Risperidone for the Treatment of Behavioral Disturbances in Dementia
Abstract
The authors describe a series of 22 patients with dementia and behavioral disturbances, including agitation, aggression, delusions, and hallucinations, who were treated with risperidone. Risperidone, in low doses, was well tolerated; 50% of patients experienced significant improvement, although 50% experienced some extrapyramidal symptoms.
Behavioral disturbances associated with dementia (BDD) are a common remediable cause of excess morbidity. Behavior such as agitation, aggression, delusions, hallucinations, anxiety, depression, shouting, and wandering can lead to significant impairment in the quality of life for both patients and their caregivers, as well as an increased risk of institutionalization. A recent review on the pharmacotherapy of BDD noted that, compared with other agents, the neuroleptics have been best studied with relatively rigorous placebo-controlled trials.1 Efficacy is modest, but concerns regarding side effects, especially extrapyramidal symptoms (EPS), have often limited their use. As a result, treatment of BDD with atypical neuroleptics such as clozapine or risperidone is potentially advantageous in view of their tendency to cause considerably fewer extrapyramidal effects. Unfortunately, there are no placebo-controlled trials of atypical neuroleptics for the treatment of BDD and very little anecdotal evidence for their use in this indication. To provide some preliminary information on the safety and effectiveness of risperidone for BDD, we report on our collective experience to date.
PATIENTS
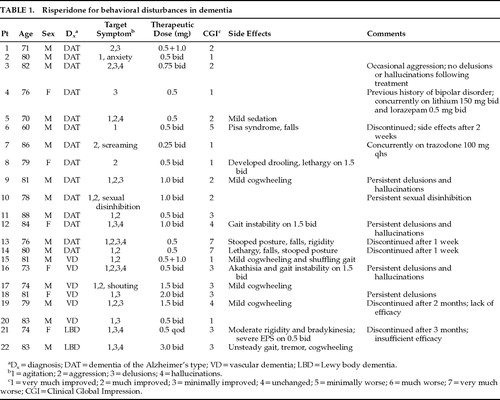
All patients met DSM-IV criteria for dementia. Specific etiological diagnoses were made clinically by academic geropsychiatrists. Twenty-two patients were included in this series, averaging 77.6±6.64 years of age (range 60–88). Of these, 73% (16 of 22) were males. There were 14 patients with dementia of the Alzheimer's type, 6 with vascular dementia, and 2 with Lewy body dementia (meeting consensus guidelines for the clinical diagnosis of dementia with Lewy bodies2). The most common target symptoms included agitation, aggression, hallucinations, and delusions. All patients except 2 (#4 and #8) had previous trials of psychotropics, including at least one unsuccessful trial of a typical neuroleptic (ineffective or not tolerated). In fact, 16 of these patients had experienced serious EPS when treated previously with typical neuroleptics. This was the clinicians' most common rationale for the institution of a trial of risperidone. The patients, the treatment, and outcome are described in Table 1.
RESULTS
The average therapeutic dose of risperidone used was 1.55±1.36 mg per day (range 0.5 mg qod to 3 mg bid). Only 2 patients received low-dose anticholinergic medication (e.g., procyclidine 2.5 mg bid). Length of trials varied from 1 week to 10 months. Risperidone was discontinued in 5 patients (23%). In 3 patients risperidone was discontinued within 1 to 2 weeks because of side effects including lethargy, stooped posture, rigidity, and falls. Risperidone was discontinued in another 2 patients because efficacy was lacking or insufficient after 2 and 3 months, respectively. EPS were noted in 11 of 22 (50%), although symptoms were mild in 4 of these. Other side effects noted in the group as a whole included sedation and drooling. There were no reported cases of orthostatic hypotension. On Clinical Global Impression,3 6 patients (27%) were rated as very much improved, 5 (23%) were rated as much improved, 6 (27%) were rated as minimally improved, 2 (9%) were rated as unchanged, 1 (5%) was rated as minimally worse, and 2 (9%) were rated as very much worse.
The following are several examples described in more detail.
Case Report
Patient 8. This 79-year-old woman was a resident in a long-term care facility. She was diagnosed as having dementia of the Alzheimer's type, Global Deterioration Scale (GDS)4 stage 6–7. Other than a history of syncope and a fractured ankle, she was medically well. She was extremely resistant to care and physically aggressive toward staff and co-residents. She would wander and make numerous attempts to leave the facility, often pulling other residents with her. Even though she had not been treated previously with a neuroleptic, her family chose treatment with risperidone as a first-line agent because of their concerns about side effects of neuroleptics. She was started on risperidone 0.25 mg bid and slowly increased to 1.5 mg bid. Behavior improved significantly. She was no longer aggressive, and staff were able to bathe and shower her with much less agitation. Over a period of 1 month on 1.5 mg bid, however, she became lethargic and would drool. Risperidone was reduced to 0.5 mg bid, with resolution of these side effects. Overall outcome was assessed as very much improved over 10 months of ongoing therapy.
Patient 20. This 83-year-old man was a resident in a long-term care facility. He was diagnosed as having vascular dementia and scored 15/30 on the Mini-Mental State Examination.5 His medical history included carcinoma of the lung, coronary artery disease, and atrial fibrillation. His only concurrent medication was digoxin. He had marked persecutory delusions of people trying to steal his belongings and attempting to harm him. These resulted in ongoing agitation manifested by repeated perseverative complaints, calling out for help, restlessness, and anxiety, which made it difficult for staff to give him care. He was previously treated with haloperidol, methotrimeprazine, and lorazepam, all of which were ineffective. He was treated with risperidone 0.5 mg bid and showed a marked decrease in delusions, agitation, calling out, restlessness, and anxiety, allowing staff to manage him quite easily. No EPS were noted, and he was assessed as being very much improved over the course of his 6-month trial.
Patient 22. This 83-year-old man was admitted to an acute psychogeriatric unit for marked delusions, hallucinations, and agitation. He was diagnosed as having dementia and fulfilled consensus guidelines for the clinical diagnosis of dementia with Lewy bodies.2 He was terrified that the Mafia was trying to kill him, and he was constantly responding to auditory hallucinations of voices telling him they were “coming to get him.” He would barricade his room at night and would not sleep for fear of being killed. Because of his distress, he was treated with loxapine in increasing doses of up to 20 mg per day. His delusions and hallucinations slowly decreased, but he experienced severe tremor, akinesia, and several falls. A brief trial of clozapine 6.25 mg was discontinued because of excessive sedation. He was started on risperidone 0.5 mg. On low doses of risperidone he had no EPS, and there was a diminution of his agitation, as evidenced by a decrease in restlessness and calling out and fewer attempts to leave the unit. His delusions and hallucinations, however, persisted. As a result, risperidone was increased slowly to a maximum of 3 mg po bid. At this dosage there was no further improvement in the psychosis, but there was an increase in EPS, including tremor, rabbit syndrome, and bradykinesia. The risperidone was discontinued after a 3-month trial as a result of lack of sufficient efficacy rather than side effects. He was assessed as being minimally improved in view of the decrease in his agitation.
Patient 14. This 80-year-old man was a resident in a long-term care facility. He was diagnosed as having dementia of the Alzheimer's type, GDS stage 6. He had a history of hypertension and B12 deficiency but was on no medications other than monthly B12 injections. He was very aggressive with staff and had injured several nurses by punching and kicking them during care. Agitation was manifested by constant pacing, restlessness, and negativism. He had several unsuccessful trials of medications, including lorazepam 0.5 mg prn (ineffective), trazodone 50 mg po qhs (excessive daytime sedation), chlorpromazine 10 mg bid (excessive sedation), and loxapine 5 mg (marked rigidity and bradykinesia). He was started on risperidone 0.5 mg, and within several days he was overly sedated, was no longer able to feed himself, and could no longer walk because of gait instability and markedly stooped posture. The risperidone was discontinued, and he improved to baseline within 3 days.
DISCUSSION
Conclusions based on the results of a case series must be modest in view of their inherent biases and lack of controls. This caution is especially important with studies on the pharmacotherapy of BDD, in which placebo-response rates can be as high as 40%.6 With this caveat in mind, the results of the series suggest that risperidone in low doses was fairly well tolerated, with 50% experiencing significant improvement and 77% experiencing at least minimal improvement. Risperidone was discontinued in only 3 patients because of side effects, and although 50% experienced at least some EPS, these were often mild in nature. The latter is particularly important in view of this group's previous experience with significant EPS when treated with typical neuroleptics. EPS occurred in both patients with Lewy body dementia (#21, #22), patients with preexisting parkinsonism (#13, #17), and patients who had previously experienced severe EPS when treated with typical neuroleptics (#6, #9, #12, #14, #15). These symptoms were often dose-related, and they diminished with dose reduction in 3 patients (#5, #12, #21). EPS occurred within the first week of treatment in 3 patients (#9, #13, #14), and within 1 week of dosage escalation in 3 others (#12, #16, #21). In the remaining 5 patients who also developed EPS, these occurred within 1 month of treatment initiation. There were no cases of reported orthostatic hypotension, a potential problem noted previously with risperidone.7 Because of the retrospective nature of this report, it is unclear whether orthostatic hypotension did not occur or simply was not assessed in this series.
Agitation manifested by restlessness, negativism, pacing, attempts to leave, and aggression seemed most responsive to risperidone. Delusions and hallucinations improved in some patients and persisted in others.
Only a small amount of anecdotal literature has been published on the use of risperidone for the treatment of BDD. In a small series of 11 geriatric inpatients, only 3 of whom had BDD, marked improvement was noted with doses of 1.5–6 mg per day (bid dosages).7 These 3 patients experienced side effects, including orthostatic hypotension, dizziness, somnolence, and nonpostural hypotension. In an unpublished preliminary report of 9 elderly patients with BDD treated prospectively with open-label risperidone, 7 showed significant decreases in scores on a behavioral rating scale, with average daily doses of 2.5 mg. Two patients dropped out because the drug was not efficacious.8 Risperidone has also been recommended as a treatment for behavioral disturbances in Lewy body dementia in view of these patients' sensitivity to neuroleptic-induced EPS. Lee et al.9 noted improvement in psychosis and agitation in a case report of a 74-year-old patient with Lewy body dementia treated with 2 mg per day of risperidone. Allen et al.10 described 3 patients with Lewy body dementia who were treated with risperidone 0.5 mg once or twice daily. All 3 patients experienced significant reductions in psychosis and agitation. Risperidone was well tolerated, with no decline in cognitive function noted. There have been two conflicting reports on the use of risperidone for the treatment of behavioral disturbances associated with Parkinson's disease. Rich et al.11 noted improvement in only 1 patient and “intolerable” exacerbations of parkinsonism in 5 of 6 patients treated with risperidone. Patients received 2–4 mg per day (average 2.2 mg) of risperidone in this series. In contrast, Meco et al.12 noted significant improvement in hallucinations and Brief Psychiatric Rating Scale scores in a group of 6 Parkinson's disease patients when they were treated with 0.25–1.25 mg per day. In this study there was no reported worsening of motor symptoms or cognition when patients were treated with these low dosages.
In summary, although there is little anecdotal evidence for the use of risperidone in the treatment of behavioral disturbances in dementia, the current series suggests that risperidone can be used safely with potentially good effectiveness. Previous clinical recommendations for the use of risperidone in elderly schizophrenic patients have included a maximum daily dose of 2 mg per day.13 Based on the current study and previous reports, recommendations for the use of risperidone in elderly demented patients with behavioral disturbances would include starting at the lowest possible dose available (0.25–0.5 mg) with slow titration upward to a maximum dose of 1.0 mg bid. In patients with preexisting parkinsonism, Lewy body dementia, or known EPS sensitivity to typical neuroleptics, maximum dosages might be even lower (0.5–1 mg per day). Side effects including lethargy, gait instability, orthostatic hypotension, and EPS should be monitored on an ongoing basis.
Besides the benefits of lower potential to cause EPS, shared by other atypical neuroleptics, risperidone may theoretically be advantageous for use in BDD considering this population's susceptibility to anticholinergic side effects.14 Risperidone has negligible effects on muscarinic receptors, whereas both olanzapine and clozapine have a high affinity for the m1 muscarinic receptor.15 Placebo-controlled trials of risperidone for the management of behavioral disturbance in dementia are urgently needed.
 |
1. Herrmann N, Lanctôt KL, Naranjo CA: Behavioral disorders in demented elderly patients: current issues in pharmacotherapy. CNS Drugs 1996; 6:280–300Crossref, Google Scholar
2. McKeith IG, Galasko D, Kosaka K, et al: Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB). Neurology 1996; 47:1113–1124Crossref, Medline, Google Scholar
3. Guy W: ECDEU Assessment Manual for Psychopharmacology (DHEW Publ No ADM 76-338). Rockville, MD, National Institute of Mental Health, 1976, pp 218–222Google Scholar
4. Reisberg B, Ferris SH, de Leon MJ, et al: The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry 1982; 139:1136–1139Crossref, Medline, Google Scholar
5. Folstein MF, Folstein SE, McHugh PR: “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198Crossref, Medline, Google Scholar
6. Schneider LS, Pollock VE, Lyness SA: A meta-analysis of controlled trials of neuroleptic treatment in dementia. J Am Geriatr Soc 1990; 38:553–563Crossref, Medline, Google Scholar
7. Madhusoodanan S, Brenner R, Araujo L, et al: Efficacy of risperidone treatment for psychoses associated with schizophrenia, schizoaffective disorder, bipolar disorder, or senile dementia in 11 geriatric patients: a case series. J Clin Psychiatry 1995; 56:514–518Medline, Google Scholar
8. Mertens C, Heylen SL: Risperidone in psychogeriatric patients with behavioral disturbances: an open long-term follow-up study. Report for trial RIS-BEL-13. Janssen Research Foundation, 1990 (data on file)Google Scholar
9. Lee H, Cooney JM, Lawlor BA: The use of risperidone, an atypical neuroleptic, in Lewy body disease. Int J Geriatr Psychiatry 1994; 9:415–417Crossref, Google Scholar
10. Allen RL, Walker Z, D'Ath PJ, et al: Risperidone for psychotic and behavioral symptoms in Lewy body dementia (letter). Lancet 1995; 346:185Crossref, Medline, Google Scholar
11. Rich SS, Friedman JH, Ott BR: Risperidone versus clozapine in the treatment of psychosis in six patients with Parkinson's disease and other akinetic-rigid syndromes. J Clin Psychiatry 1995; 56:556–559Medline, Google Scholar
12. Meco G, Alessandria A, Bonifati V, et al: Risperidone for hallucinations in levodopa-treated Parkinson's disease patients. Lancet 1994; 343:1370–1371Crossref, Medline, Google Scholar
13. Jeste DV, Eastham JH, Lacro JP, et al: Management of late-life psychosis. J Clin Psychiatry 1996; 57(suppl 3):39–45Google Scholar
14. Sunderland T, Tariot PN, Cohen RM, et al: Anticholinergic sensitivity in patients with dementia of the Alzheimer type and age-matched controls: a dose-response study. Arch Gen Psychiatry 1987; 44:418–426Crossref, Medline, Google Scholar
15. Richelson E: Preclinical pharmacology of neuroleptics: focus on new generation compounds. J Clin Psychiatry 1996; 57(suppl 11):4–11Google Scholar



