Sustained Attention Deficits Among HIV-Positive Individuals With Comorbid Bipolar Disorder
Abstract
Difficulties with sustained attention have been found among both persons with HIV infection (HIV+) and bipolar disorder (BD). The authors examined sustained attention among 39 HIV+ individuals with BD (HIV+/BD+) and 33 HIV-infected individuals without BD (HIV+/BD–), using the Conners' Continuous Performance Test–II (CPT–II). A Global Assessment of Functioning (GAF) score was also assigned to each participant as an overall indicator of daily functioning abilities. HIV+/BD+ participants had significantly worse performance on CPT–II omission errors, hit reaction time SE (Hit RT SE), variability of SE, and perseverations than HIV+/BD– participants. When examining CPT–II performance over the six study blocks, both HIV+/BD+ and HIV+/BD– participants evidenced worse performance on scores of commission errors and reaction times as the test progressed. The authors also examined the effect of current mood state (i.e., manic, depressive, euthymic) on CPT–II performance, but no significant differences were observed across the various mood states. HIV+/BD+ participants had significantly worse GAF scores than HIV+/BD– participants, which indicates poorer overall functioning in the dually-affected group; among HIV+/BD+ persons, significant negative correlations were found between GAF scores and CPT–II omission and commission errors, detectability, and perseverations, indicating a possible relationship between decrements in sustained attention and worse daily-functioning outcomes.
The prevalence of HIV among people with serious mental illness (SMI) is high,1–3 and of those with SMI, HIV infection appears to be particularly elevated among persons with bipolar disorder.4–7 Similarly, the prevalence of bipolar disorder among HIV-infected persons is elevated.7 Despite this overlap, relatively little is known about the neurocognitive and everyday functioning abilities of HIV+ persons with bipolar disorder.
Both HIV infection and bipolar disorder can cause neurocognitive impairment in a subset of affected individuals. Approximately half of HIV-infected individuals evidence at least subtle neurocognitive impairment; even among individuals with incidental comorbidities, neurocognitive impairment rates are estimated to be 40%.8 Neurocognitive impairment tends to worsen with HIV disease severity, with the greatest impairments observed among individuals with AIDS.9–11 Although the exact neurocognitive domains affected in HIV infection may be changing as a result of improved antiretroviral treatments, recent reports suggest impairments in the domains of learning, executive function, attention/working memory, memory, motor skills, speed of information-processing, and verbal fluency.10,12
Although they are not the most affected domain, attention deficits have been reported since early in the HIV epidemic.13 Similar to other neurocognitive domains, attention deficits increase with disease severity.12 In a meta-analysis of HIV-related neurocognitive impairments, Reger and colleagues, in 2002,12 showed effect sizes of neurocognitive impairment, when compared with non-HIV-infected individuals, ranging from 0.1 for asymptomatic HIV-infected individuals to 0.4 in individuals with AIDS. Other studies of HIV-infected persons have reported impairment in the areas of divided attention,9 orienting,14 response-inhibition,15 and sustained attention.16
In bipolar disorder, there is a broad consensus that neuropsychological (NP) impairments exist, as compared with age-matched persons of similar educational background.17,18 During acute manic and depressive episodes, patients typically manifest significant dysfunctions in most cognitive domains.18–21 According to recent meta-analytic reviews,22,23 neurocognitive impairments in adult euthymic BD patients cut across the domains of attention, processing-speed, verbal learning/memory, and executive functions.
In contrast to cognitive problems in HIV infection, attention impairments are commonly observed among persons with bipolar disorder. Torres and colleagues23 reported medium effect sizes (0.60–0.79) when examining performance differences between euthymic persons with bipolar disorder and healthy comparison subjects on tests of visual attention (Trails A), complex attention (Digit Symbol), and sustained attention (Continuous Performance Test; CPT).20,24–33 These studies provide evidence that attentional deficits may be a core or “trait” characteristic of BD, and that these impairments may be exacerbated during periods of mood dysregulation (i.e., mania, depression).
Studies of sustained attention (i.e., the ability to maintain a certain level of performance, especially the ability to detect the occurrence of infrequent or unpredictable events over extended periods of time34) are most commonly assessed using continuous performance tests (CPT). In CPT tasks, subjects are required to monitor a stream of stimuli (such as digits or letters), and make a response (such as a key-press) whenever a specified target appears. Subjects must focus attention on a monotonous task and avoid distraction from other stimuli. Also, CPT tests demand a level of executive control in order to 1) hold specified targets in working memory; 2) inhibit task-irrelevant stimuli; and 3) inhibit responses to task-relevant stimuli resembling targets.35 Studies of sustained attention abilities among HIV-infected persons are somewhat sparse, and findings from these studies have been mixed. One study, by Karlsen and colleagues,36 showed that HIV-infected individuals had significantly longer reaction times than healthy comparison-subjects but similar target-sensitivity. In contrast, a later study of HIV-infected persons found no deficits in either reaction time or target-sensitivity.37 More recent studies have found that greater reaction-time variability is associated with recent stimulant use,16 as well as poorer overall cognitive ability and higher HIV plasma viral load.38
Sustained-attention deficits are much more frequently evaluated among persons with bipolar disorder, as compared with HIV infection; yet, there are conflicting results in these studies, as well.26 Studies have shown decreased target-sensitivity (omission errors) on various CPT tasks evaluating euthymic patients with bipolar disorder,27,32,39,40 whereas other studies have reported no significant differences in performance between individuals with bipolar disorder and healthy-controls on any CPT measure.41,42 Furthermore, some studies have reported decreased target-sensitivity and increased false-responding (commission errors) in manic bipolar patients.39 In a review of the literature, Clark and Goodwin26 found that a majority of CPT sustained-attention studies of bipolar disorder have yielded both state- and trait-related impairments. Specifically, impaired target-detection (omission errors) was one of the most sensitive markers of the illness course in BD, appearing to be unrelated to residual mood symptomatology and medication status, and was present in patients with good functional recovery. Target-detection deficits appear to be exacerbated in manic states, whereas sustained-attention deficits are present early in the course of the disorder and become more pronounced with repeated episodes.
To our knowledge, no studies have attempted to assess sustained-attention abilities among HIV+/BD+ individuals. As a result, the present study was designed to compare sustained-attention performance among HIV+/BD+ persons relative to HIV-infected individuals without bipolar disorder (HIV+/BD–). Given that the intrinsic nature of sustained attention requires the passage of time, we also examined CPT–II performance by group over the six study blocks. Secondary analyses examined the effect of mood state (i.e., euthymia, mania, depression) on sustained attention, as well as an examination of the relationship between sustained attention and level of global daily functioning ability.
METHOD
Participants
In a cross-sectional analysis, 39 HIV+/BD+ and 33 HIV+/BD– individuals were assessed with the Conners' Continuous Performance Test II (CPT–II43). Participants also underwent neuromedical and psychiatric assessments. Participants in the HIV+/BD+ group met Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM–IV44) criteria for bipolar disorder I or II after administering the Structured Clinical Interview for DSM–IV (SCID).45 Diagnoses were made by a licensed clinical psychologist (DJM) or research associate with a master's degree in psychology, supervised by a licensed clinical psychologist and board-certified psychiatrist (JHA); ambiguous diagnoses were resolved via case discussion with a psychodiagnostic expert (JHA). Study participants were assigned a Global Assessment of Functioning (GAF) score as an indicator of overall functioning in social, occupation, and educational contexts, according to DSM–IV guidelines. A SCID-derived current mood episode diagnosis (i.e., manic, hypomanic, depressed, euthymic, or mixed) was determined for all participants, independent of group membership. Across the entire study sample, 9 participants were in a manic/hypomanic/mixed state; 16 were in a depressive state; and 47 were euthymic. Current mood symptomatology was also evaluated, using a clinician rating of mania (Young Mania Rating Scale [YMRS]46) and a self-reported indicator of depressive symptoms (i.e., Beck Depression Inventory–II [BDI–II]47). Trained interviewers administered the Composite International Diagnostic Interview (CIDI)48 to the participants to obtain diagnoses of current and past psychoactive substance abuse and dependence disorders. Diagnoses were assigned for the following psychoactive substances: alcohol, cannabis, cocaine, opioids, and methamphetamine. Abuse and dependence diagnoses were combined to form current and lifetime use-disorder categories for each substance. HIV serostatus was confirmed by enzyme-linked immunosorbent assay (ELISA). Given that the present study was embedded in a larger study that required participants to be medically treated for their respective conditions, all participants in HIV+/BD+ group were required to be prescribed antiretroviral and psychotropic medications to treat HIV infection and bipolar disorder, respectively. HIV+/BD– participants were required to have been prescribed antiretroviral medications. Finally, we examined the proportion of individuals taking psychostimulant medications.
Participants were excluded from the study if they met DSM–IV criteria for schizophrenia, mood disorder due to a general medical condition, or if they had a neurological condition known to affect cognitive functioning, such as Alzheimer's disease, stroke, traumatic brain injury, or a closed head injury with loss of consciousness for more than 30 minutes.
Procedure
The CPT–II is a computerized test of sustained attention that requires participants to press a button whenever they see a target letter appear on the screen in front of them. Participants were seated before a laptop computer and instructed to press the space bar whenever a letter other than “X” appeared. After a 1-minute practice trial, the CPT–II was administered and took approximately 14 minutes to complete. The CPT–II is divided into six time-blocks. Ninety percent (90%) of the letters in each block are targets, which require the participant to press the response button. Each block is further divided into 3 sub-blocks, each consisting of 20 trials, characterized by an inter-stimulus interval (ISI) of 1, 2, or 4 seconds. Summary variables are calculated on the basis of age- and gender-stratified normative data that generate T-scores and reflect average performance across all 6 blocks of the CPT–II. The CPT–II manual43 provides clinical interpretation for T-scores, ranging from very good performance (score <40) to markedly impaired (score >65). For this study, we examined mean T-scores and also used a T-score >60, indicating moderately atypical performance, to classify participants' performance into impaired (≥60) and non-impaired (<60). The following CPT–II measures were examined in this study: Hit Reaction Time (Hit RT), Hit RT Standard Error (SE) (see Conners' CPT–II Manual43), Omission errors, Commission errors, Variability of SE, Detectability (d′), Perseverations, and Response Bias (beta; see Levine et al.16).
Statistical Analysis
We conducted a series of one-way analyses of variance (ANOVAs) to compare mean differences in sustained-attention performance by group. Chi-square analyses were used to compare the proportion of individuals that had an impaired performance on any of the CPT–II measures. A mixed-model, repeated-measures ANOVA was used to compare the following variables across the six blocks of the CPT–II: percent Omission errors, percent Commission errors, Hit Reaction Time (Hit RT), and Hit Reaction Time SE (Hit RT SE). Since T-scores for each block are not provided by the CPT–II software, raw scores were used in the repeated-measures analyses. Secondary analyses were conducted to examine the effect of mood state on sustained attention. We divided the whole cohort by mood state into three groups: manic/hypomanic/mixed, depressive, and euthymic. We conducted a one-way ANOVA, and chi-square analysis to determine the sustained-attention performance of mood-state groups. In order to accommodate departures from normality within the various groups, we used the comparable nonparametric statistical test (e.g., the Wilcoxon rank-sum test for between-group comparisons and Spearman's ρ for correlations between CPT–II and GAF). Cohen's δ statistic (mean difference divided by the pooled SD) was used to measure the effect sizes of the group comparisons. No corrections were made for multiple comparisons.
RESULTS
The demographic and clinical characteristics of the study participants are presented in Table 1. The study cohort was predominantly Caucasian, male, and on average had a high school education. The HIV+/BD+ and HIV+/BD– study groups were comparable on demographic, psychiatric, and HIV-disease characteristics, with the exception that the HIV+/BD+ group had a significantly greater proportion of individuals who met criteria for lifetime methamphetamine-use disorder, had a significantly higher score on the Young Mania Rating Scale (YMRS), and were taking more psychotropic medications. Use of psychostimulants known to improve attention was rare among our study participants (N=2).
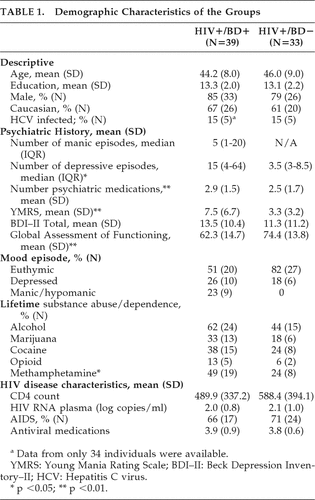 |
Groups were comparable on rates of current substance-use disorders and ADHD diagnoses. In the HIV+/BD+ group, three individuals met criteria for methamphetamine disorder, one individual met criteria for cannabis disorder, and one individual met criteria for opioid disorder, whereas only one individual in the HIV+/BD– group met criteria for cannabis disorder. Regarding ADHD diagnosis, only one individual in the HIV+/BD+ group met criteria for current ADHD, Hyperactive type, whereas one individual in the HIV+/BD– met criteria for ADHD, Inattentive type. Also, three HIV+/BD+ individuals and one HIV+/BD– met criteria for past ADHD combined type; one HIV+/BD+ met criteria for past ADHD Inattentive type, and one HIV+/BD+ patient met criteria for ADHD Hyperactive type. A high proportion (62%) of individuals in the HIV+/BD– met lifetime criteria for major depressive disorder. The mood-state groups were comparable on all demographic characteristics, but had differences in YMRS and BDI–II scores in expected directions (data not shown).
Significant differences between the HIV+/BD+ and HIV+/BD– groups were found on CPT–II Omission errors, Hit RT SE, Variability of SE, and perseverations (all p values <0.05; Table 2). Both groups had comparable Commission errors, Hit RT, Detectability, and response style. We also compared these groups on the proportion of individuals who were deemed impaired (i.e., T-score ≥60) on the CPT–II measures. Results showed that a significantly greater proportion of HIV+/BD+ individuals were impaired on Omission errors and variability of SE (all p values <0.01).
 |
We were interested in determining whether mood differences at the time of study assessment affected CPT–II performance. When individuals were compared on the basis of mood state, a one-way ANOVA yielded no significant differences for any of the CPT–II variables among the three mood-state groups. Also, the proportion of individuals impaired on the CPT–II variables did not differ across the three mood-states. Next, we examined the relationship between mania ratings (i.e., YMRS score) or depressive symptoms (i.e., BDI–II score) and CPT–II performance. The only significant association was a negative correlation between the BDI–II and Hit RT T-score within the HIV+/BD+ group, indicating that higher depressive symptomatology was associated with faster reaction times. Finally, we examined the relationship between CPT–II performance and number of cumulative lifetime manic or depressive episodes. Worse T-scores on CPT–II Detectability were significantly associated with fewer manic episodes (ρ = –0.32; p=0.03). None of the other demographic, clinical, or HIV-disease characteristics listed in Table 1 were correlated with CPT–II scores.
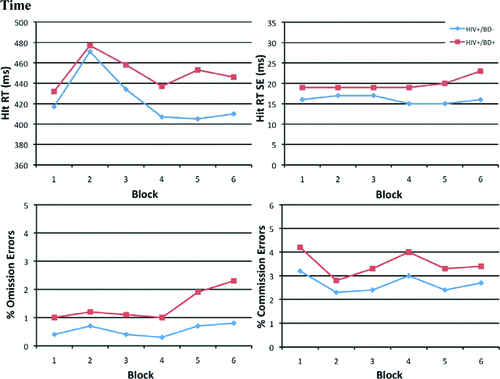
To determine whether differences existed in the group performances over the six study blocks of the CPT–II, scores on the following variables were entered in a repeated-measures ANOVA: percent Omission errors, percent Commission errors, Hit RT, and Hit RT SE. When examining the HIV+/BD+ and the HIV+/BD– groups, a main effect was found for group on percent Omission errors (F[1, 72]=7.3; p=0.009), and for Hit RT SE (F[(1, 72]=5.8; p=0.02). A main effect for Time was found for percent Commission errors (F[5, 72]=4.9; p=0.001) and Hit RT (F[5, 72]=12.9; p <0.001; Figure 1). No significant between-Group or Time interactions were observed for any of these four variables, though an interaction between Group and Time on Hit RT approached significance (p=0.09).
In order to determine whether CPT–II performance was related to daily functioning, we assessed the relationship between CPT–II performance and a global indicator of daily functioning (GAF score). The HIV+/BD+ group had significantly lower GAF scores as compared with the HIV+/BD– group (F[1, 69]=12.7; p < 0.001). Within the HIV+/BD+ group, significant correlations were found between GAF scores and Omission errors (ρ = −0.32; p=0.04), Commission errors (ρ = –0.34; p=0.05), Detectability (ρ = –0.32; p=0.05), and Perseverations (ρ = –0.33; p=0.04).
Finally, to further ensure that our findings were primarily attributable to bipolar disorder and not to other potential influences on CPT–II performance, we excluded individuals who met criteria for ADHD (either current or lifetime) and current methamphetamine abuse/dependence. The exclusion of these individuals (eight ADHD individuals and three individuals with a current methamphetamine disorder) did not change the results of the study.
DISCUSSION
The aim of the present study was to examine sustained attention in a cohort of HIV-infected individuals with comorbid bipolar disorder (HIV+/BD+) relative to HIV-infected individuals without bipolar disorder (HIV+/BD–). Results from the study show that HIV+/BD+ participants show decreased target sensitivity (Omission Errors), greater inconsistency in their response time (Hit RT SE, variability of SE), and a greater number of perseverations as compared with HIV+/BD– participants. The results also revealed that both HIV+ groups (i.e., those with and without bipolar disorder) made more false-positive errors (Commission Errors) and were slower to respond (Hit RT) as the test progressed across the six study blocks. Overall, the observed pattern of performance suggests that HIV+/BD+ individuals are able to detect target stimuli, but display inconsistent reaction times to these target stimuli. These impairments were associated with a global rating of how well persons were functioning in daily life.
We were particularly interested in determining whether current mood indicators were related to sustained-attention performance. The results from these examinations were largely non-significant. Specifically, mood state was not associated with better or worse performance on our measure of sustained attention. In examining other indicators of mood (i.e., symptom ratings scales, number of depressive/manic episodes) our findings were mostly non-significant, and the significant findings were in directions counter to what we had hypothesized (i.e., fewer manic episodes associated with worse detectability, worse self-reported depressive symptoms associated with faster reaction times). The mean CPT–II subscale scores of individuals in a manic state were consistently worse than those of individuals who were in a depressed or euthymic state, but none of these findings were statistically significant.
Our small sample size, the relative psychiatric stability of our HIV+/BD+ group, and the fact that most of our HIV+/BD+ participants were currently in a euthymic mood state likely contributed to our inability to detect significant mood-state differences on measures of sustained attention. The observation of elevated perseverative responses among HIV+/BD+ individuals, as compared with the HIV+/BD– individuals, warrants additional discussion. Perseverative responses may be the result of anticipatory (i.e., a belief that the next response can be predicted), very slow, or random responses to the preceding stimuli. Our findings appear to be most consistent with an anticipatory response pattern, which would be compatible with behaviors known to be present among some individuals with bipolar disorder—such as increased self-confidence, a belief that the future can be predicted, and impulsive behaviors. The next most plausible explanation for elevated perseverative responses in the HIV+/BD+ group is frequent and very slow responding to the preceding stimuli. This possible explanation is supported by the significantly slower reaction time in the HIV+/BD+ group. Random responding is typically only observed among persons with severe impairment, which is not consistent with our findings, given that performance on some measures was within normal limits (e.g., Detectability). An anticipatory/impulsive response style may result in specific daily functioning impairments, such as poor driving (e.g., anticipating the changing of a green light), poor financial decision-making (e.g., gambling, impulsive spending), and, most importantly, increased risk-behavior engagement (e.g., acting without thinking in high-risk-for-HIV-transmission situations).
Study results also showed that, relative to an age- and gender-matched normative sample, both HIV+ study groups had a high proportion of individuals classified as impaired on several CPT–II variables; specifically, impairments were found in Hit RT, Hit RT SE, variability of SE, and perseverations. These findings suggest that HIV infection, regardless of psychiatric comorbidity, may lead to slowed responses, inconsistent reaction times, and perseverative errors; the HIV+/BD+ group was particularly affected. These findings are consistent with previous studies, in that HIV-infected individuals have been shown to have inconsistent reaction times16 and intact detectability.36,37 In contrast, our study did not find any significant associations between reaction-time variability and higher HIV plasma viral load, as previously reported.38 These contrasting results may be due to the fact that HIV disease was relatively well controlled in our cohort.
Taking the above findings as a whole, it may be that HIV infection adversely affects response time, whereas bipolar disorder negatively affects ability to respond to relevant stimuli; however, a complete 2 x 2 design would be required to tease out the exact contribution of HIV and bipolar disorder for sustained attention-impairments. Importantly, it appears that neither condition affects the ability to detect relevant stimuli (Detectability), or the ability to inhibit a response to non-relevant stimuli (Commission Errors).
Also interesting is our lack of observed group x time interaction effects when examining the various CPT–II subscales across the six study blocks by HIV+/BD+ and HIV+/BD– group. Despite a lack of interaction, main effects of time were found for Commission Errors and Hit RT, demonstrating that both groups made more false-positive errors, and were slower to respond as the test progressed. This indicates that both groups have difficulties sustaining attention over prolonged periods of time, suggesting that any psychosocial interventions (e.g., HIV transmission risk-reduction, medication-adherence improvement) designed for these groups will need to have information presented quickly and in a targeted fashion.
Our study also highlights that HIV+/BD+ individuals have more difficulty with daily functioning than HIV+/BD– individuals. The level of daily functioning impairment in the HIV+/BD+ group was consistent with psychiatric symptoms causing some difficulty in social, occupational, or school functioning, but representative of persons who are generally functioning well in their environment, with some meaningful interpersonal relationships. In comparison, HIV+/BD– individuals, on average, rarely had psychiatric symptoms, and, if they were present, these symptoms were unlikely to cause more than slight impairment in social, occupational, or school functioning. Interestingly, when looking at the HIV+/BD+ group, significant correlations were found between daily functioning scores and Omission and Commission errors, Detectability, and Perseverations, suggesting that, sustained attention-deficits may be associated with poorer general functioning in this group. Future studies of sustained attention in cohorts such as the one studied here would benefit from more detailed examination of different domains of daily functioning.
There are several factors and study design limitations that may have contributed to the observed findings. One possibility is that our study uncovered differences in motivation between the two groups, rather than differences in sustained attention. We believe this explanation is unlikely, given that the groups differed on a subset of CPT–II variables, but not all variables. Some individuals in the study had a diagnosis of methamphetamine disorder or ADHD. Statistical analyses were repeated without these participants, and results were comparable to those found with the entire sample. A limitation of this study is the lack of an HIV-uninfected bipolar comparison group; this group would give us the opportunity to better understand the unique, independent effects of each condition on sustained attention. Finally, the present study would have benefited from a larger sample size and a better distribution of mood states (i.e., particularly, a more even distribution of depressed and manic/hypomanic/mixed groups). On the other hand, identifying and engaging HIV-infected people with co-occurring severe mental illness in research is difficult and, taking this into account, our sample of prospectively-assessed HIV+/BD+ is actually one of the largest of its kind.
A limitation that warrants special attention is the possibility that psychotropic medications contributed to sustained attention differences, as study participants were taking a wide variety of psychotropic medications. In a non-controlled study design with a small sample such at the study presented here, it is very difficult to assess how each medication may affect performance on the CPT–II. It is known that psychostimulants such as methylphenidate can enhance performance on the CPT–II;49 however, only two study participants were currently taking methylphenidate. Also, some antipsychotics, such as quetiapine, have been shown to have positive effects on general cognitive functioning and attention, in particular.50 This would argue against any hypothesis that medication negatively affected performance among HIV+/BD+ persons, given that the five individuals currently taking quetiapine belong to this group. Participants in this study were also taking antidepressants (the majority of psychiatrically-medicated participants) and mood stabilizers (e.g., lithium). There is little evidence that these medications have any effect on sustained attention tasks.51
It is important to emphasize how our findings may generalize to other HIV-infected psychiatric samples. It is unlikely that the study findings can be attributed to demographic factors (i.e., age, education, sex, or ethnicity), as the groups were comparable on these characteristics. Nevertheless, the generalizability of our findings is limited by the demographic and disease characteristics of our sample; that is, the present sample is predominantly male, Caucasian, and with relatively mild HIV disease severity. There was not a clear association between mood symptomatology and performance on sustained attention tasks; this may have been the result of the relative psychiatric stability of our HIV+ bipolar group, and our findings may not generalize to groups with more severe mood symptoms. Finally, we were not able to reach clear conclusions regarding the effect of medication on sustained attention deficits, given the small sample size and the great variability of psychotropic medication in our cohort. It is possible that different types of psychotropic medications will affect sustained attention in different ways (e.g., improving or hindering performance), and further examination of this topic is warranted.
Despite these limitations, our study further emphasizes the importance of examining neurocognitive deficits, such as sustained attention, among HIV+ individuals with co-occurring bipolar disorder. The potentially additive neurocognitive deficits of HIV infection and bipolar disorder may result in significant everyday-functioning deficits. Further studies on the impact of significant psychiatric comorbidites in the context of HIV infection, and the implication of these conditions for neurocognitive and everyday outcomes, are warranted.
1. : Risk factors for HIV, hepatitis B, and hepatitis C among persons with severe mental illness. Psychiatr Serv 2003; 54:836–841Crossref, Medline, Google Scholar
2. : Predictors of HIV and hepatitis testing and related service utilization among individuals with serious mental illness. Psychosomatics 2005; 46:573–577Crossref, Medline, Google Scholar
3. : Prevalence of HIV, hepatitis B, and hepatitis C in people with severe mental illness. Am J Public Health 2001; 91:31–37Crossref, Medline, Google Scholar
4. : HIV seroprevalence among people with severe mental illness in the United States: a critical review. Clin Psychol Rev 1997; 17:259–269Crossref, Medline, Google Scholar
5. : Prevalence, diagnosis, and pharmacological treatment of mood disorders in HIV disease. Biol Psychiatry 2003; 54:307–316Crossref, Medline, Google Scholar
6. : Schizophrenia and major affective disorder among Medicaid recipients with HIV-AIDS in New Jersey. Am J Public Health 1999; 89:1101–1103Crossref, Medline, Google Scholar
7. : Medical comorbidity in a bipolar outpatient clinical population. Neuropsychopharmacology 2005; 30:401–404Crossref, Medline, Google Scholar
8. : HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 2010; 75:2087–2096Crossref, Medline, Google Scholar
9. : Dual task performance in HIV-1 infection. J Clin Exp Neuropsychol 2000; 22:16–24Crossref, Medline, Google Scholar
10. : HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol 2011; 17:3–16Crossref, Medline, Google Scholar
11. : The HNRC 500 neuropsychology of HIV infection at different disease stages: HIV Neurobehavioral Research Center. J Int Neuropsychol Soc 1995; 1:231–251Crossref, Medline, Google Scholar
12. : A meta-analysis of the neuropsychological sequelae of HIV infection. J Int Neuropsychol Soc 2002; 8:410–424Crossref, Medline, Google Scholar
13. : Infectivity of the human immunodeficiency virus: estimates from a prospective study of homosexual men. J Infect Dis 1987; 156:189–193Crossref, Medline, Google Scholar
14. : Mild cognitive impairment in HIV disease. Nurse Pract 1995; 20: 94–97Crossref, Medline, Google Scholar
15. : Computerized and traditional Stroop task dysfunction in HIV-1 infection. Neuropsychology 1999; 13:306–316Crossref, Medline, Google Scholar
16. : The effect of recent stimulant use on sustained attention in HIV-infected adults. J Clin Exp Neuropsychol 2006; 28:29–42Crossref, Medline, Google Scholar
17. : The neuropsychology and neuroanatomy of bipolar affective disorder: a critical review. Bipolar Disord 2001; 3:106–150; discussion, 151–153Crossref, Medline, Google Scholar
18. : Neuropsychology of bipolar disorder: a review. J Affect Disord 2002; 72:209–226Crossref, Medline, Google Scholar
19. : Neuropsychological deficits and functional impairment in bipolar depression, hypomania, and euthymia. Bipolar Disord 2007; 9:114–125Crossref, Medline, Google Scholar
20. : Cognitive function across manic or hypomanic, depressed, and euthymic states in bipolar disorder. Am J Psychiatry 2004; 161:262–270Crossref, Medline, Google Scholar
21. : Decision-making cognition in mania and depression. Psychol Med 2001; 31:679–693Crossref, Medline, Google Scholar
22. : A meta-analysis of cognitive deficits in euthymic patients with bipolar disorder. J Affect Disord 2006; 93(1–3):105–115Crossref, Medline, Google Scholar
23. : Neuropsychological functioning in euthymic bipolar disorder: a meta-analysis. Acta Psychiatr Scand Suppl 2007(434):17–26Crossref, Medline, Google Scholar
24. : Neurocognitive function in clinically stable men with bipolar I disorder or schizophrenia and normal control subjects. Biol Psychiatry 2004; 56:560–569Crossref, Medline, Google Scholar
25. : Persistent cognitive dysfunctions in bipolar I disorder and schizophrenic patients: a 3-year follow-up study. Psychother Psychosom 2005; 74:113–119Crossref, Medline, Google Scholar
26. : State- and trait-related deficits in sustained attention in bipolar disorder. Eur Arch Psychiatry Clin Neurosci 2004; 254:61–68Crossref, Medline, Google Scholar
27. : Sustained attention deficit in bipolar disorder. Br J Psychiatry 2002; 180:313–319Crossref, Medline, Google Scholar
28. : Processing efficiency and sustained attention in bipolar disorder. J Int Neuropsychol Soc 2005; 11:49–57Crossref, Medline, Google Scholar
29. : Sustained attention-deficit in bipolar disorder is not a working memory impairment in disguise. Neuropsychologia 2002; 40:1586–1590Crossref, Medline, Google Scholar
30. : Cognitive impairment in euthymic bipolar patients: implications for clinical and functional outcome. Bipolar Disord 2004; 6:224–232Crossref, Medline, Google Scholar
31. : A preliminary fMRI study of sustained attention in euthymic, unmedicated bipolar disorder. Neuropsychopharmacology 2004; 29:1734–1740Crossref, Medline, Google Scholar
32. : Impulsivity and phase of illness in bipolar disorder. J Affect Disord 2003; 73(1,2):105–111Crossref, Medline, Google Scholar
33. : Neurocognitive impairment in euthymic patients with bipolar affective disorder. Br J Psychiatry 2005; 186:32–40Crossref, Medline, Google Scholar
34. : Sustained attention, in Varieties of Attention. Edited by Parasuraman RDavies DR. Orlando, FL, Academic Press, 1984Google Scholar
35. : The role of fronto-polar cortex in sub-goal processing during working memory. Neuroimage 2002; 15:523–536Crossref, Medline, Google Scholar
36. : Slowed reaction time in asymptomatic HIV-positive patients. Acta Neurol Scand 1992; 86:242–246Crossref, Medline, Google Scholar
37. : Cognitive function in asymptomatic HIV infection. Arch Neurol 1997; 54:179–185Crossref, Medline, Google Scholar
38. : Reaction-time variability in HIV-positive individuals. Arch Clin Neuropsychol 2010; 25:791–798Crossref, Medline, Google Scholar
39. : Sustained attention deficits in manic and euthymic patients with bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry 2006; 30:1097–1102Crossref, Medline, Google Scholar
40. : Deficits in sustained attention in schizophrenia and affective disorders: stable versus state-dependent markers. Am J Psychiatry 2002; 159:975–982Crossref, Medline, Google Scholar
41. : Deficits in sustained attention in schizophrenia but not in bipolar disorder. Schizophr Res 2005; 78(2,3):225–233Crossref, Medline, Google Scholar
42. : No evidence of attentional deficits in stabilized bipolar youth relative to unipolar and control comparators. Bipolar Disord 2003; 5:330–339Crossref, Medline, Google Scholar
43. : Conners' Continuous Performance Test (CPT–II) Computer Program for Windows: Technical Guide and Software Manual. Tonawanda, NY, Multi-Health Systems, Inc., 2000Google Scholar
44.
45. : Structured Clinical Interview for DSM-IV Axis I Disorders: Clinician Version: Administration Booklet. Washington, DC, American Psychiatric Association, 1997Google Scholar
46. : A rating scale for mania: reliability, validity, and sensitivity. Br J Psychiatry 1978; 133:429–435Crossref, Medline, Google Scholar
47. : Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess 1996; 67:588–597Crossref, Medline, Google Scholar
48.
49. : Methylphenidate-induced improvements of various measures of attention in adults with attention-deficit hyperactivity disorder. J Neural Transm 2006; 113:1575–1592Crossref, Medline, Google Scholar
50. : Improvement in cognitive functioning in patients with first-episode psychosis during treatment with quetiapine: an interim analysis. Br J Psychiatry Suppl 2002; 43:S45–S49Crossref, Medline, Google Scholar
51. : Sustained attention and serotonin: a pharmaco-fMRI study. Hum Psychopharmacol 2008; 23:221–230Crossref, Medline, Google Scholar



