Delayed-Onset Poststroke Psychosis Presenting as Delusional Disorder
Stroke and psychosis are extremely disabling health conditions. Each year, 16 million people worldwide have their first-ever stroke; of these, five million become permanently disabled (1). Neuropsychiatric symptoms, such as depression, anxiety, fatigue, and apathy, are common sequelae of stroke, occurring in more than 30% of stroke survivors (1). The incidence of psychotic symptoms after stroke ranges from 1% to 5.3% (2). However, when present in patients with no previous psychiatric history, these symptoms tend to be underdiagnosed and undertreated, despite their association with poor functional outcomes (3). Some of the most common features of psychosis experienced by stroke survivors are delusions (defined as fixed beliefs that are not amenable to change in light of conflicting evidence), hallucinations (defined as abnormal perceptions that are not experienced by others), and mood changes with psychotic features (i.e., major depressive disorder and bipolar disorder) (4, 5).
Here, we present a rare case in which poststroke psychosis following right-hemispheric stroke took the form of a complex delusion that combined Othello syndrome (a delusion of jealousy) with a visuospatial delusion about the structure in the patient’s home. While uncommon, this delusion exemplifies the unusual psychoses that may arise from damage to the nondominant hemisphere (6).
CASE REPORT
A 53-year-old woman presented to our hospital with a 6-week history of progressive visual hallucinations and delusions. She had no past history of psychiatric illness, but 7 months prior, she had a large ischemic stroke in the right middle cerebral artery (MCA) region (Figure 1), which was complicated by hemorrhagic conversion and cerebral edema requiring hyperosmolar therapy. She had one principal delusion involving her husband’s infidelity with a “neighbor health care worker.” She believed that her neighbor had access to her husband via a hole in the wall that connected their two apartments and that she had placed hospital beds in the apartments of the other building tenants. In addition, she had hallucinations of strangers (who she believed had entered her bedroom through the connecting hole), as well as a small child, in her bed, and this was proof of her husband’s infidelity.
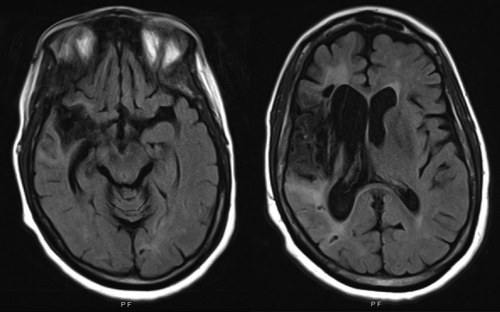
a MRI of the brain (fluid-attenuated inversion recovery sequence shown on left) at presentation 7 months after original infarct demonstrates volume loss and gliosis in the right temporal and frontal lobes, as well as the insula, and deep gray matter consistent with chronic right middle cerebral artery territory infarct. Diffusion-weighted imaging sequence did not demonstrate any new infarct.
On presentation, the patient had sinus tachycardia in the 100s and systolic blood pressure in the 140s, in addition to being afebrile. She was alert, pleasant, and well groomed. She denied depressed mood, anxiety, suicidal ideation, homicidal ideation, and auditory hallucinations. On mental status examination, she was alert, interactive, and oriented to person, place, and time. Her attention was mildly impaired, with ability to calculate serial 7s up to two calculations. Her speech was fluent without paraphasic errors or word-finding difficulties, and she had normal fund of knowledge, but her memory was impaired, with 0/5 correct items on delayed recall but 5/5 correct items with cuing. On the Montreal Cognitive Assessment (MoCA), she scored 19/30 initially and 26/30 on repeat testing several days later. Her cranial nerve examination was notable for intact visual fields bilaterally and a left-sided facial droop. On motor examination, she had left hemiparesis with upper motor neuron signs, a normal sensory examination, and no resting tremor, cogwheeling rigidity, or bradykinesia. A laboratory work-up, including urine toxicology, was unremarkable, and an MRI of the brain redemonstrated chronic ischemic changes in the right MCA region, with no acute findings. We initiated treatment with risperidone (0.75 mg nightly), and several days later, her delusions and hallucinations remitted. The patient followed up with outpatient neuropsychiatry at discharge.
DISCUSSION
Poststroke psychosis is more prevalent than previously recognized (5). It is associated with poorer functional outcome and increased mortality among stroke victims, although the reason for this additional risk is currently unknown (1, 5). A retrospective cohort study found that patients with poststroke psychosis compared with stroke patients without psychiatric disorders were 51% more times likely to die during a 10-year follow-up period (7). In this same study, patients with poststroke psychosis were found to have the lowest survival rate compared with stroke survivors with other psychiatric disorders, with cardiovascular disease being the leading cause of death.
Poststroke neuropsychiatric symptoms may have a negative effect on social and motor function, as well as on overall quality of life (3). These effects are independent of the primary neurologic sequelae of stroke itself and may influence rehabilitation and community reintegration efforts among stroke survivors. The most common symptoms are depression, anxiety, fatigue, and apathy; however, poststroke psychosis is more common than originally thought and is associated with a higher mortality rate (7). In a retrospective cohort study of 1,008 individuals that compared case subjects with poststroke psychosis to those without a poststroke psychiatric disorder, the investigators found that individuals in the former group were 51% more likely to die during a 10-year follow-up period (7). While poststroke psychosis is strongly associated with poor functional and mortality outcomes, more research on this entity is needed.
Right-hemispheric lesions cause syndromes such as hemineglect, spatial disorientation, visual perceptual disturbances, and anosognosia (8). Damage to the right hemisphere has also been strongly implicated in poststroke psychosis and poststroke delusional disorder. In one study, out of 134 patients with poststroke psychosis for whom lesion localization was described, 106 (79%) had right-hemispheric lesions, with the right parietal lobe affected most frequently (9). Among a series of several patients with right-hemispheric infarcts, size or location of the lesion was not associated with development of a delusional disorder, but underlying global atrophy appeared to increase the risk (7). Delusions described in these patients include, most commonly, delusions of persecution, delusions of infidelity (Othello syndrome), and reduplicative paramnesia.
There have been various proposed mechanisms for right-hemisphere lesion-related delusions, including the existence of preexisting psychiatric disease, delirium, or neurocognitive disorder, resulting in a “two-hit” sequence following acute brain injury (10); overactivity of preserved left-hemisphere areas unleashing a “creative narrator” leading to excessive and false explanations (10); disconnection between the limbic complex (especially the amygdala) and temporal and/or frontal cortices (areas of ventral stream processing) (11); and disruption of functional connectivity between the left retrosplenial cortex and right frontal cortex (12). Given our patient’s lack of psychiatric history, no clear signs or symptoms of delirium, and normal MoCA scores on repeat testing arguing against a major neurocognitive disorder, the proposed “two-hit” hypothesis appears less likely in this case. Left hemisphere overactivity and disconnection hypotheses are more explicative, especially considering that our patient’s right MCA stroke caused significant injury to her right medial temporal lobe structures, including the perihippocampal region and the amygdala (Figure 1).
While neurophysiologic bases of poststroke depression are better understood and are thought to involve inflammatory processes, genetic and epigenetic variations, white matter disease burden, cerebrovascular deregulation, altered neuroplasticity, and changes in glutamate neurotransmission, neuroanatomic and neurophysiologic explanations for poststroke delusional syndrome are less clear (13). Several neuroanatomic explanations have been proposed for this association. One theory is that the right hemisphere may function to prevent delusional beliefs from becoming established, by including the formation of cohesive narratives, the detection of anomalous or unusual occurrences, and the reshaping of one’s beliefs according to novel information (14). Another theory proposes that while misperceptions may be the initial cause of delusional beliefs, delusions only become established when damage to the right frontal lobe causes defects in the “belief evaluation system” (15). In our patient, stroke-related misperception of her physical surroundings, that is, a perceived absence of continuity in the walls of her apartment, may have given rise to a theory of her husband’s infidelity. Additionally, delusional jealousy has been described as a rare, isolated sequela of right cerebral infarctions even though there is no specific location for jealousy delusions, with frontal, posterior parietal, striatal, and thalamic lesions being implicated (2, 16, 17).
Interestingly, our patient experienced visual hallucinations accompanying her delusion, in spite of the fact that she did not have a visual field deficit. Hallucinations are distinguished from hallucinosis, in which unreal perceptions arise, but insight is preserved. These occur in substance use, as well as in strokes in certain areas of the brain. For instance, peduncular hallucinosis can result from lesions in the midbrain, pons, or thalamus and is characterized by nocturnal visual phenomena (18), while infarction in unilateral or bilateral occipital lobes can cause Charles Bonnet syndrome, where hallucinosis is associated with visual field deficits (19). However, lesions in the right temporal, parietal, and occipital lobes predispose stroke patients to hallucinations, where patients lack insight that their perceptions are false (20).
Our patient developed symptoms several months after her stroke. Unlike some of the more common neuropsychiatric sequelae of stroke, delayed onset of poststroke psychosis has been shown to be a relatively common presentation (5). While the majority of case reports describe psychosis appearing within the first week of the stroke, other case reports have demonstrated that poststroke psychosis can occur months after a vascular event (21). It is unclear whether delayed-onset psychosis suggests a window for early intervention at this time.
While our patient’s psychosis improved with antipsychotic medication, there are no clear guidelines on how long this treatment should be continued or also whether her symptoms will recur over time, with or without treatment. Available literature is not clear on the duration of antipsychotic treatment or on whether there are differences in early-onset and delayed-onset presentations of poststroke psychosis. Fortunately, the most common treatment outcome appears to be complete resolution of psychosis, with an average time interval to resolution being 3.5 months (5). However, given the associated morbidity and mortality noted above, future prospective studies should focus on the course and outcome of poststroke psychosis. We are not aware of any clinical trials for prevention or treatment of poststroke psychosis. For now, cross-specialty collaboration between neurologists, psychiatrists, and physiatrists may be the best approach to develop appropriate identification and management guidelines for poststroke neuropsychiatric psychotic syndromes.
1. : Neuropsychiatric outcomes of stroke. Lancet Neurol 2014; 13:525–534Crossref, Medline, Google Scholar
2. : Delusional state following acute stroke. Neurology 2004; 62:110–113Crossref, Medline, Google Scholar
3. : The neuropsychiatry of stroke. Psychosomatics 2000; 41:5–14Crossref, Medline, Google Scholar
4.
5. : Poststroke psychosis: a systematic review. J Neurol Neurosurg Psychiatry 2018; 89:879–885Crossref, Medline, Google Scholar
6. : The role of the right inferior frontal gyrus in the pathogenesis of post-stroke psychosis. J Neurol 2014; 261:600–603Crossref, Medline, Google Scholar
7. : Mortality associated with incident mental health disorders after stroke. Aust N Z J Psychiatry 2007; 41:274–281Crossref, Medline, Google Scholar
8. :
9. : The anatomic basis of delusions after right cerebral infarction. Neurology 1984; 34:577–582Crossref, Medline, Google Scholar
10. : Delusional misidentifications and duplications: right brain lesions, left brain delusions. Neurology 2009; 72:80–87Crossref, Medline, Google Scholar
11. : Capgras syndrome: a novel probe for understanding the neural representation of the identity and familiarity of persons. Proc Biol Sci 1997; 264:437–444Crossref, Medline, Google Scholar
12. : Finding the imposter: brain connectivity of lesions causing delusional misidentifications. Brain 2017; 140:497–507Crossref, Medline, Google Scholar
13. : Post-stroke depression: a review. Am J Psychiatry 2016; 173:221–231Crossref, Medline, Google Scholar
14. : Delusions and the right hemisphere: a review of the case for the right hemisphere as a mediator of reality-based belief. J Neuropsychiatry Clin Neurosci 2017; 29:225–235Link, Google Scholar
15. : Schizophrenia and monothematic delusions. Schizophr Bull 2007; 33:642–647Crossref, Medline, Google Scholar
16. : Neuroimaging correlates of chronic delusional jealousy after right cerebral infarction. J Neuropsychiatry Clin Neurosci 2008; 20:245–247Link, Google Scholar
17. : Othello syndrome after cerebrovascular infarction. J Neuropsychiatry Clin Neurosci 2014; 26:E1–E2Link, Google Scholar
18. : Visual hallucinations following a left-sided unilateral tuberothalamic artery infarction. Innov Clin Neurosci 2011; 8:31–34Medline, Google Scholar
19. : Complex visual hallucinations following stroke: epileptic origin or a deafferentation phenomenon? J Cerebrovasc Dis Stroke 2014; 1:1005–1007Google Scholar
20. : Post-stroke hallucinatory delusional syndromes. Neuropsychiatry Neuropsychol Behav Neurol 1992; 5:114–118Google Scholar
21. : Delayed-onset post-stroke delusional disorder: a case report. Behav Neurol 2013; 27:287–291Crossref, Medline, Google Scholar



