Clinical and Physiological Effects of Stereotaxic Bilateral Amygdalotomy for Intractable Aggression
Abstract
The amygdala is thought to be an important neural structure underlying the “fight-or-flight” response, but information on its role in humans is scarce. The clinical and psychophysiological effects of amygdalar destruction were studied in 2 patients who underwent bilateral amygdalotomy for intractable aggression. After surgery, both patients showed a reduction in autonomic arousal levels to stressful stimuli and in the number of aggressive outbursts, although both patients continued to have difficulty controlling aggression. The “taming effect” reported after bilateral amygdalar destruction may be due to the amygdala's inadequate processing of perceived threat stimuli that would normally produce a fight-or-flight response.
Stereotaxic amygdalotomy was first carried out by Narabayashi et al.1 for control of abnormal behaviors such as aggressiveness, violence, and hyperactivity. This approach was based in part on animal studies showing that electrical stimulation of the corticomedial region of the amygdala produced aggressive reactions2 and that lesions of this region resulted in diminished aggression and reduced fear behavior in the face of threatening situations.3,4 Reduction of aggressive behaviors after amygdalar destruction has been called the “taming effect,” and investigations demonstrating this effect provide the scientific rationale for bilateral amygdalotomy in humans to manage intractable aggression.
Following Narabayashi et al.,1 there have been more than 1,000 cases of stereotaxic amygdalotomy reported as a last-resort treatment for various behavioral disturbances—primarily assaultiveness, aggression, and hyperactivity.5–15 Aggleton16 concluded that the surgical procedures used in these amygdalotomies have shown a surprising degree of variation. For example, different approaches are used to reach the amygdala (e.g., transtemporal, dorsal/vertex), and the methods used for amygdalar destruction have included radiofrequency, cryothermy, mechanical destruction, and the injection of substances such as alcohol, oil, kaolin, and wax.5,17–21
The preferred target zones for destruction within the amygdala differ among surgical centers and have included the basal and lateral nuclei,22–24 the medial amygdala,17,25 the anteromedial amygdala,14 the corticomedial group of nuclei,26 and the bed nucleus of the stria terminalis.27 The intended size of amygdalar lesions has also varied, from between one-third and one-half,1 to three-quarters,28 to all or nearly all of the entire structure.29 The human reports of amygdalotomy also differ with regard to patient selection criteria, lateralization of lesion, length of follow-up period, and criteria for positive outcome. Despite these considerable methodological differences, the published human amygdalotomy reports have generally indicated beneficial results in reducing the severity and frequency of aggressive behaviors. Although these reports are largely anecdotal, they suggest that fear and aggression are more difficult to provoke, hyperactivity has generally decreased to normal levels, and emotional control has been enhanced.30
The mechanisms that produce this reduction in aggression and hyperactivity after amygdalotomy are unknown. A decline in the level of autonomic arousal may be related to the diminished aggression observed after bilateral destruction of the amygdala. Stimulation or ablation of various amygdalar nuclei in animals has produced alterations of autonomic nervous system functions such as heart rate, respiration, skin conductance response, and other aspects of the orienting response and emotional behavior.31–36 Amygdalectomized monkeys fail to show the heart and respiratory rate components of the orienting response, and their skin conductance responses are markedly depressed.
Unfortunately, many questions arise regarding the applicability of these animal results to humans because there may be subtle species variation in the neural structures (and their pattern of connectivity) underlying autonomic activity. Alternatively, because of the species' different behavioral repertoires, these brain systems may function differently in monkeys and humans. Moreover, the few studies that have examined skin conductance responses in humans after damage to the amygdala have yielded contradictory results.8,9,37,38
Recent work in humans has suggested that the amygdala may be vital for the acquisition of autonomic conditioned responses to both visual and auditory stimuli.39,40 Information has also accumulated implicating the human amygdala's involvement in cognitive processing of emotional stimuli, especially fear.41,42 To help determine if the amygdala is integral to autonomic functions in humans, and whether a reduction in arousal level could be an important factor in the reported reduced emotional expression (aggression), we examined the behavioral, cognitive, and psychophysiological effects of amygdalar destruction pre- and postoperatively in 2 patients who underwent bilateral stereotaxic amygdalotomy for intractable aggression.
CASE DESCRIPTIONS
Amygdalotomy Case 1 (R.L.). At the time of surgery in the late 1980s, R.L. was a 19-year-old right-handed white male who was the product of a normal pregnancy and birth and showed normal growth, development, and school performance until the age of 10 years, when he contracted viral encephalitis thought to be caused by herpes simplex. One month later, R.L. developed complex partial seizures with secondary generalization that have been well controlled with antiepileptic drugs. In the months subsequent to his initial illness, R.L. showed profound behavioral changes characterized chiefly by severe disinhibition of affect and impulsivity as evidenced by outbursts of aggressive rage. Because of the severity of his behavioral disturbance, R.L. was unable to continue his education, and he stopped attending school in the fifth grade. There were multiple psychiatric hospitalizations for violent outbursts until he was permanently institutionalized at age 13 years. Pharmacological therapy by many different psychiatrists and neurologists over 9 years (ages 10–19) did not control the aggressive outbursts.
Repeated preoperative neurological examinations were normal, the patient showing no sensory, motor, cranial nerve, reflex, or cerebellar abnormalities. Multiple EEGs were normal with the exception of one that showed spike and sharp wave activity over the right temporal region. Preoperative CT scan of the head revealed a small, low-density lesion in the left dorsomedial thalamic region but was otherwise normal. This patient has been the subject of several previous reports.9,29,43 The patient and his family were fully informed about the risks and possible benefits of the surgery, and written informed consent was obtained.
Amygdalotomy Case 2 (D.B.B.). At the time of surgery in the late 1980s, D.B.B. was a 21-year-old right-handed white male who was the product of a normal pregnancy and birth. He showed normal growth and development until he was 8 months old, when he contracted chickenpox encephalitis. Approximately one year later, he developed partial complex seizures that were well controlled with antiepileptic drugs. School performance and behavior were reportedly normal until age 12 years. At that time uncontrollable episodes of rage directed against people and objects began during puberty and gradually became more frequent and serious until he was institutionalized at age 13 years. D.B.B.'s outbursts of rage resulted in an inability to attend school beyond the ninth grade. Pharmacologic management by many different physicians over 9 years (ages 12–21) did not adequately control the aggressive outbursts.
Neurological examinations conducted over a 20-year period were generally within normal limits. One week prior to surgery, there was moderate nystagmus on vertical and horizontal gaze and slight instability during tandem walking. CT and MRI scans 2 months before surgery were normal. An EEG 2 months before surgery was abnormal due to diffuse slowing. The patient and his family were fully informed about the risks and possible benefits of the surgery, and written informed consent was obtained.
Description of Surgery. Bilateral amygdalotomies were performed on R.L. and D.B.B. under general endotracheal anesthesia with a modified Todd-Wells stereotaxic apparatus. Quarter-inch (0.64 cm) twist drill holes were placed 3 cm to either side of midline on a plane with the coronal suture. Target points were derived from examination of preoperative MRI scans, a standard stereotaxic brain atlas,44 and lateral skull X-rays obtained after the patients' heads had been fixed in the stereotaxic apparatus. Left amygdalar targets were located 18 mm and 23 mm to the left of midline. Right amygdalar targets were located 20 mm and 25 mm to the right of midline.
The deepest lesions on both sides were located at the level of the base of the dorsum sellae. A temperature-monitoring electrode with a 2.1 mm×5 mm uninsulated tip (Radionics, Cambridge, MA) was used at a temperature of 80° C for 90 seconds in all lesioning. Deep, intermediate, and superficial lesions were made at 4-mm intervals for each of the target sites. The estimated size of the completed lesions was 15 mm in diameter in both patients, and this was verified by postoperative MRI scans. This lesion size ensured complete, or nearly complete, destruction of the amygdalar complex in both patients (Figures 1, 2, and 3).
Procedures. Psychophysiological measures included electromyography (EMG) of facial muscles and skin conductance response (SCR) measured from the hands. A Grass model 7400 physiological recorder was used. For facial EMG recording, the physiological recorder was connected to a high-performance AC signal conditioner. For SCR recording, the physiological recorder was connected to an AC/DC transducer signal conditioner, interfaced with an SCA1 skin conductance adapter. The physiological recorder was equipped with a built-in computer for data analysis and storage.
Tonal habituation apparatus, to measure SCR habituation, consisted of a Macintosh PowerBook computer with a software program that delivers a 1-second, 60-db tone bilaterally through stereo earphones. There was at least a 10-second interval between the delivery of two consecutive stimuli. A change in skin conductance ≥0.5 microSiemens (μS) was considered to be a tonal response. Habituation was defined as an absence of response for six consecutive trials with no consistent series of responses occurring for the remaining trials.
SCR was measured in microSiemens with disposable Ag-Cl electrodes attached to the thenar and hypothenar eminence over the palm of the left hand. SCR was time sampled every 60 seconds during the stressor conditions by recentering the pen and noting the value. During the tonal habituation condition, SCR was monitored constantly.
Facial EMG measurements were obtained from the frontalis muscle by attaching two disposable Ag-Cl electrodes to the forehead, approximately 2.5 cm above the left eyebrow. One was an active electrode that was placed in line with the pupils and another was a ground electrode that was attached to the middle of the forehead in order to minimize 50-Hz artifact. Following electrode attachment, electrode impedance was checked; an acceptable criterion was 10 kΩ or less. EMG was determined by integrating amplitude in μV across each second and averaging every 10 seconds. Bandpass was set at 100–200 Hz.
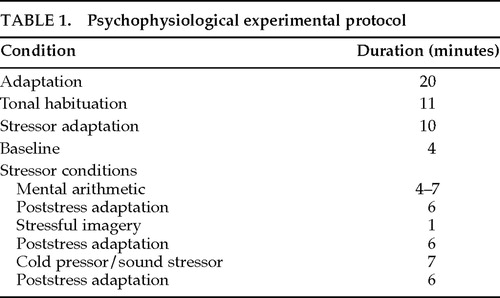
Psychophysiological assessments were performed 1 day before and 5 days after surgery on patient R.L., and 2 days before and at both 5 months and 8 years after surgery on patient D.B.B. Informed consent was obtained from both patients after the procedures had been fully explained. Autonomic measurement and stressor condition procedures differed slightly at the 8-year assessment. Sessions lasted approximately 75 minutes and consisted of an adaptation and several stressor conditions listed in Table 1.
Physiological Results. Skin conductance response habituation to the tonal stimulus did not occur after 60 trials in patient R.L. before amygdalotomy, whereas after surgery patient R.L. showed normal tonal habituation (within 14 trials). Patient D.B.B. displayed no SCR to the tonal stimuli either before or 5 months after amygdalotomy, but he displayed normal SCR tonal habituation (within 13 trials) at the 8-year assessment.
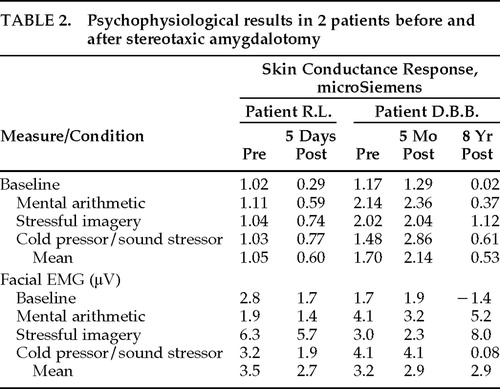
Table 2 provides SCR and facial EMG results before and after amygdalotomy for each of the four experimental conditions (baseline and three stressor conditions). As shown in Table 2, the physiological assessment revealed marked autonomic hyperarousal in both patients before surgery. The preoperative mean SCRs were 1.05 and 1.70 μS in our patients—considerably more than reported in normal control subjects, where it typically ranges between 0.115 and 0.626 mean μS.38,45
As may also be seen in Table 2, there were statistically significant reductions in SCR following amygdalotomy in both R.L. (F=17.3, df=3,37, P=0.02) and D.B.B. (pre vs. 5 mos. post vs. 8 yrs. post, F=17.3, df=3,58, P=0.003). Follow-up analyses in D.B.B. revealed no statistically significant differences between the preoperative and 5-month postoperative SCR results (Scheffé F=1.18, P>0.05), however, both preoperative SCR (Scheffé F=8.59, P<0.05) and 5-month postoperative SCR (Scheffé F=16.15, P<0.05) differed from the 8-year postoperative SCR results to a statistically significant degree.
There was a statistically significant facial EMG reduction in R.L. after surgery (F=20.53, df=3,4, P=0.02), but a similar statistically significant reduction was not seen in D.B.B. (F=0.02, df=3,8 P>0.05). None of the follow-up analyses between each of D.B.B.'s three facial EMG assessments achieved statistical significance.
Clinical Results. With regard to the efficacy of the bilateral amygdalotomy, there was a decline in the number of aggressive outbursts in both patients, but they both have continuing difficulties controlling aggressive outbursts. Similar to the physiological results, the “taming effect” following surgery was immediate in R.L. and took place more gradually in D.B.B.
Because R.L. remained in a psychiatric facility after the amygdalotomy until 1997, follow-up has been meticulously documented. Before surgery, R.L. averaged 7 assaultive outbursts each month. During the first year after surgery, R.L. had only 1 documented assaultive episode. In the second postoperative year, R.L.'s aggressive outbursts rebounded to an average of 5 assaults each month. Since that time these outbursts have again declined. R.L. has averaged approximately 1 assaultive episode every other month for the past 8 years. Aggressive outbursts have consisted of self-abuse (e.g., stabbing neck with pencil, slamming head against wall, drinking liquid laundry detergent), destruction of property (e.g., breaking windows, tearing clothing off himself, throwing and breaking ceramics, destroying furniture), and assaults (e.g., biting, kicking, hitting, and stabbing others, resulting in broken bones, teeth, dentures, and glasses). In 1997, R.L. was discharged after 16 years from a state hospital to a personal care home. He has lived there without an assaultive outburst for 7 months.
Although the effects of the amygdalotomy are more difficult to establish in D.B.B. because of frequent changes in living circumstances, the surgery ultimately must be considered a failure. In the year before surgery, D.B.B. reportedly had daily episodes of aggressive outbursts consisting of throwing, kicking, and breaking objects, and he physically attacked others 1 to 2 times each week. According to his parents, there was no change in D.B.B.'s pattern of aggressive outbursts during the first 3 months after surgery (daily angry outbursts). Beginning in the fourth postoperative month, the temper tantrums began to diminish in frequency and severity. Over the past 8 years D.B.B. has lived in many different personal care homes because his parents became unable to take care of him. Telephone interviews with his caretakers revealed that physical assault on others was an exceedingly rare event. However, impulsive outbursts not involving aggression against others (e.g., running away, spending all his money immediately after receiving it, compulsive alcohol consumption) have been more common, occurring approximately once per week. In late 1996, D.B.B. assaulted the supervisor of his personal care home, causing the supervisor to suffer significant head injury (coma lasting several days), multiple broken bones, and loss of an eye. Bystanders reported that D.B.B. stopped this assault only after being restrained by other residents of the personal care home. The patient was arrested and imprisoned until the following summer, when he was found not guilty by reason of insanity and committed to the forensic unit of a state psychiatric hospital.
With regard to cognitive outcome, repeated neuropsychological examinations over the years have shown no significant pre- to post-amygdalotomy changes in any area of cognitive functioning, including intellectual functions, new learning and memory, language, visual perceptual and visual spatial functions, sensorimotor abilities, and complex problem solving. Details of many of the cognitive examinations have been reported previously.43,46
DISCUSSION
Before surgery, the physiological assessment revealed marked autonomic nervous system hyperarousal in both patients, which is consistent with their clinical presentation of assaultive behavior in response to threats that were not apparent to observers. Following bilateral stereotaxic destruction of the amygdala, in both patients there was a marked reduction in autonomic arousal, especially in skin conductance response, and an absence of any persistent neuropsychological deficits. The diminished autonomic arousal was accompanied by a reduction in, but not elimination of, assaultive behavior. These results extend the animal investigations suggesting the amygdala is important for associating stressful stimuli with an autonomic arousal response. Further, the so-called taming effect after bilateral amygdalotomy may be due to the amygdala's inadequate processing of perceived threat stimuli that would normally produce a “fight-or-flight” response.
Because bilateral amygdalar destruction results in both a taming effect (placidity) and reduced autonomic nervous system arousal, the amygdala is probably important in mediating these behavioral and physiological events. Sympathetic hyperarousal was evident in both of our patients before surgery relative to normal controls. This preoperative hyperarousal may have been due to damage to limbic structures caused by viral encephalitis, which frequently produces necrotizing hemorrhagic damage to the orbitofrontal and ventromedial temporal regions.47 These data, in conjunction with others,48,49 suggest that both the amygdala and orbitofrontal (limbic) frontal cortex participate in the distributed neural systems that underlie emotion.
Amygdalar stimulation and cortical ablation studies in animals have suggested that reciprocal systems underlie the autonomic aspects of the fight-or-flight response. Ursin and Kaada2 reported a dissociation between aggressive (fight) behaviors and fear (flight) responses during stimulation of various amygdalar nuclei with implanted electrodes. Orbitofrontal lesions regularly produce hyperreactivity of the visceroautonomic components of the orienting response, while dorsolateral frontal resections abolish normal autonomic reactivity.50–54 Consequently, researchers have hypothesized that the dorsolateral frontal system is facilitatory and the orbitofrontal neural network is inhibitory.55 It is possible that our patients' encephalitis caused a disruption in the normal balance between these two reciprocal neural systems that resulted in a reduced capacity to inhibit aggressive impulses. Bilateral amygdalotomy may have resulted in a return to normal autonomic reactivity by restoring the balance between these reciprocal systems that mediate the fight-or-flight response.
The relationship between autonomic arousal and aggression is complex. Evidence suggests that autonomic arousal is on a continuum with fight-or-flight reactions. Low-level electrical stimulation of those portions of the hypothalamus interconnected with the amygdala can produce mild autonomic arousal in monkeys, as evidenced by postural alerting, pricking of the ears, and changes in respiration rate and blood flow.56 When electrical stimulation is increased and maintained, behaviors characteristic of the fight-or-flight response emerge, which include snarling, running, and piloerection.57 Although these observations demonstrate a clear link between arousal and aggression, this does not mean that hyperarousal causes aggression, since increased autonomic arousal levels are associated with many behaviors and emotions other than aggression (e.g., sexual activity, crying, laughter). Nevertheless, autonomic arousal appears to be an important component, either precipitating or co-occurring with aggression. Systemic hyperarousal may provide an essential context that enables fear to develop into aggression.
With regard to the clinical aspects of these cases, bilateral amygdalotomy produced a reduction in the most dangerous aspects of our patients' behavior: attacks against others. Unfortunately, the taming effect was not permanent in D.B.B.; he violently attacked another individual 8 years after surgery. The taming effect was immediate in R.L., but it emerged more gradually in D.B.B. over a 4-month period after surgery. In a similar vein, the diminished autonomic arousal was seen within a few days after surgery in R.L., but there was no autonomic effect in D.B.B. until more than 5 months after surgery. These differential amygdalotomy effects may be due to the differences in the neurological histories of our patients. R.L.'s encephalitis occurred when he was a preadolescent (10 years old), whereas D.B.B.'s brain insult occurred when he was an infant (8 months of age). Because infants' brains have a great deal of neural plasticity, it is possible that D.B.B.'s brain underwent a reorganization of neural circuitry after the encephalitis. Thus, perhaps the “normal” neuronal connections were not present in D.B.B., resulting in a different time frame for the surgical effects to appear and a poorer surgical outcome.
The desired presurgical objective of eliminating assaultive outbursts in our patients was not entirely met. Although there was a definite reduction in the number of assaults against others in both patients, this effect was not complete. Moreover, these results must be evaluated in the context of our patients' normal growth and development. That is, as expected in normal psychological development, these patients were maturing from the violence-prone years of adolescent and early adulthood into adulthood, where violence against others becomes less likely. Thus, the therapeutic success in these cases appears to be less than ideal, reinforcing the view that bilateral amygdalotomy should be undertaken only in extreme cases after all other therapeutic options have been exhausted.
ACKNOWLEDGMENTS
This study was partly supported by National Institute of Neurological Disorders and Stroke Grant P01 NS19632 to Antonio R. Damasio. Results were presented in part at the 25th annual meeting of the International Neuropsychological Society, Orlando, FL, February 1997.
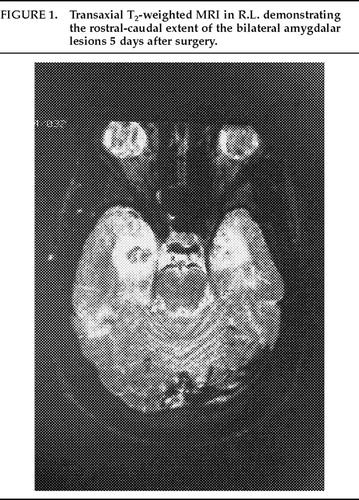
FIGURE 1. Transaxial T2-weighted MRI in R.L. demonstrating the rostral-caudal extent of the bilateral amygdalar lesions 5 days after surgery.
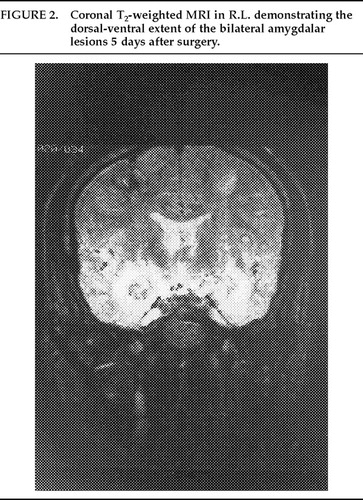
FIGURE 2. Coronal T2-weighted MRI in R.L. demonstrating the dorsal-ventral extent of the bilateral amygdalar lesions 5 days after surgery.
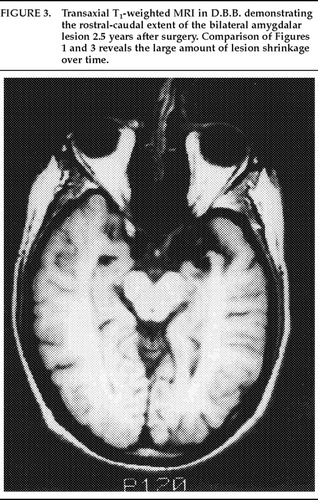
FIGURE 3. Transaxial T1-weighted MRI in D.B.B. demonstrating the rostral-caudal extent of the bilateral amygdalar lesion 2.5 years after surgery. Comparison of Figures 1 and 3 reveals the large amount of lesion shrinkage over time.
 |
 |
1. Narabayashi H, Nagao T, Saito Y, et al: Stereotaxic amygdalotomy for behavioral disorders. Arch Neurol 1963; 9:1–16Crossref, Medline, Google Scholar
2. Ursin H, Kaada BR: Functional localization within the amygdaloid complex in the cat. Electroencephalogr Clin Neurophysiol 1960; 12:1–20Crossref, Medline, Google Scholar
3. Luiten PG, Koolhaas JM, de Boer S, et al: The corticomedial amygdala in the central nervous system organization of agnostic behavior. Brain Res 1985; 332:283–297Crossref, Medline, Google Scholar
4. Maeda H, Kirata K: Two-stage amygdaloid lesions and hypothalamic rage: a method useful for detecting functional localization. Physiol Behav 1978; 21:529–530Crossref, Medline, Google Scholar
5. Heimburger RF, Whitlock CC, Kalsbeck JE: Stereotaxic amygdalotomy for epilepsy with aggressive behavior. JAMA 1966; 198:741–745Crossref, Medline, Google Scholar
6. Heimburger RF, Small IF, Small JG, et al: Stereotactic amygdalotomy for convulsive and behavior disorders: long-term follow-up. Applied Neurophysiology 1978; 41:43–51Crossref, Medline, Google Scholar
7. Hood TW, Siegfried J, Wieser HG: The role of stereotactic amygdalotomy in the treatment of temporal lobe epilepsy associated with behavioral disorders. Applied Neurophysiology 1983; 46:19–25Medline, Google Scholar
8. Kiloh LG, Gye RS, Rushworth RG, et al: Stereotactic amygdalotomy for aggressive behavior. J Neurol Neurosurg Psychiatry 1974; 37:437–444Crossref, Medline, Google Scholar
9. Lee GP, Arena JG, Meador KJ, et al: Changes in autonomic responsiveness following bilateral amygdalotomy in humans. Neuropsychiatry Neuropsychol Behav Neurol 1988; 1:119–129Google Scholar
10. Mempel E: Effect of partial amygdalectomy on emotional disturbances and epileptic seizures: preliminary report. Polish Medical Journal 1971; 10:969–974Google Scholar
11. Mempel E, Witkiewicz B, Stadnicki R, et al: The effect of medial amygdalotomy and anterior hippocampotomy on behavior and seizures in epileptic patients. Acta Neurochir 1980; 30(suppl):161–167Google Scholar
12. Narabayashi H: Lessons from amygdaloid surgery in long-term observation. Acta Neurochir 1976; 23(suppl):241–245Google Scholar
13. Narabayashi H: From experiences of medial amygdalotomy on epileptics. Acta Neurochir 1980; 30(suppl):75–81Google Scholar
14. Small IF, Heimburger RF, Small JG, et al: Follow-up of stereotaxic amygdalotomy for seizure and behavior disorders. Biol Psychiatry 1977; 12:401–411Medline, Google Scholar
15. Vaernet K, Madsen A: Stereotaxic amygdalotomy and basofrontal tractotomy in psychotics with aggressive behavior. J Neurol Neurosurg Psychiatry 1970; 33:858–863Crossref, Medline, Google Scholar
16. Aggleton JP: The functional effects of amygdala lesions in humans: a comparison with findings from monkeys, in The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction, edited by Aggleton JP. New York, Wiley-Liss, 1992, pp 485–503Google Scholar
17. Balasubramaniam V, Kanaka TS: Amygdalotomy and hypothalamotomy: a comparative study. Confin Neurol 1975; 37:195–201Crossref, Medline, Google Scholar
18. Hitchcock ER, Cairns V: Amygdalotomy. Postgrad Med J 1973; 49:894–904Crossref, Medline, Google Scholar
19. Jelasic F: Relation of the lateral part of the amygdala to pain. Confin Neurol 1966; 27:53–55Crossref, Medline, Google Scholar
20. Mark VH, Sweet WH, Ervin FR: The effects of amygdalotomy on violent behavior in patients with temporal lobe epilepsy, in Psychosurgery, edited by Hitchcock E, Laitinen L, Vaernet K. Springfield, IL, Charles C Thomas, 1972, pp 139–155Google Scholar
21. Siegfried J, Reichenbach W: Personal experiences in the stereotaxic surgery of psychiatric patients, in Special Topics in Stereotaxis, edited by Umbach W. Stuttgart, Hippokrates Verlag, 1971, pp 106–111Google Scholar
22. Kim YK: Effects of basolateral amygdalotomy, in Special Topics in Stereotaxis, edited by Umbach W. Stuttgart, Hippokrates Verlag, 1971, pp 69–81Google Scholar
23. Ramanchandran V, Balasubramaniam V, Kanaka TS: Follow up of patients treated with stereotaxic amygdalotomy. Indian Journal of Psychiatry 1974; 6:299–306Google Scholar
24. Roeder F, Muller D, Orthner H: Stereotaxic treatment of psychosis and neurosis, in Special Topics in Stereotaxis, edited by Umbach W. Stuttgart, Hippokrates Verlag, 1971, pp 82–105Google Scholar
25. Kim YK, Umbach W: Combined stereotactic lesions for treatment of behaviour disorders and severe pain, in Surgical Approaches in Psychiatry, edited by Laitinen LV, Livingston KE. Baltimore, University Park Press, 1973, pp 182–188Google Scholar
26. Narabayashi H, Shima F: Which is the better amygdala target, the medial or lateral nucleus for behavioral problems and paroxysms in epileptics? In Surgical Approaches in Psychiatry, edited by Laitinen LV, Livingston KE. Baltimore, University Park Press, 1973, pp 129–134Google Scholar
27. Burzaco JA: Fundus striae terminalis, an optional target in sedative stereotaxic surgery, in Surgical Approaches in Psychiatry, edited by Laitinen LV, Livingston KE. Baltimore, University Park Press, 1973, pp 135–137Google Scholar
28. Ramamurthi B: Stereotactic operation in behaviour disorders: amygdalotomy and hypothalamotomy. Acta Neurochir 1988; 44(suppl):152–157Google Scholar
29. Lee GP, Meador KJ, Smith JR, et al: Preserved crossmodal association following bilateral amygdalotomy in man. Int J Neurosci 1988; 40:47–55Crossref, Medline, Google Scholar
30. Halgren E: The amygdala contribution to emotion and memory: current studies in humans, in The Amygdaloid Complex, edited by Ben-Ari Y. Amsterdam, Elsevier/North-Holland Biomedical Press, 1981, pp 395–408Google Scholar
31. Bagshaw MH, Mackworth NH, Pribram KH: The effect of resections of the inferiotemporal cortex or the amygdala on visual orienting and habituation. Neuropsychologia 1972; 10:153–162Crossref, Medline, Google Scholar
32. Bonvallet M, Bobo EG: Changes in phrenic activity and heart rate elicited by localized stimulation of the amygdala and adjacent structures. Electrophysiol 1972; 32:1–16Google Scholar
33. Chin JH, Pribram KH, Drake K, et al: Disruption of temperature discrimination during limbic forebrain stimulation in monkeys. Neuropsychologia 1976; 14:293–301Crossref, Medline, Google Scholar
34. Goddard GV: Functions of the amygdala. Psychol Bull 1964; 62:89–109Crossref, Medline, Google Scholar
35. Lang H, Tourinen T, Valleala P: Amygdaloid afterdischarge and galvanic skin response. EEG Clin Electrophysiol 1964; 16:366–374Crossref, Google Scholar
36. Mogenson GJ, Calaresu FR: Cardiovascular responses to electrical stimulation of the amygdala in the rat. Exp Neurol 1973; 39:166–180Crossref, Medline, Google Scholar
37. Toone BK, Cooke E, Lader MH: The effect of temporal lobe surgery on electrodermal activity: implications for an organic hypothesis in the aetiology of schizophrenia. Psychol Med 1979; 9:281–285Crossref, Medline, Google Scholar
38. Tranel D, Damasio H: Intact electrodermal skin conductance responses after bilateral amygdala damage. Neuropsychologia 1989; 27:381–390Crossref, Medline, Google Scholar
39. Bechara A, Tranel D, Damasio H, et al: Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science 1995; 269:1115–1118Crossref, Medline, Google Scholar
40. LeDoux JE, Iwata J, Cicchetti P, et al: Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci 1988; 8:2517–2529Crossref, Medline, Google Scholar
41. Adolphs R, Tranel D, Damasio H, et al: Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature 1994; 372:669–672Crossref, Medline, Google Scholar
42. Adolphs R, Tranel D, Damasio H, et al: Fear and the human amygdala. J Neurosci 1995; 15:5879–5891Crossref, Medline, Google Scholar
43. Lee GP, Reed MF, Meador KJ, et al: Is the amygdala crucial for cross-modal association in humans? Neuropsychology 1995; 9:236–245Google Scholar
44. Schaltenbrand G, Wahren W: Atlas for Stereotaxy of the Human Brain, 2nd edition. Stuttgart, G Thieme, 1977Google Scholar
45. Mefferd RB, Sadler TG, Wieland BA: Physiological responses to mild heteromodal stimulation. Psychophysiol 1969; 6:186–196Crossref, Medline, Google Scholar
46. Lee GP, Loring DW, Meador KJ, et al: Mnemonic effects of bilateral lesions in man (abstract). J Clin Exp Neuropsychol 1991; 13:21Google Scholar
47. Leech RW, Shuman RM: Neuropathology. Philadelphia, Harper and Row, 1982, pp 59–60 Google Scholar
48. Cummings JL: Frontal-subcortical circuits and human behavior. Arch Neurol 1993; 50:873–880Crossref, Medline, Google Scholar
49. Damasio AR: Toward a neurobiology of emotion and feeling: operational concepts and hypotheses. The Neuroscientist 1995; 1:19–25Crossref, Google Scholar
50. Kimble DP, Bagshaw MH, Pribram KH: The GSR of monkeys during orienting and habituation after selective partial ablations of the cingulate and frontal cortex. Neuropsychologia 1965; 3:121–128Crossref, Google Scholar
51. Luria AR, Pribram KH, Homskaya ED: An experimental analysis of the behavioral disturbance produced by left frontal arachnoidal endothelioma (meningioma). Neuropsychologia 1964; 2:257–280Crossref, Google Scholar
52. Luria AR, Homskaya ED: Frontal lobes and the regulation of arousal processes, in Attention: Contemporary Theory and Analysis, edited by Mostofsky D. New York, Appleton-Century-Crofts, 1970, pp 303–330Google Scholar
53. Ruch TC, Shenkin HA: The relation of area 13 on orbital surface of frontal lobes to hyperactivity and hyperphagia in monkeys. J Neurophysiol 1943; 6:349–360Crossref, Google Scholar
54. Wall PD, Davis GD: Three cerebral cortical systems affecting autonomic function. J Neurophysiol 1951; 14:507–517Crossref, Medline, Google Scholar
55. Fonberg E: The normalizing effect of lateral amygdalar lesions upon the dorsomedial amygdalar syndrome in dogs. Acta Neurobiol Exp (Warsz) 1973; 33:449–466Medline, Google Scholar
56. Abrahams VC, Hilton SM: Active muscle vasodilation and its relation to the “fight and flight reactions” in the conscious animal. J Physiol 1958; 140:16–17Medline, Google Scholar
57. Abrahams VC, Hilton SM, Zbrozyna A: The role of active muscle vasodilation in the alerting stage of the defense reaction. J Physiol 1964; 171:189–202Crossref, Medline, Google Scholar



