Kisspeptin in the Limbic System: New Insights Into Its Neuromodulatory Roles
Information regarding the functions of kisspeptin (KP) in the processing of emotions, cognition, and the links between reproduction and the limbic system is emerging. In fact, KP has been recently regarded as a behavioral hormone influencing multiple structures within the limbic neural network (19, 22–24), including the hypothalamic-pituitary-gonadal (HPG) and hypothalamic-pituitary-adrenal neuroendocrine axes. These neuroendocrine circuits dictate the regulatory mechanisms of important signaling neurotransmitters and hormones (i.e., gonadal steroids and stress hormones) (25, 26). In the central nervous system (CNS), KP functions as a central endocrinological regulator for sexual development and human reproductive functions (27, 28).
Neurochemistry of KP
KP is a member of the neuropeptide family originating from the cleavage of a 145-amino acid precursor peptide encoded by the KISS1 gene, originally identified as a metastasis suppressor gene (5). This gene produces the 54-amino acid peptide (metastin or KP-54), which can be cleaved into shorter forms (i.e., KPs 14, 13, and 10) with comparable potencies (6) (Figure 1). Its function is contingent on binding to its cognate receptor (KISS1R), first known as the orphan G-protein-coupled receptor 54 (GPR54) protein (29). Herein, nomenclature regarding KISS1 and KISS1R is used in reference to the human genes, proteins, and their various gene products collectively known as KPs, as established by the Human Genome Organization Gene Nomenclature Committee (30).
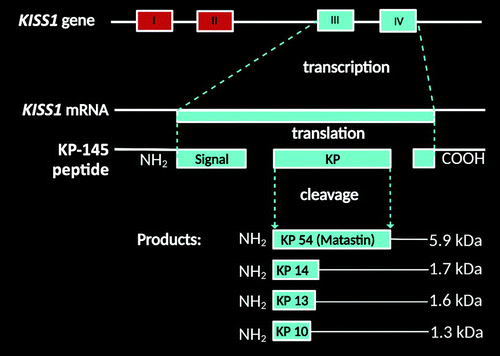
FIGURE 1. Kisspeptin (KP) isoforms in humans based on prior reports and UniProtKB data (1–4). KP accounts for a member of the neuropeptide family originating from the cleavage of a 145-amino acid precursor peptide encoded by the KISS1 gene, originally identified as a metastasis suppressor gene (5). This gene produces the 54-amino acid peptide (metastin or KP-54), which can be cleaved into shorter forms or functional fragments (i.e., KPs 14, 13, and 10) with comparable potencies (6). COOH=carboxy terminal; kDa=protein product size; NH2=amino terminal. All images created under the terms of the Creative Commons Attribution License and created with BioRender.com.
An essential component of reproduction, KP plays a fundamental role in the initiation of puberty and sexual maturation via the regulation of the HPG axis (29, 31–34). In humans, KP neurons are concentrated in the hypothalamus within the preoptic area and the infundibular nucleus (analogous to the rostral periventricular region of the third ventricle and arcuate nucleus in rodents), which are rich in gonadotrophin-releasing hormone (GnRH) cells expressing KISS1R (7, 8) (Figure 2A). Essentially, when GnRH neurons are activated by KP, they secrete GnRH, which triggers the release of gonadotropins, luteinizing hormone, and follicle-stimulating hormone from the adenohypophysis. This, in turn, triggers the release of the gonadal sex steroids (e.g., estrogens and progesterone) (9, 10) (Figure 2A).
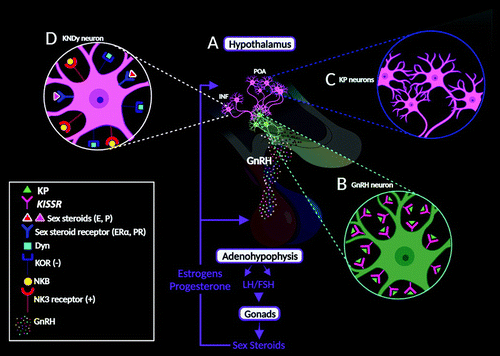
FIGURE 2. The empirical neuroanatomy and the kisspeptin (KP) gonadotrophin-releasing hormone (GnRH) pathway and the relationship between KP, neurokinin B (NKB), dynorphin (Dyn), and GnRH-secreting neurons in humans. In humans (A and B), KP neurons are concentrated in the hypothalamus within the preoptic area (POA) and the infundibular (INF) nuclei (analogous to the rostral periventricular region of the third ventricle and arcuate nucleus in rodents), which are rich in GnRH cells expressing the G-protein-coupled receptor-54 protein (GPR54, KISS1R) (7, 8). When GnRH neurons are activated by KP, they secrete GnRH, which triggers the release of gonadotropins, luteinizing hormone (LH), and follicle-stimulating hormone (FSH) from the adenohypophysis. In turn, this cascades the release of the gonadal sex steroids (i.e., estrogens, progesterone, and testosterone), forming feedback loops to regulate GnRH secretion from the hypothalamus, as well as LH, and FSH release from the adenohypophysis (9, 10). In the POA (C), the KP neurons are more numerous in females and provide input to a higher percentage of GnRH neurons (11, 12). In the INF nucleus (D), the KP cells also synthesize Dyn and NKB, thus named KP/NKB/Dyn (KNDy) neurons (13, 14). Dyn is an opioid, functioning at the kappa opioid receptor (KOR) system (15). NKB is an endogenous peptide that belongs to the family of tachykinins (12). Theoretically, NKB starts and/or accelerates synchronized KNDy neuronal responses via the neurokinin-3 (NK3) receptors (stimulatory [+]) to release KP, resulting in GnRH release via the stimulation of KISSR expressed by the GnRH neurons. Lastly, Dyn released from KNDy neurons halts KNDy stimulation via KOR (inhibitory [–]) (16, 17). E=estrogen; ERα=estrogen receptor α; P=progesterone; PR=progesterone receptor.
Studies of both animals and humans indicate that the central and peripheral administration of exogenous KP leads to the hormonal stimulation of this reproductive cascade (35, 36). KP and GnRH neurons are positioned at the upper tier of the HPG neuroendocrine axis (Figure 2B and 2C). The administration of GnRH antagonists halts the stimulatory effect of KP (37). Similar to luteinizing hormone, KP exhibits synchronized pulsatile secretions within the hypothalamus, confirming its role as a GnRH pulse generator in mammalian species, including humans (36, 38–41). However, while KP is an essential element for GnRH and luteinizing hormone rhythm control, its pulsatility per se is not required for the generation of these pulses (40). Given these characteristics and influences on neuroendocrine function, KP and its signaling pathways are potential therapeutic targets for sex hormone disorders and other endocrinologic conditions (40, 42).
Sexual Dimorphism
The KP neurons of the preoptic area and infundibular regions exhibit conspicuous anatomical variations in humans, with sexual dimorphisms being some of the most thought provoking. These sexual dimorphisms are responsible for differential behaviors, as regulated by the HPG axis between genders (3). In the preoptic area, the KP neurons are more numerous in females and provide input to a higher percentage of GnRH neurons (11, 12) (Figure 2C). In the infundibular nucleus, the KP cells also synthesize dynorphin (Dyn) and neurokinin B (NKB), and thus they are named KP/NKB/Dyn (KNDy) neurons (13, 14). Dyn is an opioid functioning in the kappa opioid receptor system (15). NKB is an endogenous peptide that belongs to the family of tachykinins (12). Tachykinins are encoded by the tachykinin-3 (TAC3) gene and act at the neurokinin-3 receptor, which is also encoded by the TAC3 gene (14, 43). To date, both NKB and Dyn peptides are regarded as cotransmitters of the KP signaling pathways (i.e., human reproduction) (3) (Figure 2D). In addition, human KP neurons coexpress other peptides, such as substance P and cocaine- and amphetamine-regulated transcripts (44, 45). The coexistence of these neurotransmitters indicates a multimodal role, with KP modulating various behavioral processes (2).
In adults, the infundibular nucleus KP neurons also exhibit a female-dominant sex difference among heterosexuals, as well as male-to-female transexuals (12, 46, 47). Results from animal and human studies indicate that KNDy neurons exert an important function in the regulation of the negative feedback effects of sex steroid hormones on GnRH neurons, regulating luteinizing hormone pulsatility and gonadotropins release (14, 48). Taken altogether, KP sex differences add to a comprehensive body of evidence on the sexual dimorphism of the HPG axis in humans (3).
KISS1 Gene/KISS1R Distribution in the CNS
While KP influences hypothalamic-mediated functions (e.g., reproduction, energy balance, food intake, and metabolism), the KISS1 gene is expressed in other locations of the human brain (2, 3, 49, 50). KISS1 has been identified in the amygdala, caudate, cingulate, globus pallidus, hippocampus, medial and superior frontal gyri, nucleus accumbens, parahippocampal gyrus, substantia nigra, putamen, thalamus, and spinal cord (2, 12, 22, 29, 34, 51). KP’s influence on behavior extends from the hypothalamus to other limbic structures and beyond, mediating (in part) anxiety, fear (as well as other emotions), and olfaction (18, 19, 22, 51).
KP in the Limbic System
Habenula.
The habenula is recognized as a phylogenetically preserved diencephalic-paired neuroanatomical structure that is present in virtually all vertebrates (52). The mammalian habenula comprises two important cellular groups or subnuclei: the medial habenula (MHb) and the lateral habenula (LHb). The medial component fibers (dopaminergic) connect with the interpeduncular nucleus, while the lateral cell group fibers (serotonergic) project to the ventral tegmental area (VTA) and raphe, respectively (52). In humans, the habenula has an approximate diameter of 5–9 mm and a total volume of 30–36 mm3 (53).
The habenula is an important component of the emotion centers of the brain, contributing to the modulation of a wide repertoire of emotions, such as fear, reward, anxiety, and depression (54, 55). Its neuroanatomical circuits include inputs from the limbic system, including the basal ganglia, as well as outputs to the midbrain, releasing dopamine (from the pars compacta of the substantia nigra and VTA) and serotonin (from the median and dorsal raphe nuclei, respectively). Thus, the habenula is involved in two major neuromodulatory monoaminergic pathways of the midbrain: the dopaminergic and serotoninergic systems (54, 55). The habenula appears to function as a processing center for emotional and aversive responses, including aversively motivated learning and emotional decision making (55–58). In addition, it appears to have an important modulatory role in the perception of pain and certain mechanisms of analgesia (59).
Emotional decision-making processes are associated with important physiological elements of reproduction (i.e., sex steroids and stress hormones) (36, 40, 41). Habenular inputs from sex centers in the hypothalamic region potentiate key hormonal regulators via the HPG axis and KP circuitry (58). Sex-hormone alterations during menopausal and pre- to postpartum transitions may lead to symptoms of depression and altered emotional processing (60). Disturbances of the habenula may contribute (in part) to psychiatric conditions, such as addiction, attention-deficit hyperactivity disorder, major depression, and schizophrenia (55, 61–64). Moreover, results from a human brain study (postmortem histological tissues) revealed less neurons in the habenula and decreased volumes of both MHb and LHb in persons diagnosed with major depression or bipolar disorder (65).
There are no significant sex differences in the structural anatomy of the habenula in humans. However, there are some important sexually dimorphic traits documented in the literature. For example, animal studies have revealed molecular dimorphisms in the expression of neurotransmitters, neuropeptides, and other neuroactive substances (e.g., glutamate, vasopressin, and tachykinins) (58). Additionally, the habenula exhibits functional sexual dimorphisms in response to stress, including sex differences in metabolic processes and neural activity (66–68). In humans, the sex differences in habenular stress responses are connected with increased susceptibility to stress-related disorders (i.e., anxiety and depression), as well as fluctuating sex steroids (69–71). Predictably, the habenula and KP have been implicated in the modulatory mechanisms of anxiety, fear, reward, and mood regulation (18, 19, 22, 51, 58, 72).
The LHb has a strategic anatomical location in the CNS, with pathways joining the forebrain to the ventral midbrain and hindbrain regions (73). Some investigators have described the LHb as the “antireward center” of the brain (74). Animal studies have revealed that it prevents behaviors leading to negative reward (i.e., punishment); however, it reinforces behaviors associated with positive reward (75, 76). Therefore, the LHb regulates the mechanisms of motivated behaviors and decision making (55). Social behaviors (e.g., avoidance, fighting, mating, and parenting) are important elements for communication in social animals (humans included), thereby critical for survival. These behaviors are regulated by neural circuits involving neuroanatomical structures (e.g., the habenula, amygdala, and prefrontal cortex), collectively referred to as the social behavior network (SBN), further integrating social decision making (77). The cellular components of the SBN are significantly influenced by sex steroids (estrogens, progesterone, and testosterone) and certain neuropeptides (i.e., KP, GnRH, and Neuropeptide Y) required for the regulation of social behaviors (77–82).
Amygdala.
KP and its cognate KISS1R gene are expressed in other key emotional structures of the limbic system, including the amygdala, in varied animal species (i.e., rodents) and humans (20). The sex differences in the amygdalar size appear to be ambiguous, with some studies indicating larger volumes in females and others reporting larger volumes in males (83–86). Despite these inconsistencies, the average volumetric size of the human amygdala ranges between 1.24 cm3 and 1.63 cm3 (87).
In addition to its roles in fear and anxiety, the amygdala also has an important role in reproduction, intervening over the release of gonadotrophic hormone, thus regulating essential mechanisms of reproductive physiology (88). Aside from its canonical involvement in fear, the regulation of reproductive and social behaviors in a sexually dimorphic manner has become a new center of attention in neurophysiology (24, 89). In humans, the peripheral administration of KP enhances functional MRI (fMRI) amygdalar activity in response to sexual and nonsexual contextual images among men (19). Similarly, men receiving peripheral intravenous KP injections exhibited higher fMRI-resting connectivity in the amygdala-cingulate circuit, indicating enhanced sexual and emotional processing (20).
Recently, the amygdala has been presented as an extrahypothalamic center, regulating body energy and metabolic homeostatic processes (90–92). There is an intimate connection between energy homeostasis and reproductive functions (90). Sex hormones are important metabolic modulators, capable of controlling foundational elements of energy homeostasis. Sex steroid dysregulation may lead to mental illnesses and eating disorders, such as anorexia nervosa, bulimia nervosa, and binge eating disorder (93–96).
Potential Therapeutic Implications
Evidence from human studies indicates that KP has possible clinical applications and therapeutic uses for psychiatric and sexual dysfunctions. For example, KP has been shown to improve brain activity, modulating the levels of gamma-aminobutyric acid in the brain and decreasing undesirable emotional responses (negative mood) and sexual aversion (18–20). Additionally, it improves neural processing by increasing attraction (perception of beauty) on the basis of olfactory (odor stimuli) and visual cues (21) (Figure 3). The role of KP in improving olfactory and limbic functions opens the possibility for its therapeutic potential in neurodegenerative conditions, such as Alzheimer’s disease and Parkinson’s disease, in which anosmia may precede the cognitive and motor dysfunctions (2, 97–99)
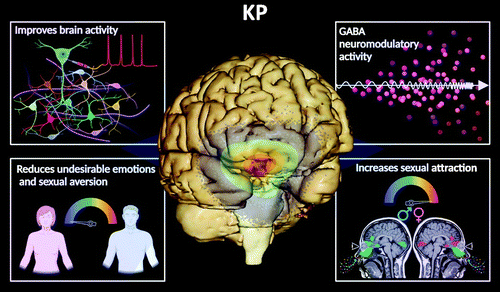
FIGURE 3. Effects of kisspeptin (KP). KP is a multifunctional neuropeptide present in the highlighted areas of the midbrain and other brain regions (spectrum of colors: yellow, green, and orange). KP has been demonstrated to improve brain activity, modulating the levels of gamma-aminobutyric acid (GABA) in the brain and decreasing undesirable emotional responses (negative mood) and sexual aversion (18–20). In addition, it improves neural processing by increasing sexual attraction (perception of beauty) on the basis of olfactory (odor stimuli) and visual cues (21). COVER. A three-dimensional virtual dissection of the human brain, emphasizing the location of the midbrain structures. Created under the terms of the Creative Commons Attribution License. Created with VH Dissector and BioRender.com.
Conclusions
KP within the limbic system is a critical neuropeptide in the regulation of reproduction and emotional behaviors. Within the hypothalamus, KP (together with the tachykinins, NKB, and Dyn) promotes the physiologic activity (i.e., oscillatory) dictating the pulsatile secretion of GnRH and synchronizing luteinizing hormone pulses; therefore, it is considered the central component of the GnRH pulse generator. In this role, KP acts as the “master regulator” of reproductive neurophysiology, integrating the emotional components of certain limbic circuits via the regulation of GnRH neurons and release of sex steroids.
The emerging information on behavioral neuroscience suggests an intriguing relationship between KP and other fundamental monoaminergic systems (aside from dopaminergic and serotonergic), potentially modulating other behavioral responses. The newly identified role of KP in these systems has potential implications for clinical and research neuropsychiatry, particularly in relation to modulating sexual and emotional behaviors in humans and its implications for psychiatric disorders associated with reproductive biological imbalances. Current innovations in neuroimaging technology and molecular biology present opportunities to advance our understanding of the multiple roles of KP in reproductive, neurodegenerative, emotional, and other neuropsychiatric disorders.
1. : UniProtKB. 2022. https://www.uniprot.org/uniprot/Q15726 Google Scholar
2. : Can the kisspeptin help us in the understanding of pathology of some neurodegenerative brain diseases? Folia Morphol (Warsz) 2021; 80:756–765Crossref, Medline, Google Scholar
3. : Kisspeptin signalling and its roles in humans. Singapore Med J 2015; 56:649–656Crossref, Medline, Google Scholar
4. : GPR54 and kisspeptin in reproduction. Hum Reprod Update 2006; 12:631–639Crossref, Medline, Google Scholar
5. : KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. J Natl Cancer Inst 1996; 88:1731–1737Crossref, Medline, Google Scholar
6. : GPR54 and kisspeptins. Results Probl Cell Differ 2008; 46:117–143Crossref, Medline, Google Scholar
7. : Hypothalamic kisspeptin and kisspeptin receptors: species variation in reproduction and reproductive behaviours. Front Neuroendocrinol 2022; 64:100951Crossref, Medline, Google Scholar
8. : Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J Clin Endocrinol Metab 2007; 92:2744–2750Crossref, Medline, Google Scholar
9. : The neuroanatomy of the kisspeptin system in the mammalian brain. Peptides 2009; 30:26–33Crossref, Medline, Google Scholar
10. : Neuroanatomy of the human hypothalamic kisspeptin system. Neuroendocrinology 2014; 99:33–48Crossref, Medline, Google Scholar
11. : Sexual dimorphism of kisspeptin and neurokinin B immunoreactive neurons in the infundibular nucleus of aged men and women. Front Endocrinol (Lausanne) 2011; 2:80 Medline, Google Scholar
12. : The kisspeptin system of the human hypothalamus: sexual dimorphism and relationship with gonadotropin-releasing hormone and neurokinin B neurons. Eur J Neurosci 2010; 31:1984–1998Crossref, Medline, Google Scholar
13. : Interactions between kisspeptins and neurokinin B. Adv Exp Med Biol 2013; 784:325–347Crossref, Medline, Google Scholar
14. : Investigating the KNDy hypothesis in humans by coadministration of kisspeptin, neurokinin B, and naltrexone in men. J Clin Endocrinol Metab 2016; 101:3429–3436Crossref, Medline, Google Scholar
15. : Dynorphin, stress, and depression. Brain Res 2010; 1314:56–73Crossref, Medline, Google Scholar
16. : Role of KNDy neurons expressing kisspeptin, neurokinin B, and dynorphin A as a GnRH pulse generator controlling mammalian reproduction. Front Endocrinol (Lausanne) 2021; 12:724632Crossref, Medline, Google Scholar
17. : The kisspeptin-GnRH pathway in human reproductive health and disease. Hum Reprod Update 2014; 20:485–500Crossref, Medline, Google Scholar
18. : Kisspeptin modulates gamma-aminobutyric acid levels in the human brain. Psychoneuroendocrinology 2021; 129:105244Crossref, Medline, Google Scholar
19. : Kisspeptin modulates sexual and emotional brain processing in humans. J Clin Invest 2017; 127:709–719Crossref, Medline, Google Scholar
20. : Modulations of human resting brain connectivity by kisspeptin enhance sexual and emotional functions. JCI Insight 2018; 3:e121958Crossref, Medline, Google Scholar
21. : Kisspeptin enhances brain responses to olfactory and visual cues of attraction in men. JCI Insight 2020; 5:e133633 Crossref, Medline, Google Scholar
22. : Emerging roles of kisspeptin in sexual and emotional brain processing. Neuroendocrinology 2018; 106:195–202Crossref, Medline, Google Scholar
23. : Kisspeptin as a behavioral hormone. Semin Reprod Med 2019; 37:56–63Crossref, Medline, Google Scholar
24. : The roles of the amygdala kisspeptin system. Semin Reprod Med 2019; 37:64–70Crossref, Medline, Google Scholar
25. : Hypothalamic-pituitary-end-organ axes: hormone function in female patients with major depressive disorder. Neurosci Bull 2021; 37:1176–1187Crossref, Medline, Google Scholar
26. : Hypothalamic-pituitary-adrenal and hypothalamic-pituitary-gonadal axes: sex differences in regulation of stress responsivity. Stress 2017; 20:476–494Crossref, Medline, Google Scholar
27. : Neuroanatomy of the kisspeptin signaling system in mammals: comparative and developmental aspects. Adv Exp Med Biol 2013; 784:27–62Crossref, Medline, Google Scholar
28. : The role of kisspeptin in sexual behavior. Semin Reprod Med 2019; 37:84–92Crossref, Medline, Google Scholar
29. : Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature 2001; 411:613–617Crossref, Medline, Google Scholar
30. Gene Symbol Report. Cambridge, United Kingdom, HUGO Gene Nomenclature Committee, 2021. https://www.genenames.org/data/gene-symbol-report/#!/hgnc_id/HGNC:4510. Accessed March 14, 2022 Google Scholar
31. : The GPR54 gene as a regulator of puberty. N Engl J Med 2003; 349:1614–1627Crossref, Medline, Google Scholar
32. : Inactivating KISS1 mutation and hypogonadotropic hypogonadism. Obstet Gynecol Surv 2012; 67:352–353 Crossref, Google Scholar
33. : A GPR54-activating mutation in a patient with central precocious puberty. N Engl J Med 2008; 358:709–715Crossref, Medline, Google Scholar
34. : The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem 2001; 276:34631–34636Crossref, Medline, Google Scholar
35. : Central and peripheral administration of kisspeptin-10 stimulates the hypothalamic-pituitary-gonadal axis. J Neuroendocrinol 2004; 16:850–858Crossref, Medline, Google Scholar
36. : Kisspeptin-54 stimulates the hypothalamic-pituitary gonadal axis in human males. J Clin Endocrinol Metab 2005; 90:6609–6615Crossref, Medline, Google Scholar
37. : A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology 2004; 145:4073–4077Crossref, Medline, Google Scholar
38. : Definition of the hypothalamic GnRH pulse generator in mice. Proc Natl Acad Sci USA 2017; 114:E10216–E10223Crossref, Medline, Google Scholar
39. : Kisspeptin and GnRH pulse generation; in Kisspeptin Signaling in Reproductive Biology. Edited by Kauffman AS, Smith JT. New York, Springer, 2013, pp 297–323. https://doi.org/10.1007/978-1-4614-6199-9_14. Accessed March 10, 2022 Crossref, Google Scholar
40. : The roles of kisspeptin and neurokinin B in GnRH pulse generation in humans, and their potential clinical application. J Neuroendocrinol (Online ahead of print, December 20, 2021)Google Scholar
41. : Kisspeptin-54 stimulates gonadotropin release most potently during the preovulatory phase of the menstrual cycle in women. J Clin Endocrinol Metab 2007; 92:3958–3966Crossref, Medline, Google Scholar
42. : Kisspeptin and the control of emotions, mood and reproductive behaviour. J Endocrinol 2018; 239:R1–R12Crossref, Medline, Google Scholar
43. : Tachykinins and tachykinin receptors: structure and activity relationships. Curr Med Chem 2004; 11:2045–2081Crossref, Medline, Google Scholar
44. : Substance P immunoreactivity exhibits frequent colocalization with kisspeptin and neurokinin B in the human infundibular region. PLoS One 2013; 8:e72369Crossref, Medline, Google Scholar
45. : Cocaine- and amphetamine-regulated transcript is a potent stimulator of GnRH and kisspeptin cells and may contribute to negative energy balance-induced reproductive inhibition in females. Endocrinology 2013; 154:2821–2832Crossref, Medline, Google Scholar
46. : Kisspeptin expression in the human infundibular nucleus in relation to sex, gender identity, and sexual orientation. J Clin Endocrinol Metab 2016; 101:2380–2389Crossref, Medline, Google Scholar
47. : Sex differences in the neurokinin B system in the human infundibular nucleus. J Clin Endocrinol Metab 2012; 97:E2210–E2220 Crossref, Medline, Google Scholar
48. : KiSS-1 messenger ribonucleic acid expression in the hypothalamus of the ewe is regulated by sex steroids and season. Endocrinology 2007; 148:1150–1157 Crossref, Medline, Google Scholar
49. : The role of kisspeptin neurons in reproduction and metabolism. J Endocrinol 2018; 238:R173–R183Crossref, Medline, Google Scholar
50. : Centrally injected kisspeptin reduces food intake by increasing meal intervals in mice. Neuroreport 2011; 22:253–257Crossref, Medline, Google Scholar
51. : AXOR12, a novel human G protein-coupled receptor, activated by the peptide KiSS-1. J Biol Chem 2001; 276:28969–28975Crossref, Medline, Google Scholar
52. : Biological significance of kisspeptin–kiss 1 receptor signaling in the habenula of teleost species. Front Endocrinol (Lausanne) 2018; 9:222Crossref, Medline, Google Scholar
53. : Habenula volume in bipolar disorder and major depressive disorder: a high-resolution magnetic resonance imaging study. Biol Psychiatry 2011; 69:336–343Crossref, Medline, Google Scholar
54. : [The structure and function of habenula]. Sheng Li Xue Bao 2017; 69:623–636Medline, Google Scholar
55. : The habenula: from stress evasion to value-based decision-making. Nat Rev Neurosci 2010; 11:503–513Crossref, Medline, Google Scholar
56. : The habenula encodes negative motivational value associated with primary punishment in humans. Proc Natl Acad Sci USA 2014; 111:11858–11863Crossref, Medline, Google Scholar
57. : Reward processing by the lateral habenula in normal and depressive behaviors. Nat Neurosci 2014; 17:1146–1152Crossref, Medline, Google Scholar
58. : Functions of habenula in reproduction and socio-reproductive behaviours. Front Neuroendocrinol 2022; 64:100964Crossref, Medline, Google Scholar
59. : Unmasking the mysteries of the habenula in pain and analgesia. Prog Neurobiol 2012; 96:208–219Crossref, Medline, Google Scholar
60. : Role of emotional processing in depressive responses to sex-hormone manipulation: a pharmacological fMRI study. Transl Psychiatry 2015; 5:e688Crossref, Medline, Google Scholar
61. : The lateral habenula in addiction and depression: an anatomical, synaptic and behavioral overview. Eur J Neurosci 2014; 39:1170–1178Crossref, Medline, Google Scholar
62. : The role of the habenula in drug addiction. Front Hum Neurosci 2014; 8:174Crossref, Medline, Google Scholar
63. : Dopaminergic circuitry and risk/reward decision making: implications for schizophrenia. Schizophr Bull 2015; 41:9–14Crossref, Medline, Google Scholar
64. : Habenula and ADHD: convergence on time. Neurosci Biobehav Rev 2013; 37:1801–1809Crossref, Medline, Google Scholar
65. : Evidence for structural abnormalities of the human habenular complex in affective disorders but not in schizophrenia. Psychol Med 2010; 40:557–567Crossref, Medline, Google Scholar
66. : Global sex differences in stress-induced activation of cerebral metabolism revealed by 2-deoxyglucose autoradiography. Horm Behav 1996; 30:611–617Crossref, Medline, Google Scholar
67. : Acute stress evokes sexually dimorphic, stressor-specific patterns of neural activation across multiple limbic brain regions in adult rats. Stress 2018; 21:136–150Crossref, Medline, Google Scholar
68. : Sex differences in the neuroadaptations of reward-related circuits in response to subchronic variable stress. Neuroscience 2018; 376:108–116Crossref, Medline, Google Scholar
69. : Dysregulation of the lateral habenula in major depressive disorder. Front Synaptic Neurosci 2018; 10:46Crossref, Medline, Google Scholar
70. : Sex differences in stress-related disorders: major depressive disorder, bipolar disorder, and posttraumatic stress disorder; in Handbook of Clinical Neurology. Edited by Lanzenberger R, Kranz GS, Savic I. Amsterdam, Elsevier, 2020, pp 335–358. https://www.sciencedirect.com/science/article/pii/B9780444641236000230. Accessed March 16, 2022 Google Scholar
71. : Why are women so vulnerable to anxiety, trauma-related and stress-related disorders? the potential role of sex hormones. Lancet Psychiatry 2017; 4:73–82 Crossref, Medline, Google Scholar
72. : The medial habenula and interpeduncular nucleus circuitry is critical in addiction, anxiety, and mood regulation. J Neurochem 2017; 142(suppl 2):130–143Crossref, Medline, Google Scholar
73. : Habenula: crossroad between the basal ganglia and the limbic system. J Neurosci 2008; 28:11825–11829Crossref, Medline, Google Scholar
74. : Circuits and functions of the lateral habenula in health and in disease. Nat Rev Neurosci 2020; 21:277–295Crossref, Medline, Google Scholar
75. : Translating the habenula: from rodents to humans. Biol Psychiatry 2017; 81:296–305Crossref, Medline, Google Scholar
76. : Peril and pleasure: an RDoC-inspired examination of threat responses and reward processing in anxiety and depression. Depress Anxiety 2014; 31:233–249Crossref, Medline, Google Scholar
77. : Role of habenula in social and reproductive behaviors in fish: comparison with mammals. Front Behav Neurosci 2022; 15:818782Crossref, Medline, Google Scholar
78. : The medial extended amygdala in male reproductive behavior: a node in the mammalian social behavior network. Ann N Y Acad Sci 1999; 877:242–257Crossref, Medline, Google Scholar
79. : The vertebrate social behavior network: evolutionary themes and variations. Horm Behav 2005; 48:11–22Crossref, Medline, Google Scholar
80. : Neuroendocrinology of social behavior. ILAR J 2009; 50:5–14Crossref, Medline, Google Scholar
81. : Neural and hormonal control of sexual behavior. Endocrinology 2020; 161:bqaa150Crossref, Medline, Google Scholar
82. : Physiological and therapeutic roles of neuropeptide Y on biological functions. Adv Exp Med Biol 2020; 1237:37–47Crossref, Medline, Google Scholar
83. : Sexual dimorphism in brain development: influence on affective disorders. J Neuropsychiatry Clin Neurosci 2021; 33:A485–A489 Link, Google Scholar
84. : A meta-analysis of sex differences in human brain structure. Neurosci Biobehav Rev 2014; 39:34–50 Crossref, Medline, Google Scholar
85. : Minute effects of sex on the aging brain: a multisample magnetic resonance imaging study of healthy aging and Alzheimer’s disease. J Neurosci 2009; 29:8774–8783Crossref, Medline, Google Scholar
86. : Sex differences and the impact of steroid hormones on the developing human brain. Cereb Cortex 2009; 19:464–473Crossref, Medline, Google Scholar
87. : Volumetry of the human amygdala: an anatomical study. Psychiatry Res 2010; 182:67–72Crossref, Medline, Google Scholar
88. : Optogenetic stimulation of kisspeptin neurones within the posterodorsal medial amygdala increases luteinising hormone pulse frequency in female mice. J Neuroendocrinol 2020; 32:e12823Crossref, Medline, Google Scholar
89. : Sexual behavior: from hormonal regulation to endocrine disruption. Neuroendocrinology 2018; 107:400–416 Crossref, Medline, Google Scholar
90. : Extrahypothalamic control of energy balance and its connection with reproduction: roles of the amygdala. Metabolites 2021; 11:837Crossref, Medline, Google Scholar
91. : Central amygdala circuits modulate food consumption through a positive-valence mechanism. Nat Neurosci 2017; 20:1384–1394 Crossref, Medline, Google Scholar
92. : Yummy or yucky? ask your central amygdala. Nat Neurosci 2017; 20:1321–1322 Crossref, Medline, Google Scholar
93. : Connecting metabolism and gonadal function: novel central neuropeptide pathways involved in the metabolic control of puberty and fertility. Front Neuroendocrinol 2018; 48:37–49Crossref, Medline, Google Scholar
94. : Estrogens and the control of energy homeostasis: a brain perspective. Trends Endocrinol Metab 2015; 26:411–421Crossref, Medline, Google Scholar
95. : Neuroanatomical framework of the metabolic control of reproduction. Physiol Rev 2018; 98:2349–2380Crossref, Medline, Google Scholar
96. : Gender-specific approach in psychiatric diseases: because sex matters. Eur J Pharmacol 2021; 896:173895Crossref, Medline, Google Scholar
97. : Olfactory dysfunction is associated with motor function only in tremor-dominant Parkinson’s disease. Neurol Sci (Online ahead of print, February 15, 2022). doi: 10.1007/s10072-022-05952-w Google Scholar
98. : Olfactory dysfunction in Parkinson disease. Nat Rev Neurol 2012; 8:329–339Crossref, Medline, Google Scholar
99. : Neuroprotective and therapeutic strategies against Parkinson’s disease: recent perspectives. Int J Mol Sci 2016; 17:904Crossref, Google Scholar



