Behavior in Huntington's Disease
Abstract
The authors examined the relationship of three dimensions of behavioral change (Apathy, Depression, and Irritability) measured by the Problem Behaviors Assessment for Huntington's Disease (PBA-HD) to cognitive and motor indices of disease severity. The Apathy subscale was highly correlated with both cognitive and motor impairment; the Irritability and Depression subscales were not. The findings suggest that certain behavioral alterations are intrinsic to the evolution and progression of HD, whereas others are more variable and are independent of other indices of disease progression.
Behavioral alterations in Huntington's disease (HD) are often the most distressing aspect of the condition for both patients and caregivers and are therefore often central to the practical clinical management of patients. Nevertheless, indices of disease severity are usually based on motor and cognitive measures. Experimental therapeutic interventions with striatal cell implants and neuroprotective agents aim to retard disease progression and restore basal ganglia function. If such treatments are to be successful, they should address behavioral as well as motor and cognitive symptoms. It is therefore important to understand how behavioral alterations are related to other features of HD.
Previous research has produced equivocal findings regarding this issue; some studies have shown a relationship between behavioral alterations and measures of disease severity;1–3 however, most have not.4–8 This has led some researchers to conclude that unlike the cognitive and motor aspects of the condition, behavioral changes are “heterogeneous, episodic, and without clear additive temporal progression.”8 However, previous studies have focused primarily on affective changes such as depression and irritability, which are not representative of the full range of behavioral changes in HD.
In addition to depression and irritability, amotivational states such as apathy and abulia are also common in HD,9 and although they are clinically striking, little is known about how they relate to other aspects of the condition. It is now well documented that there are characteristic cognitive changes in HD, particularly in the realm of executive functions. Patients exhibit difficulty with tasks that require the ability to plan, organize, sequence, shift cognitive set, and hold information “on line” in order to guide actions. It is possible that amotivational states are related to these cognitive changes. For example, an inability to plan and think ahead might be associated with a loss of initiative and drive. The hypothesis arising from this supposition is that certain aspects of behavioral change are related to cognition and so ought to be associated with cognitive impairment, whereas others are mood-based and unrelated to cognition.
Our own research using the Problem Behaviors Assessment for Huntington's Disease (PBA-HD) supports the separation of behavioral changes into different components. Craufurd et al.10 assessed 134 HD patients with the PBA-HD and, using factor analysis, distinguished three subscales of highly intercorrelated items (Table 1). The first subscale consisted of reduced activity and initiative, poor perseverance and quality of work, altered judgment, personal neglect, and emotional blunting, and was termed the Apathy subscale. The second subscale comprised symptoms of inflexibility, pathological preoccupations, poor temper control, and verbal and physical aggression, and was termed the Irritability subscale. The third subscale included low mood, depressive cognition, anxiety, and suicidal ideation, and was named the Depression subscale. We have demonstrated good interrater and test-retest reliability using this scale.
The aim of the present study was to examine how different aspects of behavioral change in HD relate to other indices of disease severity. We hypothesized that scores on the Apathy subscale of the PBA-HD would correlate with cognitive and motor measures of disease progression, whereas scores for the Irritability and Depression subscale would not.
METHODS
Patients
This cross-sectional, correlative study involved 82 patients (41 female and 41 male), with clinically diagnosed and genetically confirmed HD, who attend a regional HD clinic. The mean age of patients at the time of examination was 49 years (SD=12.6), ranging from 21 to 81 years. The mean age at disease onset was 41 years (SD=12.1), ranging from 14 to 73 years, and the mean duration of illness was 9 years (SD=4.7), ranging from 1 to 30 years.
Behavioral Assessment
Behavioral data were collected by using the Problem Behaviors Assessment for Huntington's Disease (PBA-HD). This semistructured interview includes 40 items that cover a wide range of behaviors. The scoring system is modeled on the behavioral assessment of the Unified Huntington's Disease Rating Scale (UHDRS); thus, each item is given a separate severity (0–4) and frequency (0–4) rating. A “PBA score” was also generated for each item by multiplying the severity and frequency scores for individual items. The PBA-HD is therefore compatible with the behavioral assessment of the UHDRS; however, it includes a more extensive range of behaviors than the brief UHDRS allows. Detailed operational criteria (available from the authors on request) were developed for rating each symptom and were used throughout the study. Patients were interviewed with their caregivers, and caregivers were also interviewed separately. It was felt important to take account of both patient and caregiver report because some symptoms, such as feelings of depression, may be more appropriately reported by patients, whereas symptoms such as aggressive and demanding behavior may be more objectively reported by caregivers. Interviewers were required to rate the severity and frequency of each symptom, based on patient and caregiver report and their own observations regarding the patients' mental state.
In a previous study, factor analysis of all items on the PBA-HD in a sample of 134 patients revealed that the items could be divided into three subscales (Table 1): Apathy, Irritability, and Depression. Scores for these three scales were calculated by taking the mean “PBA score” of the constituent items, thus providing a score ranging from 0 to 16 for each of the three PBA-HD subscales.
Assessment of Functional Capacity
Patients were administered the HD Functional Capacity Scale,11 which yields a total functional capacity score (TFC) ranging from 0 to 13, with lower scores indicating higher functional impairment.
Neurological Assessment
Neurological impairment was assessed with the motor examination from the Unified Huntington's Disease Rating Scale,8 which comprises standardized ratings of oculomotor function, dysarthria, chorea, dystonia, gait, and postural stability. The total UHDRS motor impairment score is the sum of all individual items, with higher scores indicating greater impairment.
Neuropsychological Assessment
The following cognitive tests were administered: 1) object memory; 2) verbal fluency; 3) Stroop test. These were selected on the basis of their sensitivity to disease progression in a longitudinal study of HD.12 The object memory test involved presentation of an array of 20 real objects for 30 seconds. Recall was assessed immediately following presentation and after a 30-minute delay. We have previously argued that this task requires self-generated, effortful encoding on the part of the patient and is therefore likely to be particularly sensitive to the memory impairment in HD, which is thought to be secondary to impaired executive skills.13 To assess verbal fluency, patients were first asked to generate as many animal names as possible in 1 minute, and then asked to generate as many words beginning with the letter “F” as possible in 1 minute (proper nouns and derivations of the same word stem not admissible). The Stroop test involved four conditions: reading black and white words, reading colored words, naming block colors, and naming the ink color of incongruous color words (interference condition). Stimuli for each condition were presented on cards containing 100 items in a 10-by-10 array.
Statistical Analysis
Data were analyzed by using SPSS for Windows V7. Nonparametric procedures were adopted because the data were not normally distributed. The Spearman rank correlation coefficient was used to assess the relationship between behavioral changes and motor, cognitive, and functional impairment. The probability level for tests of significance was set at 0.01, two-tailed.
RESULTS
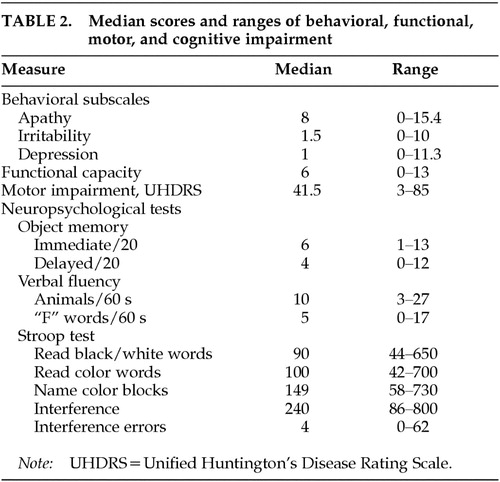
Table 2 shows the median scores and ranges for each of the test variables. It is of note that the median scores and ranges for the Irritability and Depression subscales indicate a negative skew. This reflects the fact that symptoms subsumed within these subscales are absent or of minimal severity in many patients.
Relationship Between Functional Capacity, Motor Impairment, and Cognition
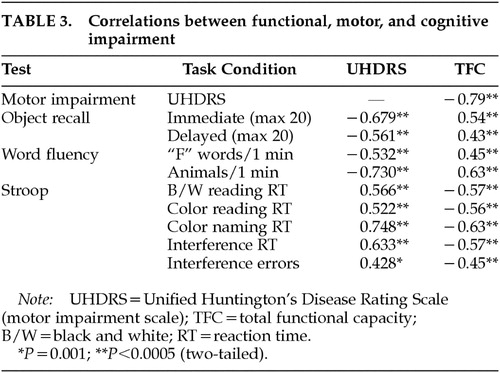
Table 3 shows the correlations between functional capacity, motor impairment, and performance on cognitive tests. There were highly significant correlations between functional capacity and motor impairment measured by the UHDRS motor scale. Functional capacity and motor impairment were both significantly correlated with performance on cognitive tests.
Relationship between Behavioral Subscales and Functional Capacity, Motor Impairment, and Cognition
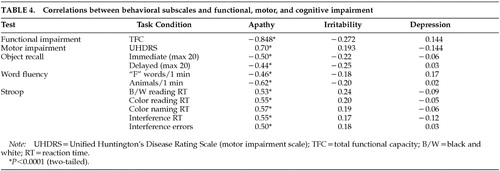
Table 4 shows correlations between behavioral subscales and functional capacity, motor impairment, and cognition. The Depression and Irritability subscales of the PBA-HD correlated poorly with functional capacity, motor impairment, and performance on cognitive tests. By contrast, scores on the Apathy subscale were significantly correlated with functional capacity, motor impairment, and performance on cognitive tests. Figure 1 shows scatterplots of the relationship between motor impairment and scores on the Apathy (1A), Irritability (1B), and Depression (1C) subscales. Visual inspection of these plots demonstrates the linear relationship between the Apathy subscale and motor impairment, and shows that scores on the Irritability and Depression subscales are negatively skewed.
Further analysis of the individual items of the Apathy subscale revealed that there were significant correlations between each of the constituent items and functional capacity, motor impairment, and performance on cognitive tasks.
Relationship of the PBA-HD Subscales to the UHDRS Behavioral Assessment
The behavioral assessment of the UHDRS includes 10 items, which can be split into 4 subscales: mood, obsessiveness, irritability, and psychosis. The total UHDRS behavioral score correlated significantly with the PBA-HD Irritability and Depression subscales (P<0.0005); the UHDRS mood score correlated significantly with the PBA-HD Depression subscale (P<0.0005); and the UHDRS irritability scale correlated significantly with the PBA-HD irritability subscale (P<0.0005). Neither the UHDRS obsessiveness nor psychosis scores were significantly correlated with the PBA-HD subscales. The PBA-HD Apathy subscale did not correlate with any of the UHDRS subscales.
DISCUSSION
We hypothesized that behavioral alterations in HD could be subdivided into those that are related to cognitive change and those that are independent of altered cognition. Consistent with our prediction, we observed strong relationships between measures of cognition and motor function and scores on the Apathy subscale of the PBA-HD. In contrast, the Irritability and Depression subscales, which reflect mood-based changes, were uncorrelated with either cognitive or motor impairment.
These findings help to resolve apparent disparities in the literature. Previous studies that found no relationship between behavioral change and other markers of disease progression4–8 focused on affective changes such as depression, anxiety, and irritability. This is consistent with our finding that the Depression and Irritability subscales did not correlate with motor and cognitive measures of disease progression. A small number of studies have shown a relationship between behavioral symptoms and other indices of disease progression. A study by Levy et al.3 is of particular relevance to our findings. Although principally concerned with the association between apathy and depression, the authors observed that while depression did not correlate with cognitive impairment (as measured by the Mini-Mental State Examination), apathy did. This is in keeping with our finding that the Apathy subscale of the PBA-HD correlated with cognitive and motor impairment.
We observed highly significant correlations between motor and cognitive impairment, both of which have been shown to correlate with PET measures of striatal dopamine binding,13,14 adding support to the notion of a common anatomical substrate. Furthermore, the Apathy subscale of the PBA-HD and its constituent items were highly correlated with motor and cognitive function as well as functional capacity. This finding supports the view that components of behavior subsumed within the Apathy scale, which includes both quantitative reductions in activity and loss of emotional response, are fundamental to the evolution and progression of HD. Functional capacity is a global measure of independence in the activities of daily living, and as such it is likely to be related to some of the items on the Apathy scale as well as to overall motor disability and cognitive impairment. However, functional capacity is a well-recognized measure of disease progression, and the finding that the Apathy subscale was so highly correlated with this and other indices of disease severity suggests that the Apathy subscale is indeed a valid measure of severity in HD.
Neither the Depression nor the Irritability subscale correlated with cognitive or motor impairment. In view of the reduced range of scores and skewed distributions of these scales, it is necessary to exercise caution in interpreting this finding. Nevertheless, our results are consistent with previous studies which have also shown that affective changes do not correlate with indices of disease severity. The absence of a correlation should not be interpreted as evidence that depression and irritability are unrelated to HD; however, it does suggest that these symptoms would be less suitable for monitoring disease progression in therapeutic trials. Nevertheless, depression and irritability are common features of HD, and therefore it would be important to monitor their occurrence, particularly in future trials designed to delay disease onset.
The nature of the relationship between the Apathy subscale and cognitive impairment is worth considering. Apathy is a common feature in neuropsychiatric disorders, and has been shown to correlate with cognitive impairment in Parkinson's disease15 and frontotemporal dementia.3 One possible explanation of the link between scores on the Apathy subscale and cognitive impairment in HD is that both arise from striatal pathology, which gives rise to a disruption in striatofrontal circuits. Specific impairments are associated with damage to these different circuits: the dorsolateral circuit is associated with impaired executive function, the anterior cingulate circuit with apathy, and the orbitofrontal circuit with disinhibition.16 Damage to these striatofrontal circuits in HD could therefore account for apathy as well as motor and cognitive impairment.
It is also possible that some of the behavioral alterations on the Apathy subscale are fundamentally related to impaired executive function—that is, that apathy and executive dysfunction both reflect a common underlying deficit. Executive functions include the planning, programming, regulation, and verification of goal-directed behavior. Reduced activity and initiative may be explained in terms of an inability to generate and execute plans. Similarly, impaired judgment, often manifested as impulsivity, may result from a failure to think plans through properly and consider their consequences. Poor perseverance could result from an inability to hold information “on line” using working memory.
Given that depression and irritability are such common features of HD, the finding that they do not appear to relate directly to progression of the disease warrants further discussion. One possible explanation is that these symptoms may not develop linearly, unlike those included on the Apathy subscale. Indeed, it has been proposed that certain types of behavioral change characterize different stages of the illness: irritability and aggression in the early stages; depression, mania and psychosis in the middle stages; and apathy and abulia in the late stages.9 It is quite possible that these affective disturbances subside as patients become more apathetic and emotionally blunted. The apparent nonlinear progression of affective symptoms may also in part be due to the responsiveness of these symptoms to psychotropic medication. These factors are unlikely to afford a complete explanation, however, as affective symptoms, although common in HD, are not the ubiquitous feature of the illness that apathy appears to be. Further research is required to begin to understand why certain individuals are more susceptible to depression and irritability. It is possible that other variables, such as genetic factors and premorbid personality characteristics, contribute to the presence and severity of affective disturbances. Longitudinal studies of behavioral alterations in HD should help to resolve these issues.
The patient group encompassed a wide range of disease severity and included some individuals with atypically early or late onset of symptoms. It is generally accepted that behavioral alterations are more severe in patients with an earlier onset and less severe in patients with a later onset. However, in a previous study we found that youthful and elderly patients did not differ significantly from the group mean in terms of overall number of behavioral symptoms or in scores on the three PBA-HD subscales, which suggests that onset age is not a factor that would affect the results of the current study.
The present study resolves some of the discrepancies in the published literature regarding the relationship of behavioral changes in HD to other indices of disease severity. The results indicate that certain behavioral changes are fundamental to the evolution and progression of HD, whereas affective changes such as depression and irritability are more variable and are not directly related to cognitive and motor measures of disease progression. This has implications for the choice of behavioral measures used to assess therapeutic efficacy in treatment trials. In explaining these findings, we highlight the link between behavior and cognition and postulate that certain behavioral alterations may be related to impaired executive functions in HD.
ACKNOWLEDGMENTS
The authors are grateful to the Huntington's Disease Association of England and Wales for financial support.
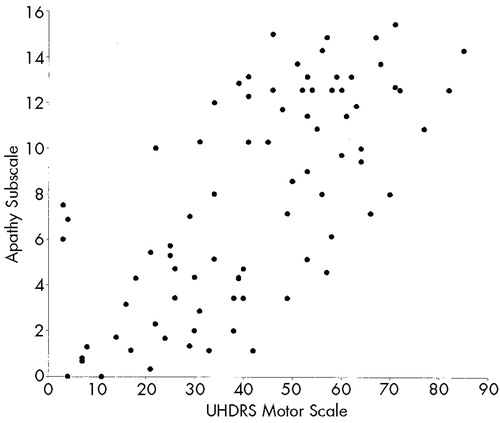
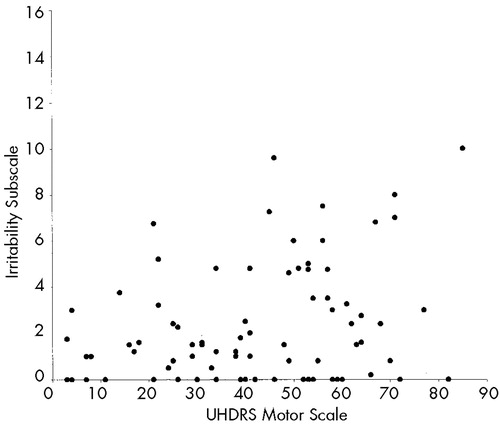
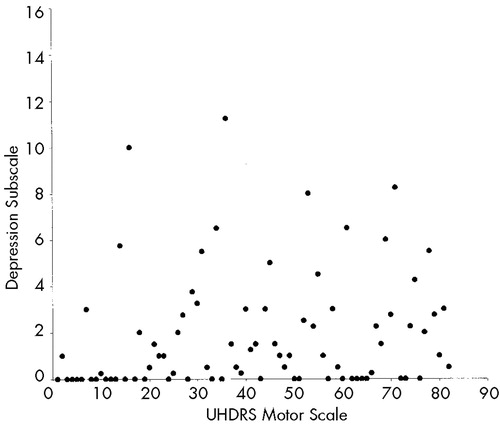
FIGURE 1. Relationship between scores on the Unified Huntington's Disease Rating Scale (UHDRS) motor scale and Problem Behaviors Assessment for Huntington's Disease (PBA-HD) behavioral subscales (Apathy; Irritability; Depression)
 |
 |
 |
 |
1 Boll TJ, Heaton R, Reitan RM: Neuropsychological and emotional correlates of Huntington's disease. J Nerv Ment Dis 1974; 158:61-68Crossref, Medline, Google Scholar
2 Shiwach RS, Patel V: Aggressive behaviour in Huntington's disease: a cross-sectional study in a nursing home population. Behav Neurol 1993; 6:43-47Crossref, Medline, Google Scholar
3 Levy ML, Cummings JL, Fairbanks LA, et al: Apathy is not depression. J Neuropsychiatry Clin Neurosci 1998; 10:314-319Link, Google Scholar
4 Caine ED, Shoulson I: Psychiatric syndromes in Huntington's disease. Am J Psychiatry 1983; 140:728-733Crossref, Medline, Google Scholar
5 Folstein SE, Folstein MF, McHugh PR: Psychiatric syndromes in Huntington's disease, in Huntington's Disease, edited by Chase TN, Wexler NS, Barbeau A. Advances in Neurology, vol 26. New York, Raven, 1979, pp 281-289Google Scholar
6 Mayberg HD, Starkstein SE, Peyser CE, et al: Paralimbic frontal lobe hypometabolism in depression associated with Huntington's disease. Neurology 1992; 42:1791-1797Crossref, Medline, Google Scholar
7 Zappacosta B, Monza D, Meoni C, et al: Psychiatric symptoms do not correlate with cognitive decline, motor symptoms, or CAG repeat length in Huntington's disease. Arch Neurol 1996; 53:493-497Crossref, Medline, Google Scholar
8 Huntington Study Group: Unified Huntington's Disease Rating Scale: reliability and consistency. Mov Disord 1996; 11:136-142Crossref, Medline, Google Scholar
9 Cummings JL: Behavioral and psychiatric symptoms associated with Huntington's disease, in Behavioral Neurology of Movement Disorders, edited by Weiner WJ, Lang AE. New York, Raven, 1995, pp 179-186Google Scholar
10 Craufurd D, Thompson JC, Snowden JS: Behavioral changes in Huntington's disease. Neuropsychiatry Neuropsychol Behav Neurol (in press)Google Scholar
11 Shoulson I, Fahn S: Huntington's disease: clinical care and evaluation. Neurology 1979; 29:1-3Crossref, Medline, Google Scholar
12 Snowden J, Craufurd D, Griffiths H, et al: Longitudinal evaluation of cognitive disorder in Huntington's disease. Journal of the International Neuropsychological Society 2001; 7:33-34Crossref, Medline, Google Scholar
13 Lawrence AD, Weeks RA, Brooks DJ, et al: The relationship between striatal dopamine receptor binding and cognitive performance in Huntington's disease. Brain 1998; 121:1343-1355Crossref, Medline, Google Scholar
14 Andrews TC, Weeks, RA, Turjanski N, et al: Huntington's disease progression: PET and clinical observations. Brain 1999; 122:2353-2363Crossref, Medline, Google Scholar
15 Starkstein SE, Mayberg HS, Preziosi TJ, et al: Reliability, validity, and clinical correlates of apathy in Parkinson's disease. J Neuropsychiatry Clin Neurosci 1992; 4:134-139Link, Google Scholar
16 Cummings JL: Anatomic and behavioral aspects of frontal-subcortical circuits. Ann NY Acad Sci 1995; 769:1-13Crossref, Medline, Google Scholar



