Is Delirium Different When It Occurs in Dementia?
Abstract
The authors studied 61 geropsychiatric patients with delirium from a cohort of 843 consecutive admissions to a geriatric clinical research unit. A central study goal was to assess how the presence of dementia affected the presentation of delirium. Eighteen delirious (D) and 43 delirious-demented (D-D) patients were compared on the Delirium Rating Scale (DRS), Mini-Mental State Examination (MMSE), Brief Psychiatric Rating Scale (BPRS), and EEG. D-D patients had lower MMSE scores, but no differences were found in total DRS or BPRS scores or in EEG grade. DRS items were similar in the two groups except that D-D had more cognitive impairment than D. An exploratory principal components analysis of DRS items identified two core factors. The authors conclude that the presentation of delirium in the setting of concurrent dementia is very similar to delirium without dementia, with subtle differences probably attributable to dementia.
Delirium and dementia are both disorders involving global cognitive impairment that can occur separately or concurrently in the elderly. Although their time course and constellation of symptoms differ, there is some overlap in symptoms that complicates differential diagnosis. Characteristics that usually assist in establishing a diagnosis of delirium include acute onset, fluctuation of symptoms, and severity of sleep–wake cycle fragmentation. Also, different types of dementias present with different symptom profiles, particularly at earlier stages of dementia progression (for instance, dementia of frontal lobe type versus Alzheimer's), further assisting in differential diagnosis. However, end-stage dementias may be indistinguishable from delirium (except by history), and some have proposed that the widespread destruction of brain in end-stage dementia actually causes a chronic delirious state.1 What is not well understood is whether and how the presence of non–end-stage dementia may alter the presentation of delirium.
Better understanding of delirium phenomenology will enhance its clinical differentiation from dementia. Unfortunately, there are few studies of delirium phenomenology. Studies that have addressed delirium symptoms have taken different approaches. Some describe the occurrence of individual symptoms.2–8 Others compare symptoms associated with hyperactive and hypoactive subtypes.9–11 Two have applied latent structure techniques (such as principal components analysis) to assess how symptoms cluster together in a delirious population.6,12 Many but not all of these studies excluded demented patients who could have manifested an alteration in the presentation of delirium phenomenology.
Liptzin et al.3 compared groups of delirious elderly patients with and without underlying dementia by using the Delirium Symptom Interview (DSI). They found no differences between these delirious groups for the presence of disorientation, fluctuating behavior, or disturbances of consciousness, sleep, perception, speech, and psychomotor behavior. The Delirium Rating Scale (DRS) distinguishes non-overlapping patient groups with either delirium or dementia;13–15 only the DRS cognitive-impairment item failed to differentiate delirious from demented patients.6
To investigate further the phenomenological impact of dementia on delirium, we used the DRS to study delirious and delirious-demented elderly patients who were admitted to a geropsychiatry clinical research inpatient unit. Mini-Mental State Examination (MMSE), Brief Psychiatric Rating Scale (BPRS), and electroencephalograms (EEG) were also administered. We expected delirium to present similarly in the two groups, except for higher age and DRS scores and lower MMSE scores in the delirious-demented group. We anticipated that factor analysis of DRS items might yield different patterns of interitem relationships between groups due to the co-occurrence of dementia affecting expression of delirium symptoms.
METHODS
Subjects
The Geriatric Clinical Research Unit (GCRU) is a 26-bed acute care unit for the assessment and treatment of late-life mental disorders at Western Psychiatric Institute and Clinic, University of Pittsburgh Medical Center; psychiatrists and an internist care for these patients. This hospital provides psychiatric care to a large urban catchment area and serves as a referral center for patients from suburban and rural southwestern Pennsylvania.
All delirious patients 60 years of age and over who were hospitalized on the GCRU between September 1, 1990, and July 1, 1993, were included in this study if they stayed for at least 3 days and completed the usual comprehensive multidisciplinary evaluation by a geriatric psychiatrist–led treatment team.14,16,17 For 2 delirious patients who had repeat admissions, only the first admission was included. Most patients had an EEG, and results were included if it was performed within 14 days of admission.
Clinical Procedures
Comprehensive evaluation included psychiatric history and mental status examination, physical examination, serum laboratory tests, chest X-ray, ECG, EEG, and brain imaging with MRI or CT. MRIs and CT scans were interpreted by following a methodology previously described,18 on the basis of radiologists' clinical reports. EEGs were interpreted as grade 0 (normal), I, II, or III dysrhythmia according to the Mayo Clinic classification system.19
Upon admission, patients were rated by a trained research clinician who was not involved in the patient's care, using a battery of instruments including the DRS,13 a standardized version of the MMSE,20,21 and an anchored version of the BPRS1 with a minimum score of 17 points. The DRS rates the severity of 10 delirium symptoms and includes symptom characterizations. Higher scores on the DRS and BPRS and lower scores on the MMSE indicate more impairment.
We assessed interrater reliability for the DRS, MMSE, and BPRS throughout the study period by using intraclass correlation coefficient procedures.22 Each intraclass correlation coefficient (ICC) was based on concurrent ratings by independent raters. During the study period, good to excellent reliability was established and maintained for the DRS (ICC from 0.59 to 0.75), MMSE (ICC from 0.98 to 1.00), and BPRS (ICC from 0.73 to 0.96), using five different nonphysician raters.
Shortly after discharge, consensus by the research team (three to six faculty psychiatrists and the research staff) was established for the admission diagnoses according to DSM-III-R criteria.23 In order to be diagnosed with delirium and dementia, patients had to meet DSM-III-R criteria for both. Dementia diagnoses had to be met in the absence of delirium on the basis of longitudinal data. Delirium diagnoses were made independently of DRS ratings; otherwise, all available clinical information from the patient, family, primary care physicians, and old records was considered. The diagnosis that corresponded to the symptoms that precipitated the admission was then recorded as the “primary psychiatric diagnosis.”
We selected all cases with a primary diagnosis of delirium on admission, and then we divided them into delirium only (D) and delirium with dementia (D-D) groups. Because different dementias have somewhat different symptom profiles, we also subdivided the D-D group into three subgroups according to DSM-III-R dementia type: Alzheimer's, multi-infarct, and other.
Statistical Analyses
All analyses were performed by using SAS statistical software. We report descriptive information (means and standard deviations) for each study measure. We used independent t-tests or chi-square to compare D and D-D groups on all variables. Chi-square was used for comparisons of EEG grade, sex, marital status, and race between D and the three D-D subgroups. Pearson correlations were used to examine associations between DRS total scores, age, MMSE, and BPRS admission scores. Statistical significance was set at P≤0.05 for all comparisons, except for Bonferroni corrections for multiple comparisons that affected the P-values set for demographic variables (P≤0.01) and DRS items (P≤0.005). Tests were two-tailed.
For dementia subtype analyses, we used one-way analysis of variance (ANOVA) to compare D and the three D-D subgroups for continuous variables, with pairwise comparisons using the Tukey Studentized range test. We used the Kruskal-Wallis one-way ANOVA test, a generalization of the Wilcoxon ranked-sums test, to compare D and the three D-D subgroups' DRS item scores. H, the test statistic associated with the Kruskal-Wallis test, is evaluated against the chi-square distribution.
Principal components analysis with varimax rotation was used to evaluate patterns of relationships among DRS items for the D and D-D groups, but this analysis was not done for the dementia subgroups because of small sample size.
RESULTS
Delirious and Delirious-Demented Groups
Sixty-one delirious elderly inpatients who met the study entry criteria were identified from among 843 consecutive geropsychiatric admissions over a 34-month period (September 1, 1990, through July 1, 1993). Mean age for all delirious patients was 74.3±7.7 years (range 61–89); 67% were female, 87% were white, 34% were married, 53% were widowed, 17% had fewer than 8 years of education, and 50% had at least 12 years of education. Specific delirium etiologies could not always be determined on a case-by-case basis, and they may have been multiple in many cases.
Eighteen patients had delirium without dementia, comprising the D group, and 43 had delirium concurrently with dementia, comprising the D-D group. Dementia types in the D-D group were further divided into three subgroups: 16 with Alzheimer's dementia (D-Alz), 11 with multi-infarct dementia (D-MID), and 16 with other dementias (D-Other; 15 with Dementia Not Otherwise Specified and 1 with alcoholic dementia).
Table 1 describes data for age, gender, DRS, MMSE, BPRS, and EEG. The D and D-D groups were similar for age, gender, and education, but fewer D were married (χ2=6.2, df=1, P=0.01). MMSE scores showed more impairment in D-D than D (t=3.6, df=49, P=0.0008). However, total DRS and BPRS scores did not differ significantly between groups.
EEGs were available for 56 patients, of whom 11 had normal EEGs, 9 had grade I dysrhythmia, and 36 had grade II dysrhythmia. There were no significant differences between D and D-D groups for EEG results, nor for the number of days between admission and the EEG evaluation (4.9±3.3 days for whole cohort).
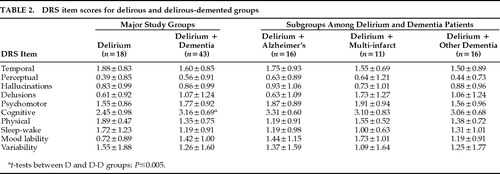
DRS items were similar for D and D-D groups (Table 2), except that D-D had more cognitive impairment than D (t=–3.3, df=59, P=0.002). In neither group was age (r=–0.09) or MMSE (r=–0.16) significantly correlated with total DRS scores. However, DRS significantly correlated with BPRS scores in D (r=0.57, P=0.017) and D-D (r=0.35, P=0.04) groups.
Dementia Subgroups
To explore whether dementia type may have contributed differentially to the D-D group, we compared the three D-D subgroups with the D group (Table 1). Demographic characteristics were similar, except for age (F=6.07, df=3,57, P=0.0012); pairwise comparisons revealed a significant difference only for D-Alz, who were older than either the D or D-Other groups. MMSE scores differed (F=6.03, df=3,47, P=0.0015) such that the D group had better scores than either the D-Alz or D-Other group. DRS and BPRS scores, EEG grade, and number of days from admission to EEG performance did not differ among the four subgroups. DRS item scores also did not differ among D and D-D subgroups.
Principal Components Analysis
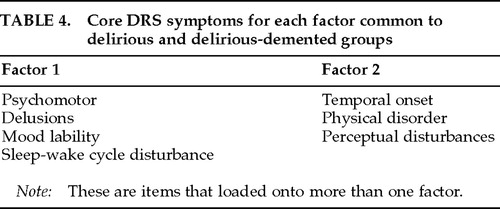
Exploratory principal components analyses of DRS items for D and D-D groups are described in Table 3. For both of these groups, two underlying factors appeared to account for the relationships among items. The two-factor structure was well defined; that is, most DRS items loaded onto only one of two extracted factors. Four items loaded onto the same factor in both groups—psychomotor behavior, delusions, temporal onset, and physical etiology. Three other items loaded similarly, but these were more fluid in that they loaded onto both factors in one of the groups—sleep–wake cycle disturbance, perceptual disturbances, and mood lability.
Including the three items that loaded onto more than one factor, both the D and D-D groups showed striking similarities in the items comprising each of the two factors. On this basis, 7 of 10 items were felt to describe two “core factors” for delirium (Table 4). However, cognitive impairment, hallucinations, and variability of symptoms were not among these core symptom groups because they loaded onto opposite factors in the D and D-D groups.
Interestingly, delusions and hallucinations loaded inversely onto factor 1 in the D group, suggesting that the absence of these psychotic symptoms was important when other factor 1 symptoms were present. Similarly, delusions loaded inversely onto factor 1 in the D-D group, suggesting that their absence in the context of other factor 1 symptoms was important in these subjects as well.
DISCUSSION
We studied 61 delirious elderly psychiatry inpatients presenting with delirium in order to distinguish the contribution of dementia to delirium phenomenology. We compared 18 delirium-only (D) with 43 delirious-demented (D-D) patients by using the DRS, a symptom severity scale for delirium. We found that delirium presented similarly whether or not dementia was also present. Only age, MMSE, and the DRS cognitive-impairment item differed between our groups. This similarity in presentation suggests that delirium dominates dementia symptoms when they occur together. Consistent with our findings, Liptzin et al.3 reported no differences between similar patient groups on the DSI.
We compared individual DRS items, corrected for multiple comparisons, and detected a difference only for cognition. When the delirious-demented group was subdivided into three dementia subtypes, we found no differences among groups for any DRS item.
Delirious-demented patients showed more severe cognitive deficits on both the DRS and MMSE. This finding might suggest a compounding effect of dementia and delirium on cognitive functions—or a selection bias, where patients with more severe dementia are at greater risk for delirium. However, Liptzin et al.3 did not find a difference in “disorientation” between similar groups of elderly patients, although orientation is only one cognitive domain and delirium affects many aspects of cognition.
An exploratory principal components analysis of DRS items did suggest some subtle differences in delirium presentation, however, depending on the presence or absence of comorbid dementia. Although 7 out of 10 delirium symptoms were identified as comprising two “core” factors that were similar for D and D-D groups, 3 symptoms (hallucinations, cognitive impairment, and variability of symptoms) clustered differently between the D and D-D groups. In addition, 3 of the 7 “core” items were fluid in that they loaded onto both factors in either one of the groups. Our n was small, and further study is needed to clarify the importance of these small differences detected. Especially needed are studies using more homogeneous dementia groups, since different dementias might differentially affect delirium presentation.
The factor structure identified in our delirious-only patients was strikingly similar to previous findings6 in nondemented adult delirious medically hospitalized patients. In both of these delirious nondemented cohorts, factor 1 loaded sleep–wake cycle, psychomotor behavior, and mood lability, and factor 2 loaded temporal onset of symptoms, symptom variability, and perceptual disturbances. Further, the three symptoms identified on factor 2 for both these reports also have been considered by others to be highly associated with and quite specific to delirium.2,5,7 Thus, although these are only tentative findings, these factors may be meaningful in indicating some underlying neurobiologic or phenomenologic construct important in delirium. Unfortunately, given the diversity of delirium symptoms and the lack of current knowledge about brain regions subserving particular psychiatric symptoms, it is not possible to ascribe a meaning to each factor without more understanding of the neuropathogenesis of individual symptoms.
Not all patients in our study had abnormal EEGs, perhaps because EEGs were not necessarily performed when the delirium was most severe. Severity of EEG slowing did not distinguish our delirious from delirious-demented elderly, possibly because the delirium overshadowed the effect of dementia on EEGs. Jacobson et al.24 and Koponen et al.25 have used quantitative EEG techniques to successfully distinguish delirious from nondelirious elderly patients, but they have not compared delirious with delirious-demented patients.
Two strengths of this study are that DRS and other ratings were performed upon admission by research clinicians who were not directly involved in the patients' care, and admission psychiatric diagnoses were finalized by a group of research geriatric psychiatrists who were blind to symptom ratings for delirium. Weaknesses include a relatively small number of patients, diagnostic heterogeneity in the demented group, and a possible bias because patients were admitted to a psychiatry unit instead of a medical-surgical unit.
Our findings are largely consistent with the clinical maxim that one should consider a confusional state to be a delirium until proven otherwise, before diagnosing a patient as demented. Delirium symptoms overshadow dementia symptoms, except for severity of cognitive impairment. However, this finding needs to be reassessed in a larger sample before we can conclude that dementias do not have any effect on delirium phenomenology.26 Differences in even a few symptoms may be important because individual delirium symptoms have been shown to have prognostic implications in elderly hospitalized patients.27
ACKNOWLEDGMENTS
The authors thank Michelle Gregor, M.S.W., for data management and analysis. This work was supported in part by National Institute of Mental Health Grants MH01153, MH30915, MH49786, and MH52247. It was presented in part at the annual meetings of the American Neuropsychiatric Association in Pittsburgh, PA, October 1995, and the Academy of Psychosomatic Medicine in Palm Springs, CA, November 1995.
 |
 |
 |
 |
1. Mulsant BH, Rosen J: Dementia (Alzheimer's disease), in Handbook of Prescriptive Treatments for Adults, edited by Hersen M, Ammerman T. New York, Plenum, 1994, pp 31–51Google Scholar
2. Morse RM, Litin EM: The anatomy of a delirium. Am J Psychiatry 1971; 128:111–116Crossref, Medline, Google Scholar
3. Liptzin B, Levkoff SE, Gottlieb GL, et al: Delirium (Background Papers for DSM-IV). J Neuropsychiatry Clin Neurosci 1993; 5:154–160Link, Google Scholar
4. Sirois F: Delirium: 100 cases. Can J Psychiatry 1988; 33:375–378Crossref, Medline, Google Scholar
5. Rockwood K: The occurrence and duration of symptoms in elderly patients with delirium. J Gerontol Med Sci 1993; 48:M162–M166Google Scholar
6. Trzepacz PT, Dew MA: Further analyses of the Delirium Rating Scale. Gen Hosp Psychiatry 1995; 17:75–79Crossref, Medline, Google Scholar
7. Levkoff SE, Evans DA, Liptzin B, et al: Delirium: the occurrence and persistence of symptoms among elderly hospitalized patients. Arch Intern Med 1992; 152:334–340Crossref, Medline, Google Scholar
8. Johnson JC, Gottlieb GL, Sullivan E, et al: Using DSM-III criteria to diagnose delirium in elderly general medical patients. J Gerontol Med Sci 1990; 45:M113–M119Google Scholar
9. Ross CA, Peyser CE, Shapiro I, et al: Delirium: phenomenologic and etiologic subtypes. Int Psychogeriatr 1991; 3:135–147Crossref, Medline, Google Scholar
10. Liptzin B, Levkoff SE: An empirical study of delirium subtypes. Br J Psychiatry 1992; 161:843–845Crossref, Medline, Google Scholar
11. Kobayashi K, Takeuchi O, Suzuki M, et al: A retrospective study on delirium type. Japanese Journal of Psychiatry 1992; 46:911–916Medline, Google Scholar
12. van der Mast R: Delirium After Cardiac Surgery. Thesis, Erasmus University, Rotterdam, Netherlands; Amsterdam, Benecke Consultants, 1994, pp 78–89Google Scholar
13. Trzepacz PT, Baker RW, Greenhouse J: A symptom rating scale for delirium. Psychiatry Res 1988; 23:89–97Crossref, Medline, Google Scholar
14. Rosen J, Sweet RA, Mulsant BH, et al: The DRS in a psychogeriatric inpatient setting. J Neuropsychiatry Clin Neurosci 1994; 6:30–35Link, Google Scholar
15. Rockwood K, Goodman J, Flynn M, et al: Cross-validation of the Delirium Rating Scale in older patients. JAGS 1996; 44:839–842Crossref, Google Scholar
16. Mulsant BH, Stergiou A, Keshavan MS, et al: Schizophrenia in late-life: elderly patients admitted to an acute care psychiatric hospital. Schizophr Bull 1993; 19:709–721Crossref, Medline, Google Scholar
17. Zubenko GS, Rosen J, Sweet RA, et al: Impact of psychiatric hospitalization on behavioral complications of Alzheimer's disease. Am J Psychiatry 1992; 149:1484–1491Crossref, Medline, Google Scholar
18. Zubenko GS, Sullivan P, Nelson JP, et al: Brain imaging abnormalities in mental disorders of late life. Arch Neurol 1990; 47:1107–1111Crossref, Medline, Google Scholar
19. Mayo Clinic: Clinical Examinations in Neurology, 6th edition. St. Louis, MO, Mosby Year Book, 1991Google Scholar
20. Folstein MF, Folstein SE, McHugh PR: “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198Crossref, Medline, Google Scholar
21. Molloy DW, Alemayehu E, Roberts R: A standardized Mini-Mental State Examination (SMMSE): its reliability compared to the traditional Mini-Mental State Examination. Am J Psychiatry 1991; 148:102–105Crossref, Medline, Google Scholar
22. Fliess JL: The Design and Analysis of Clinical Experiments. New York, Wiley, 1986Google Scholar
23. American Psychiatric Association: DSM-III-R: Diagnostic and Statistical Manual of Mental Disorders, 3rd edition, revised. Washington, DC, American Psychiatric Association, 1987Google Scholar
24. Jacobson SA, Leuchter AF, Walter DO: Conventional and quantitative EEG in the diagnosis of delirium among the elderly. J Neurol Neurosurg Psychiatry 1993; 56:153–158Crossref, Medline, Google Scholar
25. Koponen H, Partanen J, Paakkonen A, et al: EEG spectral analysis in delirium. J Neurol Neurosurg Psychiatry 1989; 52:980–985Crossref, Medline, Google Scholar
26. Trzepacz PT: Neuropathogenesis of delirium: a need to focus our research. Psychosomatics 1994; 35:374–391Crossref, Medline, Google Scholar
27. Wada Y, Yamaguchi N: Delirium in the elderly: relationship of clinical symptoms to outcome. Dementia 1993; 4:113–116Medline, Google Scholar



