuropsychiatry Clin Neurosci 2018; 30:183–187Link, Google Scholar
25. : Apathy profile in Parkinson’s and Huntington’s disease: a comparative cross-sectional study. Eur Neurol 2018; 79:13–20Crossref, Medline, Google Scholar
26. : Rating apathy in Huntington’s disease: patients and companions agree. J Huntingtons Dis 2015; 4:49–59Crossref, Medline, Google Scholar
27. : Apathy is not depression in Huntington’s disease. J Neuropsychiatry Clin Neurosci 2009; 21:266–270Link, Google Scholar
28. : Imbalanced basal ganglia connectivity is associated with motor deficits and apathy in Huntington’s disease. Brain 2022; 145:991–1000Crossref, Medline, Google Scholar
29. : Impaired face-like object recognition in premanifest Huntington’s disease. Cortex 2020; 123:162–172Crossref, Medline, Google Scholar
30. : Structural and functional brain network correlates of depressive symptoms in premanifest Huntington’s disease. Hum Brain Mapp 2017; 38:2819–2829Crossref, Medline, Google Scholar
31. : Plasma cytokine levels in relation to neuropsychiatric symptoms and cognitive dysfunction in Huntington’s disease. J Huntingtons Dis 2016; 5:369–377Crossref, Medline, Google Scholar
32. : Irritability in a prospective cohort of Huntington’s disease mutation carriers. J Neuropsychiatry Clin Neurosci 2015; 27:206–212Link, Google Scholar
33. : Suicidality in Huntington’s disease. J Affect Disord 2012; 136:550–557Crossref, Medline, Google Scholar
34. : Incidence, course, and predictors of apathy in Huntington’s disease: a two-year prospective study. J Neuropsychiatry Clin Neurosci 2011; 23:434–441Link, Google Scholar
35. : Hypokinesia in Huntington’s disease co-occurs with cognitive and global dysfunctioning. Mov Disord 2010; 25:1612–1618Crossref, Medline, Google Scholar
36. : Correlates of apathy in Huntington’s disease. J Neuropsychiatry Clin Neurosci 2010; 22:287–294Link, Google Scholar
37. : A comparison of self-report and caregiver assessment of depression, apathy, and irritability in Huntington’s disease. J Neuropsychiatry Clin Neurosci 2005; 17:378–383Link, Google Scholar
38. : Psychopathology in patients with degenerative cerebellar diseases: a comparison to Huntington’s disease. Am J Psychiatry 2002; 159:1306–1314Crossref, Medline, Google Scholar
39. : Reliability and validity of the Apathy Evaluation Scale. Psychiatry Res 1991; 38:143–162Crossref, Medline, Google Scholar
40. : Reliability, validity, and clinical correlates of apathy in Parkinson’s disease. J Neuropsychiatry Clin Neurosci 1992; 4:134–139Link, Google Scholar
41. : Frontal behavioral syndromes in cortical and subcortical dementia. Assessment 1996; 3:327–337Crossref, Google Scholar
42. : Frontal Systems Behavior Scale Professional Manual. Lutz, FL, Psychological Assessment Resources, Inc, 2001Google Scholar
43. : The impact of anosognosia on clinical and patient-reported assessments of psychiatric symptoms in Huntington’s disease. J Huntington’s Dis 2020; 9:291–302Crossref, Medline, Google Scholar
44. : Apathy is related to cognitive control and striatum volumes in prodromal Huntington’s disease. J Int Neuropsychol Soc 2019; 25:462–469Crossref, Medline, Google Scholar
45. : Executive impairment is associated with unawareness of neuropsychiatric symptoms in premanifest and early Huntington’s disease. Neuropsychology 2018; 32:958–965Crossref, Medline, Google Scholar
46. : Emotion recognition correlates with social-neuropsychiatric dysfunction in Huntington’s disease. J Int Neuropsychol Soc 2018; 24:417–423Crossref, Medline, Google Scholar
47. : Examining Huntington’s disease patient and informant concordance on frontally mediated behaviors. J Clin Exp Neuropsychol 2015; 37:981–987Crossref, Medline, Google Scholar
48. : Impact of cognitive and behavioural changes on quality of life in Huntington’s disease. Basal Ganglia 2013; 3:123–126Crossref, Google Scholar
49. : “Frontal” behaviors before the diagnosis of Huntington’s disease and their relationship to markers of disease progression: evidence of early lack of awareness. J Neuropsychiatry Clin Neurosci 2010; 22:196–207Link, Google Scholar
50. : Behavioural abnormalities contribute to functional decline in Huntington’s disease. J Neurol Neurosurg Psychiatry 2003; 74:120–122Crossref, Medline, Google Scholar
51. : Factor analysis of the Frontal Systems Behavior Scale (FrSBe). Assessment 2003; 10:79–85Crossref, Medline, Google Scholar
52. : Neuropsychiatric assessment of Gilles de la Tourette patients: comparative study with other hyperkinetic and hypokinetic movement disorders. Mov Disord 2001; 16:1098–1104Crossref, Medline, Google Scholar
53. : Neuropsychiatric aspects of Huntington’s disease. J Neurol Neurosurg Psychiatry 2001; 71:310–314Crossref, Medline, Google Scholar
54. : Neuropsychiatric assessment of patients with hyperkinetic and hypokinetic movement disorders. Arch Neurol 1998; 55:1313–1319Crossref, Medline, Google Scholar
55. : The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994; 44:2308–2314Crossref, Medline, Google Scholar
56. : Assessing the impact of neuropsychiatric symptoms in Alzheimer’s disease: the Neuropsychiatric Inventory Caregiver Distress Scale. J Am Geriatr Soc 1998; 46:210–215Crossref, Medline, Google Scholar
57. : Embodied emotion impairment in Huntington’s disease. Cortex 2017; 92:44–56Crossref, Medline, Google Scholar
58. : Clinical and genetic characteristics in patients with Huntington’s disease from China. Neurol Res 2016; 38:916–920Crossref, Medline, Google Scholar
59. : Neuropsychiatric symptoms in a European Huntington’s disease cohort (REGISTRY). J Neurol Neurosurg Psychiatry 2014; 85:1411–1418Crossref, Medline, Google Scholar
60. : Suicidal ideation in a European Huntington’s disease population. J Affect Disord 2013; 151:248–258Crossref, Medline, Google Scholar
61. : Characterization of the Huntington intermediate CAG repeat expansion phenotype in PHAROS. Neurology 2013; 80:2022–2027Crossref, Medline, Google Scholar
62. : Huntington’s disease from the patient, caregiver and physician’s perspectives: three sides of the same coin? J Neural Transm 2012; 119:1361–1365Crossref, Medline, Google Scholar
63. : Disease onset in Huntington’s disease: when is the conversion? Mov Disord Clin Pract 2021; 8:352–360Crossref, Medline, Google Scholar
64. Unified Huntington’s Disease Rating Scale: reliability and consistency. Mov Disord 1996; 11:136–142Crossref, Medline, Google Scholar
65. : Behavioral symptoms in premanifest Huntington disease correlate with reduced frontal CB(1)R levels. J Nucl Med 2019; 60:115–121Crossref, Medline, Google Scholar
66. : Acute-phase proteins in relation to neuropsychiatric symptoms and use of psychotropic medication in Huntington’s disease. Eur Neuropsychopharmacol 2014; 24:1248–1256Crossref, Medline, Google Scholar
67. : Course of irritability, depression and apathy in Huntington’s disease in relation to motor symptoms during a two-year follow-up period. Neurodegener Dis 2014; 13:9–16Crossref, Medline, Google Scholar
68. : Measurement of psychopathology in Huntington’s disease: the critical role of caregivers. J Nerv Ment Dis 2010; 198:329–333Crossref, Medline, Google Scholar
69. : Behavioural problems in Huntington’s disease using the Problem Behaviours Assessment. Gen Hosp Psychiatry 2008; 30:155–161Crossref, Medline, Google Scholar
70. : Behavior in Huntington’s disease: dissociating cognition-based and mood-based changes. J Neuropsychiatry Clin Neurosci 2002; 14:37–43Link, Google Scholar
71. : Utility of Huntington’s disease assessments by disease stage: floor/ceiling effects. Front Neurol 2021; 12:595679Crossref, Medline, Google Scholar
72. : Sex differences in Huntington’s disease: evaluating the Enroll-HD database. Mov Disord Clin Pract 2021; 8:420–426Crossref, Medline, Google Scholar
73. : Structural brain correlates of irritability and aggression in early manifest Huntington’s disease. Brain Imaging Behav 2021; 15:107–113Crossref, Medline, Google Scholar
74. : Gray matter vulnerabilities predict longitudinal development of apathy in Huntington’s disease. Mov Disord 2021; 36:2162–2172Crossref, Medline, Google Scholar
75. : Cognitive and behavioral associated changes in manifest Huntington disease: a retrospective cross-sectional study. Brain Behav 2021; 11:e02151Crossref, Medline, Google Scholar
76. : Age of onset and behavioral manifestations in Huntington’s disease: an Enroll-HD cohort analysis. Clin Genet 2021; 99:133–142Crossref, Medline, Google Scholar
77. : Apathy predicts rate of cognitive decline over 24 months in premanifest Huntington’s disease. Psychol Med 2020; 51:1338–1344Crossref, Medline, Google Scholar
78. : Early-motor phenotype relates to neuropsychiatric and cognitive disorders in Huntington’s disease. Mov Disord 2020; 35:781–788Crossref, Medline, Google Scholar
79. : Utility of the Parkinson’s Disease-Cognitive Rating Scale for the screening of global cognitive status in Huntington’s disease. J Neurol 2020; 267:1527–1535Crossref, Medline, Google Scholar
80. : White matter cortico-striatal tracts predict apathy subtypes in Huntington’s disease. Neuroimage Clin 2019; 24:101965Crossref, Medline, Google Scholar
81. : Apathy associated with impaired recognition of happy facial expressions in Huntington’s disease. J Int Neuropsychol Soc 2019; 25:453–461Crossref, Medline, Google Scholar
82. : Cortical atrophic-hypometabolic dissociation in the transition from premanifest to early-stage Huntington’s disease. Eur J Nucl Med Mol Imaging 2019; 46:1111–1116Crossref, Medline, Google Scholar
83. : Apathy and atrophy of subcortical brain structures in Huntington’s disease: a two-year follow-up study. Neuroimage Clin 2018; 19:66–70Crossref, Medline, Google Scholar
84. : Relationships among apathy, health-related quality of life, and function in Huntington’s disease. J Neuropsychiatry Clin Neurosci 2018; 30:194–201Link, Google Scholar
85. : Cognitive performance and apathy predict unemployment in Huntington’s disease mutation carriers. J Neuropsychiatry Clin Neurosci 2018; 30:188–193Link, Google Scholar
86. : Structural and metabolic brain correlates of apathy in Huntington’s disease. Mov Disord 2018; 33:1151–1159Crossref, Medline, Google Scholar
87. : Spanish validation of the Problem Behaviors Assessment–Short (PBA-s) for Huntington’s disease. J Neuropsychiatry Clin Neurosci 2017; 29:31–38Link, Google Scholar
88. : Neuropsychiatric symptoms are very common in premanifest and early stage Huntington’s Disease. Parkinsonism Relat Disord 2016; 25:58–64Crossref, Medline, Google Scholar
89. : Neuropsychiatry and white matter microstructure in Huntington’s disease. J Huntingtons Dis 2015; 4:239–249Crossref, Medline, Google Scholar
90. : The structural correlates of functional deficits in early Huntington’s disease. Hum Brain Mapp 2013; 34:2141–2153Crossref, Medline, Google Scholar
91. : Quality of life in Huntington’s disease: a comparative study investigating the impact for those with pre-manifest and early manifest disease, and their partners. J Huntingtons Dis 2013; 2:159–175Crossref, Medline, Google Scholar
92. : Clinical impairment in premanifest and early Huntington’s disease is associated with regionally specific atrophy. Hum Brain Mapp 2013; 34:519–529Medline, Google Scholar
93. : Potential endpoints for clinical trials in premanifest and early Huntington’s disease in the TRACK-HD study: analysis of 24 month observational data. Lancet Neurol 2012; 11:42–53Crossref, Medline, Google Scholar
94. : Biological and clinical changes in premanifest and early stage Huntington’s disease in the TRACK-HD study: the 12-month longitudinal analysis. Lancet Neurol 2011; 10:31–42Crossref, Medline, Google Scholar
95. : Biological and clinical manifestations of Huntington’s disease in the longitudinal TRACK-HD study: cross-sectional analysis of baseline data. Lancet Neurol 2009; 8:791–801Crossref, Medline, Google Scholar
96. : Understanding the outcomes measures used in Huntington disease pharmacological trials: a systematic review. J Huntingtons Dis 2014; 3:233–252Crossref, Medline, Google Scholar
97. : Developing a new apathy measurement scale: Dimensional Apathy Scale. Psychiatry Res 2014; 219:658–663Crossref, Medline, Google Scholar
98. : The brief Dimensional Apathy Scale: a short clinical assessment of apathy. Clin Neuropsychol 2020; 34:423–435Crossref, Medline, Google Scholar
99. : The Lille Apathy Rating Scale (LARS), a new instrument for detecting and quantifying apathy: validation in Parkinson’s disease. J Neurol Neurosurg Psychiatry 2006; 77:579–584Crossref, Medline, Google Scholar
100. : Assessing apathy in everyday clinical practice with the short-form Lille Apathy Rating Scale. Mov Disord 2013; 28:2014–2019Crossref, Medline, Google Scholar
101. : Non-motor symptoms in Huntington’s disease: a comparative study with Parkinson’s disease. J Neurol 2019; 266:1340–1350Crossref, Medline, Google Scholar
102. : Genetic risk underlying psychiatric and cognitive symptoms in Huntington’s disease. Biol Psychiatry 2020; 87:857–865Crossref, Medline, Google Scholar
103. : Timing and impact of psychiatric, cognitive, and motor abnormalities in Huntington disease. Neurology 2021; 96:e2395–e2406Crossref, Medline, Google Scholar
104. : The non-motor symptom complex of Parkinson’s disease: a comprehensive assessment is essential. Curr Neurol Neurosci Rep 2005; 5:275–283Crossref, Medline, Google Scholar
105. : Resources and Services. EHDN, 2016. http://www.ehdn.org/about-ehdn/resources-and-services. Accessed Jan 6, 2022Google Scholar
106. : Huntington disease: clinical care and evaluation. Neurology 1979; 29:1–3Crossref, Medline, Google Scholar
107. : Where is cingulate cortex? A cross-species view. Trends Neurosci 2020; 43:285–299Crossref, Medline, Google Scholar
108. : The role of the posterior cingulate cortex in cognition and disease. Brain 2014; 137:12–32Crossref, Medline, Google Scholar
109. : Midcingulate cortex: structure, connections, homologies, functions and diseases. J Chem Neuroanat 2016; 74:28–46Crossref, Medline, Google Scholar
110. : Brain mechanisms underlying apathy. J Neurol Neurosurg Psychiatry 2019; 90:302–312Crossref, Medline, Google Scholar
111. : Rating scales for behavioral symptoms in Huntington’s disease: critique and recommendations. Mov Disord 2016; 31:1466–1478Crossref, Medline, Google Scholar
112. : Apathy as a behavioural marker of cognitive impairment in Parkinson’s disease: a longitudinal analysis. J Neurol 2020; 267:214–227Crossref, Medline, Google Scholar
113. : Symptoms of apathy are associated with progression from mild cognitive impairment to Alzheimer’s disease in non-depressed subjects. Dement Geriatr Cogn Disord 2012; 33:204–209Crossref, Medline, Google Scholar
114. : Apathy as a risky neuropsychiatric syndrome of progression from normal aging to mild cognitive impairment and dementia: a systematic review and meta-analysis. Front Psychiatry 2021; 12:792168Crossref, Medline, Google Scholar
115. : The anatomy of apathy: a neurocognitive framework for amotivated behaviour. Neuropsychologia 2018; 118:54–67Crossref, Medline, Google Scholar



