Discriminant Analysis of Beck Depression Inventory and Hamilton Rating Scale for Depression in Huntington's Disease
Abstract
Depression is common in Huntington's disease, but standard rating scales have doubtful validity in this population. Using data from the European Huntington's Disease REGISTRY study, the authors examined the discriminant value of items on the Beck Depression Inventory (N=843) and the Hamilton Rating Scale for Depression (N=768). Good discriminators of depression, apart from “depressed mood,” were “guilt,” “loss of interest,” and “suicidality.” Items that discriminated poorly were “weight loss,” “sleep disturbance,” “loss of appetite,” “psychomotor retardation,” “agitation,” and “irritability.” These findings highlight the limited usefulness of these scales within the area of Huntington's disease.
Huntington's disease (HD) is a progressive neurodegenerative disorder that affects around 1 in 10,000 people. Depression in HD is very common, with prevalences varying from 33% to 69%, depending on measurement tools used and the disease-stage studied.1 Recent data have suggested that there may be a peak in depressive symptoms before the motor onset of HD.2
Accurate measurement of depression is necessary for screening, diagnosis, and measuring the effect of treatment. However, standard rating scales for depression in HD are problematic. At face value, they appear to contain a number of items relating to aspects of HD that are altered in the absence of depression, such as weight loss and sleep disturbance.
A recently published study3 showed that the Beck Depression Inventory–II (BDI–II)4 performed relatively poorly in distinguishing depressed from nondepressed people with HD, using the Schedules for Clinical Assessment in Neuropsychiatry (SCAN).5 Scales that contained fewer “somatic” items, such as the Hospital Anxiety and Depression Scale (HADS)6 and a visual scale, the Depression Intensity Scale Circles (DISCS),7 were better able to identify depressed individuals with HD.
The European Huntington's Disease Network (EHDN) REGISTRY Study is a Europe-wide collaboration that performs yearly standardized assessments on about 5.000 people with HD in many European countries. Participating centers gather data on motor, cognitive, and psychiatric symptoms as well as biomarkers. In addition to a standard behavioral rating scale, the Unified Huntington's Disease Rating Scale (UHDRS) Behavioral section,8 a number of centers also administer standard depression rating scales, such as the BDI9 and the Hamilton Rating Scale for Depression (Ham-D).10 This study aimed to find out how specific items from these two scales performed in the discrimination of depressed from nondepressed people with HD.
METHOD
The Outcome Scores of the Ham-D, BDI, and UHDRS Behavioral Section were Collected from the REGISTRY Database.
All participants were divided into two groups (those experiencing depressed mood or no/minimal depressed mood) according to the item “depressed mood” of the UHDRS Behavioral section. This item measures the frequency (score range: 0–4) and severity (score range: 0–4) of depressed mood that the person has been experiencing over the last month. Lower numbers represent less frequent and less severe depressed mood. Participants were classified as depressed if they scored 6 or more; this item score being calculated by multiplying the frequency and severity scores. The cutoff score of 6-or-more represents depressed mood being experienced at either a minimum of at least once per week of a moderate severity that causes distress or a minimum of several times a week at a mild severity. Data were analyzed using a discriminant analysis method (correlation between items and the discriminant function) with SPSS Version 16.0 for Windows.
The discriminant model looks at how to identify HD patients experiencing depressed mood versus those with no or minimal depressed mood, using all items on the Ham-D and BDI as predictors. Correlation coefficients were produced for all items of the rating scales, and the higher the coefficient, the more that item contributed to discriminating HD patients endorsing depressed mood from those with no/minimal depressed mood patients as defined above. Wilks' lambda (λ) was used to test the significance of the discriminant model.
RESULTS
In all, we analyzed 834 BDI scales and 768 Ham-D scales.
Beck Depression Inventory
Of all patients with a BDI score, 180 patients (21.6%) were classified as endorsing depressed mood according to the criterion of a score of ≥6 on the “depressed mood” item of the UHDRS Behavioral section. The average score on the BDI was 19.18 (standard deviation [SD]: 10.77) for those experiencing depressed mood and 7.56 (SD: 7.57) for HD patients without depressed mood. Wilks' λ was highly significant, at 0.684; χ2=312.10; df: 21; p<0.0001. We calculated the correlations between the items and the discriminant function. The results of the discriminant analysis of the BDI are presented in Table 1.
 |
Hamilton Rating Scale for Depression (Ham-D)
Of all patients with a Ham-D score, 175 patients (22.8%) were classified as endorsing depressed mood according to the criterion of a score of ≥6 on the “depressed mood” item of the UHDRS Behavioral section.
The items “severity of diurnal variation” and “loss of insight” on the Ham-D were excluded because these were unrecorded on a majority of forms.
The average score on the Ham-D was 14.45 (SD: 6.57) for HD patients experiencing depressed mood and 6.66 (SD: 5.75) for those without depressed mood. Again, Wilks' λ was highly significant, at 0.623; χ2=358.29; df: 20; p<0.0001. We calculated the correlations between the items and the discriminant function; the results from the discriminant analysis of the Hamilton Rating Scale for Depression are shown in Table 2.
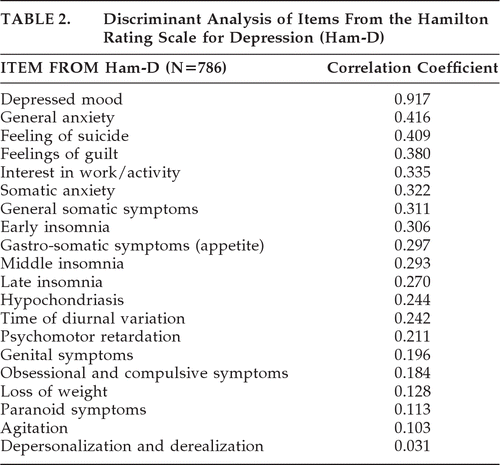 |
DISCUSSION
Choosing a “Gold Standard” for the Analysis
The concept of “depression” within the setting of a neurological disorder such as HD is problematic for two main reasons. The first reason is that symptoms of HD overlap with those of major depressive disorder. For instance, weight loss is a common symptom of HD regardless of mood status. The second reason is that a depressive syndrome in patients with HD may be different from “major depressive disorder” because the cause is different (for instance, the depressive syndrome in HD may be related to specific pathological changes in the striatum).
So far, there is no clear gold standard for discriminating HD patients with depressive symptoms from those with no such symptoms. We chose a simple definition for a gold standard, using the item “depressed mood” from the UHDRS Behavioral section. This equated to a score of at least 6 points on the “depressed mood” item. When analyzing the results, however, it is not surprising that the corresponding items on the BDI and Ham-D (“depressed mood” and “feeling sad”) highly correlate with the gold standard, as they are effectively measuring the same factor.
Which Items Performed Best on the Whole?
In general, items from the BDI were more likely than those from the Ham-D to discriminate HD patients with depressed mood from those without or with minimal depressed mood. With the exception of the items actually measuring depressed mood, the 14 next-best discriminators all came from the BDI. A number of these items measured thoughts or attitudes around mood, such as “discouraged about the future” (correlation coefficient = 0.653), “satisfaction in life”(0.640), “disappointed in self” (0.589) “feel like a failure”(0.540) “feel to blame” (0.481) and “feel being punished” (0.461).
Anxiety (which is measured by the Ham-D, but not the BDI) was not a particularly good discriminator (0.416) between individuals with and without depressed mood.
Which Items Did Not Discriminate Depressed Mood in HD?
“Irritability” or “agitation” did not discriminate between HD patients with and without depressed mood (0.321 on the BDI and 0.065 on the Ham-D). Also, weight loss had poor discriminatory power (0.194 for BDI and 0.128 for Ham-D). In general, items measuring sleep disturbance were poor discriminators. The best sleep item on the BDI was the measure of early awakening (0.401). Of the three sleep items on the Ham-D, the early (initial) insomnia item (0.306) was the best discriminator. Poorly discriminating items, which appeared in only one of the two scales, included “loss of sexual interest” (0.240), “paranoid symptoms” (0.113), “depersonalization and derealization” (0.031), and “obsessive-compulsive symptoms” (0.184). Three items were relatively uncommon in the HD population studied: paranoid symptoms (0.099), depersonalization and derealization (0.092), and obsessive and compulsive symptoms (0.200).
CONCLUSION
This study found that, apart from depressed mood, the best discriminators between HD patients endorsing depressed mood and those with no or minimal depressed mood (as we had defined them) were related to loss of interest, guilt, and suicidality. Vegetative symptoms related to sleep and appetite were poor discriminators, as were items in relation to externalizing behaviors such as agitation and irritability. The study has the advantage of using a large sample of people with HD. In essence, it looks at those items from the BDI and Ham-D that go together with significant depressed mood. Those symptoms that discriminate well are mainly concerned with depressive cognitions and anxiety. The study further reinforces the ideas, as stated by others,11–13 that depressed mood and anxiety constitute a relatively discrete syndrome in HD and that irritability is a relatively separate entity and has no value in the differential diagnosis of depression. It is known that people with HD are prone to weight loss14 and to have disturbed sleep,15 independently of depression, so it is not surprising that weight loss and sleep items are poor discriminators. The use of “depressed mood” on the UHDRS as a gold standard allows for a much larger sample to be recruited and makes only limited assumptions about the nature of depression in HD.
Further study in this area should elucidate the syndrome of depression in the setting of HD, including the temporal course of depressive symptoms and the role of somatization, with the aim of producing a measurable and valid concept of depression in Huntington's disease.
1. : Psychopathology in verified Huntington's disease gene carriers. J Neuropsychiatry Clin Neurosci 2007; 19:441–448Link, Google Scholar
2. : Psychiatric symptoms in Huntington's disease before diagnosis: The Predict-HD Study. Biol Psychiatry 2007; 62:1341–1346Crossref, Medline, Google Scholar
3. : Validation of self-report depression rating scales in Huntington's disease. Mov Disord 2010; 25:91–96Crossref, Medline, Google Scholar
4. : Manual for the Beck Depression Inventory-II. San Antonio, TX, Psychological Corporation, 1996Google Scholar
5. : SCAN: Schedules for Clinical Assessment in Neuropsychiatry. Arch Gen Psychiatry 1990; 47:589–593Crossref, Medline, Google Scholar
6. : The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 1983; 67:361–370Crossref, Medline, Google Scholar
7. : The Depression Intensity Scale Circles (DISCS): a first evaluation of a simple assessment tool for depression in the context of brain injury. J Neurol Neurosurg Psychiatry 2005; 76:1273–1278Crossref, Medline, Google Scholar
8.
9. : An inventory for measuring depression. Arch Gen Psychiatry 1961; 4:561–571Crossref, Medline, Google Scholar
10. : Development of a rating scale for primary depressive illness. Br J Soc Clin Psychiatry 1967; 6:278–296Crossref, Medline, Google Scholar
11. : Behavioural changes in Huntington's disease. Neuropsychiatry Neuropsychol Behav Neurol 2001; 14:219–226Medline, Google Scholar
12. : Rate of functional decline in Huntington's disease. Neurology 2000; 54:452–458Crossref, Medline, Google Scholar
13. : Factor analysis of behavioural symptoms in Huntington's disease. J Neurol Neurosurg Psychiatry 2011; 82:411–412Crossref, Medline, Google Scholar
14. : Weight loss in early stage of Huntington's disease. Neurology 2002; 59:1325–1330Crossref, Medline, Google Scholar
15. : Sleep features in Tourette's syndrome, neuroacanthocytosis, and Huntington's chorea. Neurophysiol Clin 1995; 25:66–77Crossref, Medline, Google Scholar



