Correlation Between Rostral Dorsomedial Prefrontal Cortex Activation by Trauma-Related Words and Subsequent Response to CBT for PTSD
Abstract
Objective:
Trauma-focused cognitive-behavioral therapy (CBT) is an important component of evidence-based treatment for posttraumatic stress disorder (PTSD), but the efficacy of treatment varies from individual to individual. It is hypothesized that some of this variability is derived from interindividual differences in the brain’s intrinsic response to trauma-related stimuli and in activity of executive functional regions. The authors sought to characterize these differences using functional MRI (fMRI) in patients about to undergo CBT for PTSD.
Methods:
Blood-oxygenation-level-dependent signal was measured in 12 individuals with PTSD related to sexual and/or physical trauma while they read words with positive, neutral, and negative content. Some negative words had PTSD-related themes, while others did not. It was hypothesized that PTSD-related words would evoke emotional processes likely to be engaged by the CBT process and would be most likely to activate brain circuitry important for CBT success.
Results:
A group-level analysis showed that the rostral dorsomedial prefrontal cortex (rdmPFC) was activated to a greater degree in response to PTSD-related words compared with other word types. This activation was strongest among patients with the best CBT responses, particularly in the latter part of the task, when differences between individuals were most pronounced.
Conclusions:
The rdmPFC activation observed in this study may reflect the engagement of neural processes involved in introspection and self-reflection. CBT may be more effective for individuals with a greater ability to engage these processes.
Posttraumatic stress disorder (PTSD) is characterized by an often disabling constellation of persistent symptoms following a traumatic life experience. Symptoms include intrusive re-experiencing of the trauma via flashbacks or nightmares, emotional dysregulation, hyperarousal, and avoidant behaviors. Trauma-focused cognitive-behavioral therapy (CBT), a mainstay of PTSD treatment, often combines exposure therapy with cognitive therapy (1). The cognitive component is aimed at challenging maladaptive trauma-related appraisals and to teach patients to think more realistically about the trauma and their symptoms. Exposure therapy is based on the theory that avoidance of negative affective states interferes with extinction learning, a process mediated by interactions between the amygdala, dorsal anterior cingulate cortex (dACC), and ventromedial prefrontal cortex (vmPFC), whereby learned threat associations are updated and extinguished when the previously learned response is not reinforced (2). The effects of the cognitive component of trauma-focused CBT presumably depend on prefrontal regions, such as the dorsolateral prefrontal cortex (dlPFC) and dorsomedial prefrontal cortex (dmPFC), which are involved in higher order cognitive operations (3).
Despite its apparent efficacy, response to trauma-focused CBT is incomplete, with as many as 60% of patients in some trials still meeting criteria for PTSD diagnosis following treatment (4). Skills training in affect and interpersonal regulation (STAIR) is a particular form of trauma-focused CBT with demonstrated benefit in PTSD. When a STAIR protocol was combined with standardized exposure therapy (STAIR/exposure), full remission (Clinician-Administered PTSD Scale [CAPS] score <20) (5) was achieved in 27% of patients, with 61% of patients no longer meeting criteria for PTSD diagnosis compared with 33% of those treated with exposure therapy alone (6). Although individuals in the STAIR/exposure group experienced more improvement than those in exposure therapy alone, more than a third of patients still met criteria for PTSD following combined treatment. Although most patients benefit from treatment, the degree of improvement is variable. A better understanding of who is likely to benefit from particular forms of treatment may help guide treatment selection for individual patients and can lead to improved understanding of the differential mechanisms by which these treatments work.
Functional neuroimaging studies have revealed differences in pretreatment brain function that are associated with subsequent CBT response, although findings may depend in part on the nature of the particular task used. Studies comparing pretreatment neural responses to emotionally negative images versus matched neutral or positive images have shown that greater activations in the amygdala (7, 8) and dACC (9, 10) correlate with poorer response to treatment. However, greater dACC was associated with better treatment response when it was observed in response to anticipation of negative versus positive images, in contrast to the opposite effect during image viewing (9). Fonzo and colleagues (8) found that when participants viewed faces depicting expressions associated with threat and fear, compared with neutral facial expressions, greater dACC activation (as well as greater activation in other frontal regions and in the anterior insula) was associated with better treatment response, an effect apparently opposite that shown by Aupperle and colleagues (9). An opposite effect on amygdala activation in response to facial expressions associated with threat and fear compared with neutral expressions was also demonstrated in a population of adolescent girls with PTSD (11), where CBT nonresponders demonstrated less differential amygdala activation. However, it was noted that this reflected greater activation in response to neutral stimuli among nonresponders, suggesting an overactive threat response to neutral stimuli. Studies using behavioral inhibition tasks have shown that greater activation in the dorsal striatum, prefrontal networks (12), and inferior parietal lobule (13) was associated with better response to treatment. In general, these studies have been interpreted as demonstrating that greater activation in emotion-responsive regions prior to treatment indicates poorer prognosis, whereas greater activation in brain areas involved in cognitive control and emotion regulation indicates better prognosis.
All of the above studies used standard emotion or cognitive tasks to probe the relevant circuitry. To our knowledge, trauma-specific stimuli have not been used in the context of an activation task for the purpose of identifying neural circuitry associated with PTSD treatment response. By way of their semantic content, visually presented trauma-specific words can convey themes that are highly personally and emotionally relevant to individuals with PTSD while controlling for low-level visual features. Trauma-related words can elicit early amygdala hyperactivation in patients with PTSD to a degree not seen with nontrauma-related emotionally negative words (14). We hypothesized initially that amygdala activation would correlate negatively with treatment response, whereas activation in higher-order frontal lobe regions involved in emotion regulation (such as the vmPFC, dACC, and dlPFC) would correlate positively with treatment response. Thus, we sought to investigate the relationship between trauma-related brain activation and subsequent response to trauma-focused CBT. Significant correlations in these specific brain regions were not found in our analyses. However, we conducted a whole-brain analysis to address a more exploratory hypothesis: that brain activations specifically associated with reading trauma-related words, even when outside of regions typically associated with PTSD, can have relevance for how the brain processes trauma-related content during CBT and can therefore have bearing on CBT success. Certain neural responses to trauma-related content may facilitate better response to CBT, whereas others may interfere. We therefore sought to identify trauma-word-related brain activations that correlated with subsequent response to treatment.
Methods
Participants
Among 36 participants who enrolled in the study, four dropped out before starting treatment, and 12 dropped out during treatment. Complete data sets, including imaging pre- and posttreatment CAPS scores, were available for 12 participants. Of these 12 participants, all were right-handed females, with a mean age of 35.4 years (range, 23–48 years), who met DSM-IV criteria for PTSD related to sexual and/or physical assault as their primary diagnosis. All participants were native English speakers and were free of other psychiatric diagnoses, substance abuse, and significant neurological or medical disorders. The CAPS (5) was used to establish a diagnosis of PTSD. To meet criteria for a diagnosis of PTSD, the following symptoms had to be present: from criterion B (re-experiencing symptoms), at least one symptom with a frequency rating of 1 and an intensity rating of 2; from criterion C (avoidance and numbing symptoms), at least three symptoms; and from criterion D (startle response/hyperarousal symptoms), at least two symptoms. All participants provided informed consent prior to participation in the study, which was part of a protocol approved by the institutional review board at New York-Presbyterian Hospital/Weill Medical College of Cornell University. Data analyses and manuscript preparation for this protocol were approved by the Partners Human Research Committee. Participants were assessed using CAPS before and after CBT.
CBT Protocol
Following the functional MRI (fMRI) scanning session, patients underwent a 16-session course of CBT with the STAIR/exposure protocol, a CBT program specifically targeting PTSD related to childhood abuse. The treatment consisted of eight 1-hour weekly sessions on STAIR, followed by eight 1-hour biweekly exposure therapy sessions (6). The first four STAIR sessions focused on identifying and labeling feelings, emotion management, distress tolerance, and acceptance of feelings, while the next four focused on exploration and revision of maladaptive schemas, effective assertiveness, awareness of social context, and flexibility in interpersonal expectations and behavior. Exposure sessions involved reviewing trauma narratives. The protocol was flexibly applied, allowing up to 21 actual sessions to take into account circumstances such as crisis sessions and the need to repeat sessions. The degree of treatment response was estimated by subtracting the post-CBT total CAPS score from the pre-CBT total CAPS score, such that a higher positive response score reflected a greater degree of symptom improvement. Normalized CAPS improvement indexes were calculated using the following equation: (CAPS TotalPre – CAPS TotalPost)/CAPS TotalPre.
fMRI Scans
Task-based fMRI experiment: emotional word paradigm.
Before beginning CBT, fMRI scans were obtained while participants completed an emotional word task.
Stimuli consisted of 48 negative or anxiety-provoking words (PTSD-related, N=24; panic disorder-related, N=24), 48 neutral words, and 48 positive words, balanced across categories for frequency, length, and part of speech. The words used in this study were cultivated by our laboratory and based on a similar list of words that was piloted for 34 healthy volunteers who rated the three word categories (positive, negative, and neutral) as significantly different in valence. PTSD-related words were designed to be relevant to individuals with a history of physical and/or sexual trauma, and panic disorder-related words were designed to relate to panic attack symptoms and somatic or illness-related anxiety (a negative control condition). Words were selected for suitability for this task by a panel of three experienced clinicians.
The three valences of words were presented within a block design (six words per block, eight blocks per valence). Each word appeared for 2 seconds, followed by an interstimulus interval jittered around an average of 2.8 seconds, for a total of 28.8 seconds per block. During presentation of the stimuli, participants were instructed to read each word silently and then immediately press a button under their right index finger. After the scan, participants were tested for their ability to recognize the words from among a list of similar distractors and to rate the valence of each word on a scale of −3 to +3. Recognition performance was quantified as a sensitivity index (d′). Ratings, recognition performance, and reaction times were compared across conditions using two-tailed Wilcoxon signed-rank tests. Further details of the task are presented elsewhere (14).
fMRI image acquisition and analysis.
Details of the fMRI methods are presented in the online supplement. Briefly, images were acquired with a research-dedicated GE Signa 3-Tesla MRI scanner (maximum gradient strength, 40 militesla per meter; maximum slew rate, 150 Tesla per meter per second). Blood-oxygenation-level-dependent (BOLD) contrast imaging was conducted using a gradient echo-planar imaging sequence (TR=1,200 ms, TE=30 ms, 15 or 21 slices of 5 mm thickness, 1-mm gap, field of view=240 mm, matrix=64×64). Functional image analysis was performed using customized fMRIstat software (15). A two-level voxel-wise linear fixed-effects model was utilized. Effects at every brain voxel were estimated using the expectation maximization algorithm, and effects of word type were then compared using linear contrasts. At the group level, the within-group pretreatment effects of the hypothesis-driven contrasts (PTSD-related versus neutral words, PTSD-related versus panic disorder-related words, and PTSD-related versus positive words) were examined for their association with the normalized CAPS improvement index via a multiple regression model, with the CAPS improvement index as the main regressor and age and scanning protocol as covariates of no interest. To examine the effects of experiment time, we also tested the effects of each half of the experiment via linear contrasts within the voxel-wise general linear model. Maps of the t statistic were thresholded initially at a voxel-wise two-tailed p value <0.01 and a cluster spatial extent >250 mm3. The p values at the peak voxels were then corrected for multiple comparisons using random field theory based on family‐wise error rate over the whole brain at a p value <0.05. The group-level correlation effect of interest at a peak coordinate was considered significant if the corrected p value (pcorr) was <0.05 based on family-wise error rate correction of the voxel-wise p values over the entire brain.
Results
Behavioral Analysis
The mean total CAPS score before treatment was 63.2 (range, 46–84). Following treatment, the mean total CAPS score was 36.9 (range, 9–54). The mean reduction in CAPS score following treatment (CAPS total score posttreatment − CAPS total score pretreatment) was 26.3 (range, 1–57). There were no significant differences in reaction time between any pair of word types. The mean reaction time ranged from 448 ms to 1,586 ms, with a grand mean of 950 ms. Following the scan, participants were asked to rate the words for valence on a scale of −3 to +3. Rating data were available for 11 participants. PTSD-related words were rated significantly more negative than all other word types (PTSD-related words: mean=−2.27 [SD=0.45]; neutral words: mean=0.28 [SD=0.33]; panic disorder-related words: mean=−1.84 [SD=0.52]; positive words: mean=2.03 [SD=0.32]; p<0.001 for all three comparisons). Memory for the words viewed in the scanner was assessed by computing a d′ value comparing hits with false positives during a postscan recognition test. Participants exhibited significantly better recall with PTSD-related words compared with neutral words (PTSD-related words: mean d′=1.98 [SD=0.49]; neutral words: mean d′=1.46 [SD=0.58]; p=0.002). Recall was also significantly better with PTSD-related words compared with positive words (positive words: mean d′=1.30 [SD=0.70]; p<0.001), but the difference between PTSD-related and panic disorder-related words was not significant (panic disorder-related words: mean d′=1.75 [SD=0.92]; p=0.13).
Imaging
At the group level, responses to PTSD-related words were compared against responses to panic disorder-related words, emotionally positive words, and emotionally neutral words. PTSD-related words activated the rostral dorsomedial prefrontal cortex (rdmPFC) more than panic disorder-related, neutral, and positive words (Figure 1). The differences between PTSD-related and neutral words were more pronounced than the differences between PTSD-related words and words in the other emotion conditions (panic disorder and positive). PTSD-related words also activated a network of thalamic, basal ganglia, and midbrain regions more than panic disorder-related, neutral, and positive words. The left amygdala was more activated during the PTSD condition than during the neutral condition but not during the panic disorder-related and positive conditions (for further details, see Tables S1–S3 in the online supplement).
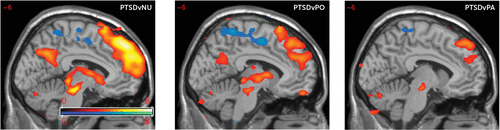
FIGURE 1. Activation in the rostral dorsomedial prefrontal cortex (rdmPFC) among female participants with a history of sexual or physical traumaa
a Shown are greater posttraumatic stress disorder (PTSD)-related word activation in the rdmPFC compared with neutral (NU) word activation (left) (t scores are presented with an initial voxel-wise threshold p value <0.001); greater PTSD-related word activation in the rdmPFC compared with positive (PO) word activation (center); and greater PTSD-related word activation in the rdmPFC compared with panic-disorder (PA) word activation (right).
Correlations were calculated for each voxel for the PTSD versus neutral effect against normalized treatment response (Table 1). The most significant positive correlation with treatment response was in the rdmPFC (left: pcorr=0.002; right: pcorr=0.015). This correlation reflects greater activation in response to PTSD-related words compared with neutral words among patients with the greatest symptomatic improvement in response to treatment (Figure 2A and 2B). This effect was primarily driven by the second half of the experiment (Figure 2C). During the first half of the task, even patients with poorer response to treatment demonstrated a differential activation in this region, but these patients did not maintain this differential activation throughout the experiment as those with better treatment response.
| Correlation and brain hemisphere | Brain region | Brodmann’s area | Montreal Neurological Institute Coordinates (x, y, z) | z | p | Corrected p value | Cluster | Cluster size (mm3) |
|---|---|---|---|---|---|---|---|---|
| Positive | ||||||||
| Left | Medial frontal gyrus | 8 | –6, 54, 33 | 4.887 | <0.001 | 0.002 | 1 | 8,127 |
| Superior frontal gyrus | 8 | 0, 45, 45 | 4.707 | <0.001 | 0.004 | 1 | ||
| Left | Medial frontal gyrus | 8 | –3, 57, 33 | 4.702 | <0.001 | 0.005 | 1 | |
| Medial frontal gyrus | 9 | 0, 60, 30 | 4.659 | <0.001 | 0.005 | 1 | ||
| Left | Superior frontal gyrus | 8 | –9, 54, 42 | 4.444 | <0.001 | 0.013 | 1 | |
| Left | Superior frontal gyrus | 6 | –3, 21, 63 | 5.093 | <0.001 | 0.001 | 2 | 2,943 |
| Right | Superior frontal gyrus | 6 | 9, 24, 66 | 4.415 | <0.001 | 0.015 | 2 | |
| Left | Inferior semi-lunar lobule | –9, –72, –39 | 4.546 | <0.001 | 0.009 | 3 | 1,188 | |
| Left | Cerebellar uvula | –6, –69, –36 | 4.507 | <0.001 | 0.01 | 3 | ||
| Right | Cerebellar tonsil | 48, –51, –36 | 4.185 | <0.001 | 0.036 | 4 | 891 | |
| Left | Lingual gyrus | –30, –78, 6 | 4.635 | <0.001 | 0.006 | 5 | 1,188 | |
| Left | Cuneus | 18 | –6, –81, 30 | 4.549 | <0.001 | 0.009 | 6 | 1,431 |
| Right | Medial frontal gyrus | 9 | 12, 54, 18 | 4.258 | <0.001 | 0.027 | 7 | 756 |
| Right | Middle frontal gyrus | 10 | 36, 60, –21 | 4.248 | <0.001 | 0.028 | 8 | 459 |
| Left | Cerebellar tonsil | –30, –33, –45 | 4.392 | <0.001 | 0.016 | 10 | 459 | |
| Negative | ||||||||
| Left | Superior parietal lobule | 7 | –45, –57, 60 | 5.516 | <0.001 | <0.001 | 1 | 6,885 |
| Left | Inferior parietal lobule | 40 | –45, –54, 57 | 5.444 | <0.001 | <0.001 | 1 | |
| Left | Supramarginal gyrus | 40 | –48, –42, 39 | 4.192 | <0.001 | 0.035 | 1 | |
| Right | Inferior occipital gyrus | 18 | 51, –84, –6 | 4.494 | <0.001 | 0.011 | 2 | 2,808 |
| Right | Superior parietal lobule | 7 | 45, –54, 57 | 4.947 | <0.001 | 0.002 | 3 | 4,077 |
| Right | Postcentral gyrus | 5 | 51, –42, 69 | 4.44 | <0.001 | 0.013 | 3 | |
| Right | Inferior frontal gyrus | 45 | 72, 27, 12 | 5.257 | <0.001 | <0.001 | 4 | |
| Right | Inferior frontal gyrus | 46 | 63, 36, 6 | 4.426 | <0.001 | 0.014 | 4 | |
| Right | Cuneus | 18 | 9, –93, 21 | 4.94 | <0.001 | 0.002 | 5 | 3,537 |
| Right | Cuneus | 17 | 9, –96, 9 | 4.617 | <0.001 | 0.006 | 5 | |
| Right | Superior frontal gyrus | 6 | 30, 6, 72 | 4.453 | <0.001 | 0.013 | 6 | 1,890 |
| Left | Postcentral gyrus | 7 | –15, –48, 78 | 4.413 | <0.001 | 0.015 | 7 | 3,240 |
| Left | Middle frontal gyrus | 46 | –57, 36, 24 | 4.524 | <0.001 | 0.01 | 8 | 2,538 |
| Right | Superior temporal gyrus | 38 | 33, 9, –42 | 4.275 | <0.001 | 0.025 | 9 | 1,377 |
| Left | Inferior temporal gyrus | 37 | –69, –51, –9 | 4.794 | <0.001 | 0.003 | 10 | 1,485 |
| Right | Superior parietal lobule | 7 | 36, –75, 57 | 4.217 | <0.001 | 0.032 | 11 | 1,890 |
| Right | Inferior frontal gyrus | 47 | 45, 24, –30 | 4.291 | <0.001 | 0.024 | 13 | 675 |
| Left | Superior parietal lobule | 7 | –12, –69, 66 | 4.418 | <0.001 | 0.015 | 14 | 918 |
| Left | Precuneus | 7 | –15, –72, 66 | 4.299 | <0.001 | 0.023 | 14 | |
| Left | Superior frontal gyrus | 6 | –12, –9, 78 | 4.119 | <0.001 | 0.045 | 18 | 702 |
| Right | Supramarginal gyrus | 40 | 42, –42, 36 | 4.47 | <0.001 | 0.012 | 19 | 567 |
TABLE 1. PTSD compared with neutral correlation with cognitive-behavioral therapy response among female participants with a history of sexual or physical traumaa
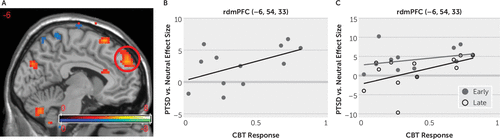
FIGURE 2. Correlations between cognitive-behavioral therapy (CBT) treatment response and activations in the rostral dorsomedial prefrontal cortex (rdmPFC) in response to PTSD-related words relative to neutral words among female participants with a history of sexual or physical traumaa
a Panel A shows a sagittal slice (x=−6) with a t-statistic presented with an initial voxel-wise threshold p value <0.001. Reds and yellows represent a positive correlation, while blues and greens represent a negative correlation. The scatter plots in panels B and C show the correlations at the peak voxel for the rostral dorsomedial prefrontal cortex (rdmPFC) cluster (illustrated with the circle in panel A). Panel C shows the correlations separately for the early and late portions of the task, demonstrating a stronger correlation during the late part of the experiment. The x-axis represents the normalized treatment response, with higher numbers indicating greater symptom improvement. PTSD=posttraumatic stress disorder
A trend toward a positive correlation was observed in the dACC and a negative correlation in the ventromedial prefrontal cortex (vmPFC), neither of which survived whole-brain correction. This effect was driven by the early part of the task.
Discussion
While the dACC (among other regions) has been previously highlighted in the literature on PTSD treatment response, activations in the dmPFC have received little attention as mediators of CBT response. Here, we showed that stronger activation in the rostral dmPFC in response to the reading of trauma-related words prior to treatment with CBT correlated with better response to treatment.
The portions of the medial prefrontal cortex that are dorsal and rostral to the anterior cingulate cortex, comprising the medial aspects of Brodmann’s areas 6, 8, 9, and 10, are often referred to as the dmPFC or medial frontal gyrus. The dmPFC can be further subdivided into regions with distinct functional associations. The most posterior portions of the dmPFC are concerned with motor functions and include the supplementary motor area (SMA) and pre-SMA. Anterior to this region is the mid-dmPFC, which is involved in internal monitoring of action, is particularly sensitive to error or conflict, and is involved in the regulation of behavior by monitoring the value of potential future actions (16). Our results point toward a region of the dmPFC anterior to the mid-dmPFC, referred to here as the rdmPFC, which encompasses the medial portions of Brodmann’s areas 9 and 10 and is most closely linked with social cognition. This area has not been closely associated with emotion down-regulation as have other areas of the prefrontal cortex, such as the vmPFC, which makes strong connections with the amygdala and other limbic regions and is implicated in emotion regulation, particularly in the context of extinction of a learned threat response (2, 17).
Although there are few direct connections between the dmPFC and amygdala, the dmPFC has been invoked (along with other brain areas such as the dorsolateral PFC and dACC) in reappraisal, a method of emotion regulation whereby a deliberate effort is made to alter the emotional interpretation of an emotionally laden stimulus. However, meta-analyses have suggested that it is primarily the mid-dmPFC rather than the rdmPFC that subserves this function (18–21). Why, then, might activation in the rdmPFC in response to reading trauma-related words correlate with a better response to subsequent CBT? We propose that differences in the degree of activation in this region among patients with PTSD may reflect an individual’s capacity for introspection and emotional self-awareness, psychological characteristics likely to mediate treatment response.
Among all PTSD patients in our sample about to undergo CBT, the rdmPFC was activated in response to trauma-related words but not in response to neutral words at the group level. Positive words and panic disorder-related words were activated in this region more than neutral words but less than trauma-related words. Taken together, these findings suggest that the rdmPFC may respond more strongly to personally relevant emotion stimuli. Although the stimuli were standardized across participants and were not specific to an individual’s personal history, they were chosen to reflect the experiences and preoccupations of patients with PTSD related to sexual and/or physical trauma, a history common to all of the participants in this study. The panic disorder-related words would be expected to be less personally relevant to this population. The particular relevance of the PTSD-related words may evoke stronger self-reflection compared with the other categories of stimuli.
Previous studies have demonstrated that the rdmPFC is activated by a variety of types of emotion and nonemotion tasks that involve mentalizing, or the meta-representation of one’s own or another’s mental processes or attributes (16). For example, while viewing affectively charged photographs, the rdmPFC was activated when individuals reflected on their own feelings or on the feelings of the central character in the picture but not when asked to make a determination about whether the same pictures were taken outdoors or indoors (22, 23). In a task in which participants were asked to either up-regulate or down-regulate their negative emotions in response to aversive images, the rdmPFC was activated with up-regulation but not with down-regulation (24), again arguing against a direct role for this region in down-regulation of negative emotion. The activation in the rdmPFC in response to up-regulation of negative emotion in that study may have been due to the self-focused method by which half of the participants were taught in order to increase negative emotion: they were instructed to imagine themselves in the negative situation depicted in the picture. By contrast, the method by which they were taught to decrease negative emotion involved viewing the image from a more detached perspective, in which self-referential processes are expected to be less engaged. Thus, the effect of that task on the rdmPFC may have related more to the differing degree of self-examination between the two tasks rather than on the directionality of emotional self-regulation The rdmPFC demonstrates greater activity when individuals make judgments about self-attributes (mental or otherwise) compared with impersonal semantic judgments (25) or judgments about the accuracy of impersonal factual statements (26), further supporting a role for this region in self-referential processing. The rdmPFC also tends to be relatively active during the resting state (27, 28), a finding that has been attributed to the types of self-generated mental activities that occur spontaneously under unconstrained resting conditions, many of which are likely introspective or self-referential (29). Whitfield-Gabrieli and colleagues (30) found an overlap in the rdmPFC between the self-referential network and the resting-state default-mode network.
The rdmPFC is also activated by tasks that require participants to make inferences about the attributes (25) and mental states (31, 32) of other people, and even of dogs (33), suggesting a role for this region in social cognition. Taken together, the previous literature suggests that the rdmPFC is involved with meta-representation of one’s own mind or the minds of others and supports cognitive functions such as self-examination, introspection, and awareness of one’s emotional state. In fact, patients with alexithymia, a condition characterized by difficulty identifying and describing one’s own feelings and emotions, demonstrated reduced activation in the rdmPFC in response to a theory-of-mind task (34).
The STAIR treatment module, which precedes the exposure module, aims to help patients become more aware of their emotional responses during emotional events and recall of traumatic memories (35). During the STAIR phase of therapy, patients practice skills, including identifying and labeling feeling states and identifying and altering interpersonal schemas (36), to enhance their capacity to use exposure therapy. It is possible, based on the evidence reviewed above, that the rdmPFC supports an individual’s capacity to practice such skills. Some research indicates that treatments that capitalize on patients’ strengths produce better outcomes (37). Thus, patients who already have the ability to reflect on their emotions may benefit more from therapies that include self-reflective exercises, and this may be an avenue for future investigation. It remains to be seen whether individuals who have less reflective capacity can benefit more from a therapy that teaches them these skills as opposed to therapies that use more concrete behavioral strategies that may be more consistent with current skills or preferences.
Individuals differ in their ability to reflect upon their own emotions, and these intrinsic characteristics could inform the type of psychotherapy recommended. We might predict that these differences in abilities would be supported by differences in the mediating functional neuroanatomy under relevant probe conditions. Ultimately, it is hoped that functional neuroimaging at the individual level, along with psychological profiling, will be clinically useful for the design or selection of an individualized psychotherapy program.
A previous study found that amygdala BOLD responses to these linguistic stimuli in PTSD patients compared with healthy control subjects change from the early part of the task to the late part of the task, highlighting variable degrees of habituation or sensitization in patients and in healthy control subjects, depending on the stimulus type (14). Because neural processes engaged by the stimuli change over time as the individual gains experience with the task and as the stimulus effects build, examining effects of experiment time on the BOLD responses can provide further insight into the nature of these processes. Interestingly, examination of the effects of experiment time on rdmPFC activation reveals that the correlation with treatment response is largely driven by the second half of the task. During the first half of the task, there was activation in the rdmPFC, even among poor responders, but they were less able than the stronger responders to sustain this response during the latter part of the task, and thus the rdmPFC activation during the late portion of the task demonstrated a stronger correlation with treatment response. This finding could potentially be explained by a tendency of the poorer responders (but not the stronger responders) to be unable to sustain their emotional engagement and self-reflection throughout the scanning session as a result of either fatigue or a tendency to dissociate under the stress of the repeated exposure to the emotionally distressing stimuli.
Limitations to this study include the small sample size and the fixed-effects analysis, which may limit the generalizability of the findings beyond the particular group studied. In particular, this study included only female participants with PTSD symptoms related to sexual or physical assault. It is unclear whether these findings will generalize to men or to PTSD related to other types of trauma. The lack of a control group comprised of individuals who did not undergo CBT makes it difficult to demonstrate the extent to which improvements in symptom scores were a result of treatment, but the efficacy of STAIR when combined with exposure therapy has been previously shown to be effective compared with exposure without STAIR in a randomized controlled trial (6). Although we surmise that the rdmPFC effect can be explained by an intrinsic capacity among the strong responders toward introspection and self-reflection in the context of negative emotion, we did not assess this behavioral characteristic in our participants. Future studies might use scales such as the Self-Consciousness Scale (38), the Self-Reflection and Insight Scale (39), or the Toronto Alexithymia Scale (40), to examine the extent to which these behavioral measures correlate with treatment response or rdmPFC activation.
Conclusions
In summary, individuals with PTSD related to sexual and/or physical assault demonstrate differential activation in the rdmPFC while reading words related to PTSD and sexual or physical trauma, and greater activation in this region prior to treatment is associated with a better response to CBT with STAIR/exposure therapy. We propose that rdmPFC activation during this task may be driven by self-referential thought and that the relationship between activation of this region and response to treatment may reflect a greater capacity to benefit from STAIR among individuals with intrinsically greater ability to mobilize neural resources for self-examination.
1 : Practice guideline for the treatment of patients with acute stress disorder and posttraumatic stress disorder . Am J Psychiatry 2004 ; 161 ( Suppl ): 3 – 31 Crossref, Medline, Google Scholar
2 : Fear extinction as a model for translational neuroscience: ten years of progress . Annu Rev Psychol 2012 ; 63 : 129 – 151 Crossref, Medline, Google Scholar
3 : Dissociating bottom-up and top-down mechanisms in the cortico-limbic system during emotion processing . Cereb Cortex 2016 ; 26 : 144 – 155 Crossref, Medline, Google Scholar
4 : Empirically supported psychological treatments for adult acute stress disorder and posttraumatic stress disorder: a review . Depress Anxiety 2009 ; 26 : 1086 – 1109 Crossref, Medline, Google Scholar
5 : The development of a Clinician-Administered PTSD Scale . J Trauma Stress 1995 ; 8 : 75 – 90 Crossref, Medline, Google Scholar
6 : Treatment for PTSD related to childhood abuse: a randomized controlled trial . Am J Psychiatry 2010 ; 167 : 915 – 924 Crossref, Medline, Google Scholar
7 : Amygdala and ventral anterior cingulate activation predicts treatment response to cognitive behaviour therapy for post-traumatic stress disorder . Psychol Med 2008 ; 38 : 555 – 561 Crossref, Medline, Google Scholar
8 : PTSD psychotherapy outcome predicted by brain activation during emotional reactivity and regulation . Am J Psychiatry 2017 ; 174 : 1163 – 1174 Crossref, Medline, Google Scholar
9 : Neural responses during emotional processing before and after cognitive trauma therapy for battered women . Psychiatry Res 2013 ; 214 : 48 – 55 Crossref, Medline, Google Scholar
10 : Predicting treatment outcome in PTSD: a longitudinal functional MRI study on trauma-unrelated emotional processing . Neuropsychopharmacology 2016 ; 41 : 1156 – 1165 Crossref, Medline, Google Scholar
11 : Amygdala response predicts trajectory of symptom reduction during trauma-focused cognitive-behavioral therapy among adolescent girls with PTSD . J Psychiatr Res 2015 ; 71 : 33 – 40 Crossref, Medline, Google Scholar
12 : Inhibitory neural activity predicts response to cognitive-behavioral therapy for posttraumatic stress disorder . J Clin Psychiatry 2013 ; 74 : 895 – 901 Crossref, Medline, Google Scholar
13 : Neural correlates of inhibition and contextual cue processing related to treatment response in PTSD . Neuropsychopharmacology 2015 ; 40 : 667 – 675 Crossref, Medline, Google Scholar
14 : Differential time courses and specificity of amygdala activity in posttraumatic stress disorder subjects and normal control subjects . Biol Psychiatry 2005 ; 57 : 464 – 473 Crossref, Medline, Google Scholar
15 : A general statistical analysis for fMRI data . Neuroimage 2002 ; 15 : 1 – 15 Crossref, Medline, Google Scholar
16 : Meeting of minds: the medial frontal cortex and social cognition . Nat Rev Neurosci 2006 ; 7 : 268 – 277 Crossref, Medline, Google Scholar
17 : Extinction learning in humans: role of the amygdala and vmPFC . Neuron 2004 ; 43 : 897 – 905 Crossref, Medline, Google Scholar
18 : Emotional processing in anterior cingulate and medial prefrontal cortex . Trends Cogn Sci 2011 ; 15 : 85 – 93 Crossref, Medline, Google Scholar
19 : The functional neuroanatomy of reappraisal: time matters . Neurosci Biobehav Rev 2009 ; 33 : 1215 – 1226 Crossref, Medline, Google Scholar
20 : Rethinking feelings: an FMRI study of the cognitive regulation of emotion . J Cogn Neurosci 2002 ; 14 : 1215 – 1229 Crossref, Medline, Google Scholar
21 : Bottom-up and top-down processes in emotion generation: common and distinct neural mechanisms . Psychol Sci 2009 ; 20 : 1322 – 1331 Crossref, Medline, Google Scholar
22 : Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function . Proc Natl Acad Sci USA 2001 ; 98 : 4259 – 4264 Crossref, Medline, Google Scholar
23 : Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other . J Cogn Neurosci 2004 ; 16 : 1746 – 1772 Crossref, Medline, Google Scholar
24 : For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion . Neuroimage 2004 ; 23 : 483 – 499 Crossref, Medline, Google Scholar
25 : Metacognitive evaluation, self-relevance, and the right prefrontal cortex . Neuroimage 2004 ; 22 : 941 – 947 Crossref, Medline, Google Scholar
26 : Neural correlates of self-reflection . Brain 2002 ; 125 : 1808 – 1814 Crossref, Medline, Google Scholar
27 : The human brain is intrinsically organized into dynamic, anticorrelated functional networks . Proc Natl Acad Sci USA 2005 ; 102 : 9673 – 9678 Crossref, Medline, Google Scholar
28 : Searching for a baseline: functional imaging and the resting human brain . Nat Rev Neurosci 2001 ; 2 : 685 – 694 Crossref, Medline, Google Scholar
29 : The resting state questionnaire: an introspective questionnaire for evaluation of inner experience during the conscious resting state . Brain Res Bull 2010 ; 81 : 565 – 573 Crossref, Medline, Google Scholar
30 : Associations and dissociations between default and self-reference networks in the human brain . Neuroimage 2011 ; 55 : 225 – 232 Crossref, Medline, Google Scholar
31 : Reading the mind in cartoons and stories: an fMRI study of “theory of mind” in verbal and nonverbal tasks . Neuropsychologia 2000 ; 38 : 11 – 21 Crossref, Medline, Google Scholar
32 : Children’s and adults’ neural bases of verbal and nonverbal “theory of mind” . Neuropsychologia 2007 ; 45 : 1522 – 1532 Crossref, Medline, Google Scholar
33 : General and specific contributions of the medial prefrontal cortex to knowledge about mental states . Neuroimage 2005 ; 28 : 757 – 762 Crossref, Medline, Google Scholar
34 : Impaired self-awareness and theory of mind: an fMRI study of mentalizing in alexithymia . Neuroimage 2006 ; 32 : 1472 – 1482 Crossref, Medline, Google Scholar
35 : Neural correlates of self-reflection in post-traumatic stress disorder . Acta Psychiatr Scand 2012 ; 125 : 238 – 246 Crossref, Medline, Google Scholar
36 : Therapeutic alliance, negative mood regulation, and treatment outcome in child abuse-related posttraumatic stress disorder . J Consult Clin Psychol 2004 ; 72 : 411 – 416 Crossref, Medline, Google Scholar
37 : The compensation and capitalization models: a test of two approaches to individualizing the treatment of depression . Behav Res Ther 2012 ; 50 : 699 – 706 Crossref, Medline, Google Scholar
38 : The Self-Consciousness Scale: a revised version for use with general populations . J Appl Soc Psychol 1985 ; 15 : 687 – 699 Crossref, Google Scholar
39 : The Self-Reflection and Insight Scale: a new measure of private self-consciousness . Soc Behav Personal 2002 ; 30 : 821 – 835 Crossref, Google Scholar
40 : The twenty-item Toronto Alexithymia Scale, I: Item selection and cross-validation of the factor structure . J Psychosom Res 1994 ; 38 : 23 – 32 Crossref, Medline, Google Scholar



