Enigmatic Neural Pathways: Potentiating Viral Neuroinvasion Into the CNS
The blood-brain barrier (BBB) and blood-cerebrospinal fluid barrier (BCSFB) are exceptional anatomical structures and intricate physiological complexes providing functional and chemical stability to the central nervous system (CNS), including protection from pathogens such as viruses. Despite their extraordinary biochemical and protective efficiency, these structures are considered to be a major target for viral entry into the CNS (4, 6). Neurotropic viruses are categorized based on their unique tendency for invading and attacking the CNS (7). A caveat regarding the BBB is that its permeability is not infallible, and neurotropic viruses have evolved to interrupt and evade its shielding properties (8). A plethora of pathogenic viruses have been identified (e.g., arbovirus, bunyaviruses, cytomegalovirus, enterovirus, flaviviruses, herpes simplex virus, human immunodeficiency virus, human T-cell leukemia virus, influenza virus, lymphocytic choriomeningitis virus, and measles virus). Other neurotropic viruses are the morbillivirus, mouse adenovirus type 1, parainfluenza virus, rhabdovirus mumps virus, parvovirus B19, and most recently discovered, the severe acute respiratory syndrome-related coronavirus-2 (SARS-CoV-2), responsible for the severe pneumonia coronavirus disease 2019 (COVID-19) (7, 9–12).
Viral infections affecting the CNS structures are less common than those attacking other body organs (13). The hematogenous route has been categorized as one of the main pathways for CNS infection (neuroinvasion) for certain viruses (e.g., poliovirus, hepatitis B) (14). Viremia is a term that describes the presence of viruses in the blood after the virus trespasses the epithelial barrier and spreads to local and distant sites coursing through lymphatics and the bloodstream (15). The efficiency of the BBB and BCSFB depends on their molecular structure and seamless integrity, restricting the entry of unwanted microbes into the CNS (4, 15). In the case of the single-stranded RNA genome of SARS-CoV-2, once the virus enters the bloodstream, it binds to the angiotensin converting enzyme 2 (ACE 2) receptors of the endothelial membrane within blood vessels, disrupting the structural and functional composition of the BBB and consequently fiercely accessing the CNS (16–18). Other neurotropic invasions occur via the infection of Trojan horse-like immune cells (i.e., phagocytes) that are competent to cross the BBB, thereby transporting viruses into the nervous tissue (13, 19). Once in the CNS, pathogens are released from the “Trojan horses,” spreading the infection throughout important neural structures (e.g., limbic system, cerebral cortex) (19).
CNS viral infections may present with acute, latent, or chronic illness, depending on the constant “virtual competition” between the pathogen infection and the immune resistance of the host (6, 13). However, specific neurological conditions, such as encephalitis and olfactory dysfunctions (i.e., anosmia-hyposmia), posit an interesting argument suggesting a direct invasive route (i.e., retrograde axonal transport) through the olfactory nerve components (e.g., olfactory neuronal axons, olfactory bulbs and tracts). For example, the olfactory nerve (CN I) is considered one of the strongest route contenders for SARS-CoV-2 neuroinvasion due to its proximity to the epithelial lining (olfactory mucosa) within the nasal cavity (20).
The olfactory mucosa consists of a pseudostratified columnar epithelium composed of varied cell populations, including the microvillar cells (at apical surface), olfactory receptor neurons, sustentacular cells, and two types of basal cells (i.e., globose and horizontal) situated at the base of the olfactory epithelium (Figure 1). The lamina propria within the subepithelial region consist of connective tissues with blood and lymphatic vessels, nerve fibers (olfactory unmyelinated axons), and the olfactory glands of Bowman’s disease (i.e., branched tubulo-alveolar structures) (1, 2). The olfactory neuropil (bundles of unmyelinated axons forming the olfactory fila) originating from the olfactory receptor cells (bipolar neurons) travels through the mucosa toward the cribriform plate of the ethmoid bone in the skull. The small olfactory fila are surrounded by glial-like cells (olfactory ensheathing cells). It pierces the cribriform plate foramina forming CN I and ultimately reaches the glomeruli (approximately 2,000) and the dendrites of the neurons (mitral cells) within the olfactory bulb, where it integrates into varied synaptic connections (1–3). The olfactory bulb has a multilayered allocortex structure, such as the cerebral neocortex, but is functionally more complex. The principal cortical neurons are the mitral cells (approximately 50,000) that synapse with the olfactory nerve fibers and give rise to the olfactory tract. The smell signal transduction mechanisms start with the periglomerular cells and continue with the granule cells that refine the odorant information (Figure 1). The axons emerging from the olfactory bulb constitute the bilateral olfactory tract, which further divide at the olfactory trigone in front of the anterior perforated substance giving rise to the medial (minor) and lateral (major) olfactory striae. The medial stria contains axons from the anterior olfactory nucleus projecting to the septal area and others coursing the anterior commissure connecting to the opposite tract. The lateral stria terminates in the piriform cortex of the anterior temporal lobe (i.e., ventral portion of the parahippocampal gyrus, amygdala, and uncus) (4). Altogether, these constitute the main neuroanatomical projections of the olfactory sensory information to varied regions of the CNS (e.g., limbic structures, entorhinal cortex, thalamus) (1, 4) (Figure 2).
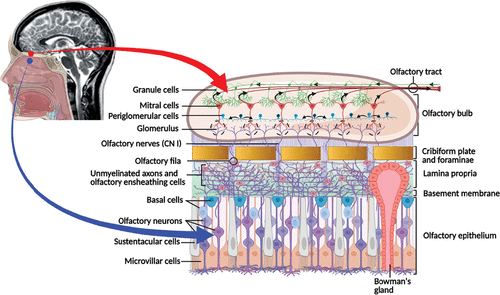
FIGURE 1. Olfactory epithelium and olfactory bulbs. The olfactory mucosa consists of a pseudostratified columnar epithelium composed of varied cell populations, including the microvillar cells (at apical surface), olfactory receptor neurons, sustentacular cells, and two types of basal cells (i.e., globose and horizontal) situated at the base of the olfactory epithelium. The lamina propria within the subepithelial region consists of connective tissues with blood and lymphatic vessels, nerve fibers (olfactory unmyelinated axons), and the olfactory glands of Bowman’s (i.e., branched tubulo-alveolar structures) (1, 2). The olfactory bulb has a multi-layered allocortex structure. The principal cortical neurons are the mitral cells (approximately 50,000), which synapse with the olfactory nerve fibers (CN I). These axons merge into glomeruli (approximately 2,000) innervated by the mitral cells, whose axons give rise to the olfactory tracts, conveying the olfactory stimuli to downstream olfactory areas of the brain (1–3). Created under the terms of the Creative Commons Attribution License. Created with BioRender.com.
FIGURE 2. (A) Basal view and (B) Coronal view of the olfactory racts. These bilateral structures further divide at the olfactory trigone in front of the anterior perforated substance giving rise to the medial (minor) and lateral (major) olfactory striae. The medial stria contains axons from the anterior olfactory nucleus projecting to the septal area and to other nerve fibers, traveling in the anterior commissure connecting to the opposite tract. The lateral stria terminates in the piriform cortex of the anterior temporal lobe (i.e., ventral portion of the parahippocampal gyrus, amygdala, and uncus) {4}. Altogether, these nerve components constitute the main neuroanatomical projections of the olfactory sensory information to varied regions of the brain (e.g., limbic structures, entorhinal cortex, thalamus) (1, 4). Created under the terms of the Creative Commons Attribution License. Created with BioRender.com.
Interestingly, the cells of the olfactory mucosa, as well as those of the respiratory epithelium, express the angiotensin-converting enzyme 2 (ACE 2) and the transmembrane serine protease 2 (TMPRSS2) receptors, which contain the essential molecular elements for binding, replication, and accumulation of varied viruses, including SARS-CoV-2 (21–23). A recent study conducted by Klingenstein and colleagues (23) using human donor samples demonstrated that ACE 2 and TMPRSS2 are expressed strictly in the sustentacular cells and Bowman glands cells of the olfactory epithelium, however, not in the olfactory receptor neurons (immature or mature) nor in the basal cells of the olfactory mucosa. This important discovery provides further anatomical evidence confirming the histological array of cells expressing ACE 2 and TMPRSS2 in the nose, olfactory epithelium, and olfactory bulb, respectively. It has been theorized that the viral neuroinvasion occurs via the olfactory receptor neurons, thereby affecting their functionality leading to anosmia. However, an important concept to further elucidate is how the olfactory neurons acquire the virus if they are devoid of ACE 2 and TMPRSS2 receptors. An alternative explanation is that either the sustentacular cells or the Bowman gland’s cells (possibly both) may represent the main targets for the SARS-CoV2 virus, considering that they express both of the entry proteins (i.e., ACE 2 and TMPRSS2) (23, 24).
The sustentacular cells are supporting nonsensory epithelial cells exhibiting an interesting microvilliated apical surface (2, 3) (Figure 1). These cells have junctional complexes (i.e., tight and adherens junctions) connecting them directly to the olfactory receptor cells and with other neighboring sustentacular cells (3). Therefore, they provide physical support, nourishment, and electrical insulation to the olfactory cells and are essential for the structural and physiological mechanisms of olfaction. Sustentacular cells provide mechanical stability comparable to glia cells and perform phagocytosis; thereby they are potentially responsible for protective immunologic mechanisms by expressing antiviral and antibacterial agents (23–25). Taken altogether, the viral neuroinvasion of the olfactory receptor neurons may take place indirectly via the sustentacular cells, possibly via their intercellular junctional complexes (23, 24). This provides insights that could possibly explain the smell dysfunction caused by the olfactory neural pathways disruption, generating long-term anosmia during and after COVID-19 (22, 23). In addition, the expression of ACE 2 and TMPRSS2 entry proteins seems to increase with age in experimental animals, further aligning (assuming human reciprocity) with the higher infectious risk of SARS-CoV-2 in the elderly population (24).
In addition to the neurotropic viruses mentioned earlier, varied reports from both human and animal studies indicate that several other viruses can enter the brain specifically via the olfactory mucosa and the nerve fibers of CN I. These include adenoviruses, bunyaviruses, chikungunya virus, herpesviruses, influenza A virus, Japanese encephalitis, La Crosse virus poliovirus, mouse hepatitis virus, parainfluenza virus, paramyxoviruses, rabies virus, vesicular stomatitis, and West Nile virus (10, 26–35).
Aside from CN I, there are other important neuroanatomical structures associated with the nasal septum, providing different modalities of innervation to the olfactory mucosa. These include the notable trigeminal nerve (fifth cranial nerve, CN V), the enigmatic nervus terminalis or cranial nerve zero (CN 0), and the autonomic fibers of the cervical ganglia (1, 5). Given the anatomical location of these structures and their proximity to the olfactory mucosa, they may be potential targets for viral neuroinvasion (10) (Figure 3). For example, clinical presentations due to the impairment of CN V and other cranial nerves (i.e., facial nerve, CN VII) have been reported in a significant number of patients with COVID-19 (36). Herpes zoster ophthalmicus is a viral infection affecting CN V, particularly its ophthalmic division (V1) (37). During its latent phase, the virus resides within the sensory nerve ganglia of CN V after traveling retrogradely via the CN V axons. During the reactivation phase, it moves anterogradely reaching the superficial tissues of the face where it reemerges with ocular manifestations, including pain, photophobia, reduced vision, and others (38).
FIGURE 3. Nerves of the nasal cavity and nasal mucosa. Cranial nerve I is one of the principal nerves of the olfactory region within the nasal cavity. However, there are other important nerves associated with the nasal septum, providing different modalities of innervation to the olfactory mucosa and olfactory region. These include the notable trigeminal nerve (fifth cranial nerve, CN V), the enigmatic nervus terminalis (NT) or cranial nerve zero (CN 0), and the autonomic fibers of the cervical ganglia (1, 5). Created under the terms of the Creative Commons Attribution License. Created with BioRender.com.
COVER. Three-dimensional digital virtual dissection of the cranial nerves of the human brain and spinal cord, emphasizing the olfactory and trigeminal neural systems (yellow). Created under the terms of the Creative Commons Attribution License. Created with VH Dissector and BioRender.com.
An investigation by Schäfer and colleagues reported (39, 40) a considerable number of patients with sexual impairment as a concomitant complaint of olfactory dysfunction. More recently, a study showed varied cases of postviral olfactory impairment and reduced sexual function in COVID-19 patients (41). Normal olfactory functions support the quality and quantity of sexual behaviors (42). The conscious perception of certain odorants along with the psycho-neuroendocrinological effects of “pheromones” (i.e., odorless social and sexual chemosignal molecules) increase sexual arousal (43). The CN 0 plays a pivotal role in the maturation of the hypothalamic-pituitary-gonadal gonadotropin-releasing hormone (GnRH) axis, the perception of pheromones, and for triggering sexual responses independently or together with other neural pathways (i.e., kisspeptin neuronal network) (5, 44–46). In addition, the CN 0 has been implicated in Kallmann syndrome, a type of congenital hypogonadotropic hypogonadism correlated with various degrees of olfactory dysfunction, consequential to faulty GnRH, and the impaired migration of olfactory neurons (5, 44, 46–48).
There is substantial evidence to support the pathophysiological mechanisms for viral neuroinvasion via cranial nerves fibers and other neuroanatomical structures within the sinonasal passages, allowing viruses to enter the brain spreading the infection and affecting the nervous tissue (10, 36, 49). The CNS sophistication and exquisite neuronal cytoarchitectural arrangement, including its characteristic physiological processes of intracellular conveyance, are regrettably favorable for viral nourishment and replication. The paramount neuronal circuits with their extensive neuropil and widespread synapses somehow facilitate a natural highway for viral infection and neural sepsis (6). Within the CNS, viruses can cause neurological disturbances, such as induced cell death (i.e., vesicular stomatitis virus), direct lysis (i.e., cytomegalovirus), or indirect injury affecting the release of glutamate, DNA structure, or release of other toxic substances (13). Viruses such as rabies corrupt cellular transcriptional processes commanding the expression of viral genes within neurons and altering their physiological processes, although leaving their structural composition intact upon routine pathological examination (13, 50).
The pathophysiological mechanisms of CNS sepsis often include an acute brain dysfunction, typically associated with increased morbidity and mortality rates. The sequelae include inflammatory and noninflammatory alterations of the nervous tissue, resulting in excessive microglial activation, impaired cerebral perfusion, BBB dysfunction, and altered neurotransmission (51). Unfortunately, one of the most concerning aspects regarding viral infections is that they can produce long-term neurological issues, manifesting years after, or even decades from, the original exposure. For example, the postpolio syndrome (PPS) presents with new clinical manifestations (e.g., limbs paresis, fatigue, arthralgia) in recovered poliomyelitis patients decades after initial polio infection (52, 53). Some reports have drawn attention comparing the polio epidemics in Europe and the United States during the early 20th century with the current COVID-19 pandemic, warning about a potential disease sequelae (54). Despite the limited information regarding the long-term outcomes and possible neurologic disturbances of COVID-19, vigilance concerning the chronic effects of viral infections based on the lessons learned from PPS and other viral pandemics is imperative (53).
From the neuropsychiatric standpoint, another thought-provoking virus is Borna disease virus 1 (BoDV-1), causing behavioral abnormalities and lethal CNS infections in a wide range of species (e.g., cats, chicken, dog, fox, horse, llama, monkey, ostrich, rats, and others), including humans (13, 55, 56). The virus’ natural reservoir is the bicolored white-toothed shrew (Crocidura leucodon) (57). The neurotropic BoDV-1 is the prototype of the family Bornaviridae (Mammalian 1 orthobornavirus). It is an enveloped, non‐cytolytic, non‐segmented, negative-strand RNA virus with slowly replicating capabilities within the nucleus of the infected cells. It can access the brain via the olfactory mucosa and its neural pathways, spread through the limbic system structures to most cortical areas, brainstem, cerebellum, and spinal cord, and remain active in the CNS for years (13, 55, 58, 59). Although the virus’ relationship with mental disorders was initially established in 1985, the first cases of human encephalitis due to BoDV-1 infection were formally introduced in 2018 (60–62). Subsequent reports have indicated a possible link between BoDV-1 infection and mental illnesses, such as bipolar disorder, chronic fatigue syndrome, schizophrenia, and unipolar depression (61, 63, 64). However, other investigators have argued against any involvement of the BoDV-1 virus in the pathogenesis of these psychiatric disorders (65, 66). More recently, a systemic review and metanalysis conducted by Azami et al. (67) demonstrated a possible role of this virus and its potential involvement in the etiology of schizophrenia. In addition, the results from a double-blind placebo-controlled randomized clinical trial study conducted by Dietrich et al. (68) further supports the established link between BoDV-1 virus infection and depression.
Despite the controversy over the association of certain CNS viral infections with human neuropsychiatric illnesses, neurotrophic viruses posit an intriguing model for the study of psychiatry conditions such as anxiety, depression, and schizophrenia. In addition, peripheral viral infections (nonneurotropic) warrant attention, as their clinical manifestations do not appear to occur from the primary infection. Instead, the secondary neuropathology may manifest much later, presenting syndromic-like clinical scenarios (e.g., Guillian-Barré syndrome, acute necrotizing encephalopathy) (69).
In summary, infectious diseases are emerging or re-emerging every year, with COVID-19 as the most recent and vivid example. Consequentially, neuroinfectious conditions may occur as outbreaks in small local regions or may spread rapidly over larger geographical territories. Viruses neuroinvasion leads to varied CNS illnesses that may cause a broad spectrum of neurological presentations, such as encephalitis, Guillian-Barré-like-syndromes, meningitis, meningoencephalitis, special senses disabilities (e.g., anosmia, ageusia), and many others. Infections due to neurotropic and nonneurotropic viruses may cause acute and dormant neuropathologic responses; however, some could lead to chronic neuropsychiatry disturbances as a long-term repercussion. It is anticipated that a significant number of COVID-19 survivors may experience innumerable neurological deficiencies and CNS disorders. Thus, an increased awareness regarding the vulnerability of the nervous system to viral infections is critical. It is imperative to increase collective research efforts to improve scientific understanding of the pathophysiology of neurotropism to better prepare for future neurological treatment strategies.
1. : Nose, nasal cavity and paranasal sinuses, in Gray’s Anatomy. Edited by Standring S. https://www.clinicalkey.com/#!/content/book/3-s2.0-B9780702077050000392?scrollTo=%23hl0001899Google Scholar
2. : Histology and cell biology: an introduction to pathology, in Histology and Cell Biology: An Introduction to Pathology, 5th ed. Edited by Kierszenbaum AL, Tres LL. https://www.clinicalkey.com/#!/content/book/3-s2.0-B9780323673211500 171?scrollTo=%23hl0000298Google Scholar
3. : Respiratory System, in Textbook of Histology, 5th ed. Edited by Gartner LP. https://www.clinicalkey.com/#!/content/book/3-s2.0-B9780323672726000151?scrollTo=%23hl0000568Google Scholar
4. : Blood supply of the brain, in Fitzgerald’s Clinical Neuroanatomy and Neuroscience. https://www.clinicalkey.com/#!/content/book/3-s2.0-B978070207909 2000048?scrollTo=%23hl0000670Google Scholar
5. : Function of gonadotropin-releasing hormone in olfaction. Keio J Med 2001; 50:81–85Crossref, Medline, Google Scholar
6. : Neurons under viral attack: victims or warriors? Neurochem Int 2010; 56:727–735Crossref, Medline, Google Scholar
7. : Molecular biology and epidemiology of neurotropic viruses. Cureus 2020; 12:e9674Medline, Google Scholar
8. : Emerging and re-emerging viruses affecting the nervous system. Neurol Res Pract 2019; 1:20Crossref, Medline, Google Scholar
9. : CNS implications of COVID-19: a comprehensive review. Rev Neurosci 2020; 32:219–234Crossref, Medline, Google Scholar
10. : The olfactory nerve: a shortcut for influenza and other viral diseases into the central nervous system. J Pathol 2015; 235:277–287Crossref, Medline, Google Scholar
11. : How viruses infiltrate the central nervous system. Acta Virol 2017; 61:393–400Crossref, Medline, Google Scholar
12. : Function of membrane rafts in viral lifecycles and host cellular response. Biochem Res Int 2011; 2011:245090Crossref, Medline, Google Scholar
13. : Viral infection leading to brain dysfunction: more prevalent than appreciated? Neuron 2009; 64:17–20Crossref, Medline, Google Scholar
14. : Unraveling the possible routes of SARS-COV-2 invasion into the central nervous system. Curr Treat Options Neurol 2020; 22:37Crossref, Medline, Google Scholar
15. General pathology of infectious diseases. https://www.clinicalkey.com/#!/content/book/3-s2.0-B9780323353175000090?scrollTo=%23hl0001170Google Scholar
16. : Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). J Med Virol 2020; 92:699–702Crossref, Medline, Google Scholar
17. : The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol 2020; 92:552–555Crossref, Medline, Google Scholar
18. : Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus: a first step in understanding SARS pathogenesis. J Pathol 2004; 203:631–637Crossref, Medline, Google Scholar
19. : False friends: phagocytes as Trojan horses in microbial brain infections. PLoS Pathog 2017; 13:e1006680Crossref, Medline, Google Scholar
20. : Evidence of central nervous system infection and neuroinvasive routes, as well as neurological involvement, in the lethality of SARS-CoV-2 infection. J Med Virol 2021; 93:1304–1313Crossref, Medline, Google Scholar
21. : Infection mechanism of SARS-COV-2 and its implication on the nervous system. Front Immunol 2021; 11:621735Crossref, Medline, Google Scholar
22. : Background mechanisms of olfactory dysfunction in COVID-19: expression of ACE2, TMPRSS2, and Furin in the nose and olfactory bulb in human and mice. bioRxiv 2020; 2020.05.15.097352Google Scholar
23. : Evidence of SARS-CoV2 entry protein ACE2 in the human nose and olfactory bulb. Cells Tissues Organs 2020; 209:155–164Crossref, Medline, Google Scholar
24. : Expression of the SARS-CoV-2 entry proteins, ACE2 and TMPRSS2, in cells of the olfactory epithelium: identification of cell types and trends with age. ACS Chem Neurosci 2020; 11:1555–1562Crossref, Medline, Google Scholar
25. : Supporting cells as phagocytes in the olfactory epithelium after bulbectomy. J Comp Neurol 1996; 376:509–517Crossref, Medline, Google Scholar
26. : Fatal influenza A(H1N1)pdm09 encephalopathy in immunocompetent man. Emerg Infect Dis 2013; 19:1005–1007Crossref, Medline, Google Scholar
27. : Herpes simplex encephalitis: an immunohistological study of the distribution of viral antigen within the brain. J Neurol Sci 1982; 54:209–226Crossref, Medline, Google Scholar
28. : Olfactory transmission of neurotropic viruses. J Neurovirol 2005; 11:129–137Crossref, Medline, Google Scholar
29. : Human herpesvirus-6 entry into the central nervous system through the olfactory pathway. Proc Natl Acad Sci USA 2011; 108:13734–13739Crossref, Medline, Google Scholar
30. : Localizations of the virus poliomyelitis in the central nervous system during the preparallytic period, after intranasal instillation. J Exp Med 1933; 57:933–954Crossref, Medline, Google Scholar
31. : Influence of the host factors on neuroinvasiveness of vesicular stomatitis virus. J Exp Med 1938; 67:201–228Crossref, Medline, Google Scholar
32. : Viral replication in olfactory receptor neurons and entry into the olfactory bulb and brain. Ann N Y Acad Sci 1998; 855:751–761Crossref, Medline, Google Scholar
33. : Brain lesions induced by experimental intranasal infection of Japanese encephalitis virus in piglets. J Comp Pathol 2009; 141:156–162Crossref, Medline, Google Scholar
34. : Mode of entry of a neurotropic arbovirus into the central nervous system: reinvestigation of an old controversy. Lab Invest 1983; 48:399–410Medline, Google Scholar
35. : Changing patterns of chikungunya virus: re-emergence of a zoonotic arbovirus. J Gen Virol 2007; 88:2363–2377Crossref, Medline, Google Scholar
36. : Cranial nerve involvement in COVID-19. Am J Otolaryngol 2021; 42:102999Crossref, Medline, Google Scholar
37. : Herpes zoster ophthalmicus. Treasure Island, Fla, StatPearls Publishing, 2021. https://www.ncbi.nlm.nih.gov/books/NBK557779Google Scholar
38. : Herpes zoster in the ophthalmic branch of the trigeminal ganglia obscuring cavernous sinus thrombosis due to Streptococcus constellatus subsp. Constellatus: a case report. Anesth Pain Med (Seoul) 2020; 15:205–208Crossref, Medline, Google Scholar
39. : Sexual desire after olfactory loss: quantitative and qualitative reports of patients with smell disorders. Physiol Behav 2019; 201:64–69Crossref, Medline, Google Scholar
40. : Human olfactory dysfunction: causes and consequences. Cell Tissue Res 2021; 383:569–579Crossref, Medline, Google Scholar
41. : More than smell–COVID-19 is associated with severe impairment of smell, taste, and chemesthesis. Chem Senses 2020; 45:609–622Crossref, Medline, Google Scholar
42. : Olfactory function relates to sexual experience in adults. Arch Sex Behav 2018; 47:1333–1339Crossref, Medline, Google Scholar
43. : Changes in men’s salivary testosterone and cortisol levels, and in sexual desire after smelling female axillary and vulvar scents. Front Endocrinol 2013; 4:159Crossref, Medline, Google Scholar
44. : Cranial pair 0: the nervus terminalis. Anat Rec (Hoboken) 2019; 302:394–404Crossref, Medline, Google Scholar
45. : The neglected cranial nerve: nervus terminalis (cranial nerve N). Clin Anat 2014; 27:46–53Crossref, Medline, Google Scholar
46. : Neuroanatomy, cranial nerve 0 (terminal nerve). Treasure Island, Fla, StatPearls Publishing, 2021 http://www.ncbi.nlm.nih.gov/books/NBK459159Google Scholar
47. :
48. : Olfactory phenotypic spectrum in idiopathic hypogonadotropic hypogonadism: pathophysiological and genetic implications. J Clin Endocrinol Metab 2012; 97:E136–E144Crossref, Medline, Google Scholar
49. : Neuroinvasion, neurotropic, and neuroinflammatory events of SARS-CoV-2: understanding the neurological manifestations in COVID-19 patients. Neurol Sci 2020; 41:2657–2669Crossref, Medline, Google Scholar
50. : Perspectives in diagnosis and treatment of rabies viral encephalitis: insights from pathogenesis. Neurotherapeutics 2016; 13:477–492Crossref, Medline, Google Scholar
51. : Understanding brain dysfunction in sepsis. Ann Intensive Care 2013; 3:15Crossref, Medline, Google Scholar
52. : Assessment and differential diagnosis for post-polio syndrome. Orthopedics 1991; 14:1209–1217Crossref, Medline, Google Scholar
53. : The value of a post-polio syndrome self-management programme. J Thorac Dis 2020; 12(Suppl 2):S153–S162Crossref, Medline, Google Scholar
54. : What the history of polio can teach us about COVID-19. Time, May 5, 2020. https://time.com/5831740/polio-coronavirus-parallelsGoogle Scholar
55. : The neuropathology of fatal encephalomyelitis in human Borna virus infection. Acta Neuropathol 2019; 138:653–665Crossref, Medline, Google Scholar
56. : Are human Borna disease virus 1 infections zoonotic and fatal? https://www.clinicalkey.com/#!/content/playContent/1-s2.0-S1473309920303807?returnurl= https:%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS14 73309920303807%3Fshowall%3Dtrue&referrer=https:%2F%2 Fpubmed.ncbi.nlm.nih.gov%2FGoogle Scholar
57. : Shedding of infectious Borna disease virus-1 in living Bicolored white-toothed shrews. PLoS One 2015; 10:e0137018Crossref, Medline, Google Scholar
58. : Intranasal borna disease virus (BoDV-1) infection: insights into initial steps and potential contagiosity. Int J Mol Sci 2019; 20:(6)1318Crossref, Google Scholar
59. : Natural and experimental Borna disease virus infections–neuropathology and pathogenetic considerations. APMIS 2008; (124):53–57Crossref, Google Scholar
60. : Fatal encephalitis associated with Borna disease virus 1. N Engl J Med 2018; 379:1375–1377Crossref, Medline, Google Scholar
61. : The pathogenesis of bornaviral diseases in mammals. Anim Health Res Rev 2016; 17:92–109Crossref, Medline, Google Scholar
62. : Borna disease virus: a possible etiologic factor in human affective disorders? Arch Gen Psychiatry 1985; 42:1093–1096Crossref, Medline, Google Scholar
63. : Detection of serum antibodies to Borna disease virus in patients with psychiatric disorders. Science 1985; 228:755–756Crossref, Medline, Google Scholar
64. : Detection of Borna disease virus-reactive antibodies from patients with affective disorders by western immunoblot technique. J Affect Disord 1993; 27:61–68Crossref, Medline, Google Scholar
65. : Borna disease virus–fact and fantasy. Virus Res 2011; 162:162–172Crossref, Medline, Google Scholar
66. : Absence of evidence for bornavirus infection in schizophrenia, bipolar disorder and major depressive disorder. Mol Psychiatry 2012; 17:486–493Crossref, Medline, Google Scholar
67. : The association between Borna disease virus and schizophrenia: a systematic review and meta-analysis. Asian J Psychiatr 2018; 34:67–73Crossref, Medline, Google Scholar
68. : Antiviral treatment perspective against Borna disease virus 1 infection in major depression: a double-blind placebo-controlled randomized clinical trial. BMC Pharmacol Toxicol 2020; 21:12Crossref, Medline, Google Scholar
69. : Neurological damage by coronaviruses: a catastrophe in the queue! Front Immunol 2020; 11:565521Crossref, Medline, Google Scholar



