Handedness and Cerebral Dominance
In a BBC radio program called “The Brain's Trust,” the philosopher C. M. Joad typically began his answers with, “It all depends on what you mean by ‘x’.” Neglect of this proviso for “handedness” has serious consequences. Here are two examples. The argument for earlier mortality in left- than right-handers1 depends on the premise that relative frequencies have not changed over this century. An early study2 classified 15.7% of students as left-handed but did not explain the criterion. Left-handed writing has certainly changed, such that left-writers today are younger on average than right-writers. Left-handers were 9 years younger among the recently deceased in California,1 but they are also 9 years younger among the living in the United Kingdom.3 The second example concerns cerebral dominance for speech (CD). No right-handers were found among aphasics with right-sided lesions in two influential series, but in both series the proportion of left-handers among all aphasics was considerably higher than among all nonaphasics,4,5 suggesting that the absence of right-handers among right-lesioned aphasics was due to generous criteria of left-handedness in critical cases.
The right shift (RS) theory6 was founded on empirical study of types of handedness. This review aims to describe my approach to classification, outline its theoretical implications, point up some contrasts with other theories, and persuade readers to examine their data as needed for critical tests.
HANDEDNESS AS A CONTINUOUS VARIABLE AND CLASSIFICATIONS AS THRESHOLDS
My starting assumption was that the preferred hand is more skilled, and my first surprise was that many people prefer different hands for different skilled tasks. Inconsistent preferences used to be attributed to social pressures on natural left-handers (shifted sinistrality), but this does not account for left-writers who prefer the right hand for other actions. When preferences for several actions were studied in samples of children, service recruits, and undergraduates, the proportions were remarkably constant, at about 3% to 4% consistent left-handers, 25% to 33% mixed, and 60% to 70% consistent right-handers; in nonhumans the corresponding proportions were about 25%, 50%, and 25%.7 The problem of mixed-handedness is usually swept under the carpet, along with a statement to the effect that “ambidexterity is rare.” This is true (about 3 per 1,000 for writing), but mixed-handedness is 100 times more common. The variability of incidences between studies and the puzzle of relationships with CD could be due to arbitrary classification of some 30% of the population. How should mixed-handers be classified? A computer-run analysis of questionnaire responses found a large number of patterns but no obvious major divisions. My conclusion was that handedness varies continuously between strong left and strong right and that dichotomous classifications are arbitrary.8
Medical diagnosis may be influenced by several factors, including the cultural milieu, the patient's attitude to illness, the number of tests administered, and criteria of severity. Diagnosis can be considered to depend on thresholds of ascertainment that vary along a continuum.9 Handedness classification, too, depends on culture, self-appraisal, tests, and criteria. Incidences indicate where the continuum was cut, but little else. Thresholds are sensitive to variables such as volunteer biases and methods of sampling, measurement, and analysis, so that incidences are not comparable unless these features were identical. Further, right-handers take handedness for granted and are often unaware of left-handedness in relatives.
The studies that led to the RS theory were based on “captive” samples, such as class groups of children and undergraduates. Participants were observed performing several actions and timed performing an objective test of skill (peg moving) by each hand. Children were tested individually, but undergraduates observed and timed each other in practical classes. Questionnaires were sent to homes for personal completion by relatives when possible.
A key question was, “How does the continuum of hand preference relate to the continuum of relative hand skill?” This was investigated empirically by examining differences between the hands for peg-moving time (L−R for time or R−L for skill) in groups distinguished for patterns of preference.8 Eight classes (or subgroups) were defined: classes 1 and 8 were consistent right- and left-handers, respectively; classes 2–5 (later redefined as 2–4) were mixed right-handers; and classes 6–7 were mixed left-handers, as shown in Figure 1 and described further in Table 1. When the study was replicated in Open University students, the L−R means were virtually identical for several subgroups.10 The subgroups were found later to be ordered for frequency of left-eye and left-foot preferences.11 They were ordered also for frequency of left-handed relatives.12 The subgroup classification gives a reliable and valid map of degrees of preference along an asymmetry continuum.
CHANCE AS UNIVERSAL PLUS RIGHT SHIFT IN HUMANS
Figure 2 shows how the RS theory was founded on this analysis of hand preference. At the base is the threefold classification of handedness, and then eight subgroups are ordered along an R–L continuum. The distribution of peg-moving differences takes the form of a unimodal normal (Gaussian) curve. For some years I puzzled how the various distributions of hand and paw preferences and the normal distributions of R-L skill and grip strength13 might be related. An answer presented itself when I took the threshold model seriously enough to look up the locations of the cut-points needed to distinguish mixed-handers from consistent handers in humans and in nonhumans (the standard deviations or z values that define areas to the left: for humans, equal to 4% for consistent left-handers and 34% for left- plus mixed-handers; for nonhumans, equal to 25% and 75%, respectively; see dashed lines in Figure 2). The distance was about the same, 1.34z. That is, the relative proportions of left-, mixed-, and right-handers in humans and nonhumans were consistent with a normal distribution, which was symmetrical about 0 for nonhumans but displaced slightly to the right for humans. This was the “aha!” experience on which the RS theory was founded.6 It neatly solved several puzzles about distributions but immediately raised new questions: What causes the normal distribution? What causes the dextral shift? My strategy in seeking answers was to stay as close as possible to the empirical evidence and test the simplest explanations first. More complex hypotheses could be entertained, of course, but the simplest assumptions at each stage of the argument have led to several further happy surprises.
The normal distribution of R-L asymmetry could be common to all creatures capable of independent limb movement. Breeding studies in rats14 and mice15 found no evidence that paw preferences are inherited, but they are congenital.16 Paw preferences depend on factors that influence the development of each side of the body in utero and give equal numbers of left- and right-preferent animals, the majority not strongly biased to either side. The distribution is as expected for the combined effect of many small differences occurring at random. If a Gaussian distribution of asymmetry occurs nongenetically in mice, it could occur in humans also. That is, human handedness could be due to chance asymmetries of early growth.
What causes the displacement of the chance distribution to the right in humans but not in other primates? The first hypothesis must be that the cause is something that gives a relative advantage to the human left hemisphere for CD. Inspection of the overlapping curves in Figure 2 suggests that this “factor” acts like a mathematical constant that is added to the chance distribution without changing its shape. Therefore, the causes of typical CD could be independent of the causes of handedness but could influence handedness by weighting the probabilities in favor of the right hand.
RS AS CAUSED BY A GENE FOR LEFT HEMISPHERE ADVANTAGE
The idea that an RS factor (later, RS+ gene17) could be inherited but absent in some people, who were then without systematic bias to either side, was supported by findings for R-L skill in children with two left-handed parents.18,19 At the level of “cerebral asymmetry” in Figure 2 are two normal curves. The one symmetrical about 0 is associated with absence of the typical pattern of cerebral asymmetry and with RS−− genotype. RS− is intended to represent one or more alleles that are indifferent to laterality and probably common to our primate heritage. The curve displaced to the right is not displaced so far as to make everyone right-handed. The RS+ gene is often misunderstood as “for” right-handedness. On the contrary, it is “for” typical CD and probably originated in our hominid ancestors.
The threshold for left-handed writing is likely to be to the left of 0 under both curves because cultural pressures make people who are balanced for skill use the right hand (see the base of Figure 3). If this is true, aphasics with right-hemisphere lesions (all RS−− genotypes) are predicted to include more right- than left-handers. How could this surprising prediction be tested? The war wound series described the effects of acute brain lesions in young males from the healthy general population, and inclusion was independent of laterality. German Second World War cases included 18 aphasics with right unilateral lesions, and of these, 61% were right-handed.20 British cases included 27 aphasics, of whom 70% were right-handed.21 Examination of other apparently contrary samples led to the discovery of the criterion shifts between aphasics and non-aphasics described above.22
How many people have right-sided cerebral speech? The question has been asked many times for right-handers and left-handers separately, but data are needed for the population as a whole. Among cases with unilateral lesions and aphasia (necessarily lesioned in speech areas) the proportions of right-sided cases in four series were 9%,20 10%,4 11%,21 and 5%,23 averaging 9.27%. The estimate was confirmed in a recent survey of stroke, which found 9.23% right hemisphere lesions among unilateral cases with aphasia.24 If all right hemisphere speech is due to chance asymmetry within RS−− genotypes, then for every individual with a right-sided lesion there must be another RS−− with a left-sided lesion. Twice 9.27% gives 18.54%. The idea that so many people lack the agent for typical CD is often met with incredulity. Recall that these estimates were made without reference to handedness. Previous calculations for separate handedness groups have distorted the evidence.
Alexander and Annett25 examined the implications for neurology of random lateralizations of cerebral asymmetries and described 10 new illustrative cases. Quantitative predictions were tested against findings for several samples, and the fits were good provided that the predictions were matched to the incidences as required for a threshold model.26 For example, Rasmussen and Milner's27 findings for handedness and speech hemisphere fit the model well at the 10% threshold for left-handers and the 30% threshold for right-handers (see X and Y in Figure 4). This is understandable on the assumption that strict criteria were adopted for both groups. It implies, however, that some 20% mixed-handers were excluded, and the findings are not representative, therefore, of the population as a whole. The RS analysis suggests that when there are 10 left-handers per 100 population, the ratio is 7 left- to 3 right-sided speech (3/10 or 30% atypical) among left-handers and 84 left- to 6 right-sided speech (6.7% atypical) among right-handers. When 30 nonright-handers are identified per 100 population, the ratios are 24:6 (20% atypical) among nonright-handers and 67:3 (4.3% atypical) among consistent right-handers.
My early approaches to the genetics of handedness28,29 were superseded by the idea of a single gene (RS+) dominant for left CD.17 If 18.54% are RS−−, the frequency of the RS− gene is the square root (0.43) and the frequency of RS+ is 0.57. The genotype proportions at the top of Figure 3 follow by simple Mendelian rules. Extent of shift was estimated from the aphasia analysis also. This allowed a test of the model that treated RS+ as dominant for handedness as well as for left cerebral speech (see further below). In family studies in the literature and in my own samples, incidences of nonright-handers range from about 4%30 to 40%. Each incidence implies a threshold, and each threshold implies specific genotype proportions. The latter were multiplied for each type of family (R×R, L×R, and L×L) to find the genotypes of children in each study. The incidence in children defines their threshold and hence the number of left-handers for each genotype and type of family. The match between predictions and observations in almost all samples was one of the great surprises of the RS theory. Predictions for the dominant model were reworked and extended to new samples.31 An additive version of the model for handedness (see below) gives good fits to most samples also.11
At first sight, twins pose serious problems for genetic theories because monozygotic (MZ) twins may be of opposite handedness (RL pairs) and, further, the RL proportion is about the same for MZ and dizygotic (DZ) pairs.32 The normal curves in Figure 2 suggest that handedness depends on chance (the normal distribution) plus right shift. Chance implies random accidents of development that affect every individual, including each individual twin. When both twins (or siblings or unrelated pairs) carry the RS+ gene, there is a fairly high probability of opposite handedness (by chance). When both are non–gene carriers, handedness is fully at random. The RS+ gene makes twins more often right-handed than left-handed, like everyone else, but the genetic variability is tiny in comparison with chance variability.
Tests of the RS genetic model for twins found that the extent of shift must be smaller for both MZ and DZ pairs than for the single-born.17 Smaller shift implies reduced expression of the RS+ gene and increased proportion of nonright-handers. Doubts as to whether twins are more often left-handed have been used to cast doubt on the RS model,33 but the prediction was strongly supported.3 A factor shared by MZ and DZ twins is that growth must be restrained before birth so that two fetuses can be accommodated in the womb. This suggests that expression of the RS+ gene might be a function of growth in utero. Females are slightly more mature at birth than males, and slightly more often right-handed. Children of very low birth weight include a high proportion of nonright-handers.34 Findings for twinning, sex, and birth weight are consistent with the possibility that any factors that affect early growth are likely to influence RS. The influence of such factors would account for the raised incidence of nonright-handedness among the learning disabled and other “at-risk” groups, without implying causal relationships between handedness and disability.
A GENETIC BALANCED POLYMORPHISM WITH HETEROZYGOTE ADVANTAGE
The genotypes are illustrated in Figure 4, with shifts of 1z and 2z for RS+− and RS++, respectively, as estimated for an additive effect on handedness in males.11 (For females, the corresponding shifts would be 1.2z, 2.4z.). Although the genetic effect is dominant for CD, the possibility that this effect is additive for underlying mechanisms is suggested by the genotype proportions. Why are heterozygotes (RS+− genotype) most frequent (49%) and both homozygotes substantial although less frequent? The proportions suggest a genetic balanced polymorphism for the RS locus, with heterozygote advantage (BP+HA).17 How this might work psychologically has been the focus of my research for the past 20 years. The question is whether cognitive, social, and motor skills vary with different patterns of cerebral specialization. The wider question for human biology is how these differences might influence reproductive success.
Figure 3 sets out a schematic summary of some possibilities. It was obvious from the beginning6 that people who lack something that facilitates typical CD (RS−− genotypes) might be at risk for speech learning and dyslexia, but what risks beset the RS++ genotype? The idea that there might be costs for typical CD was unprecedented. I surmised that there might be overdependence on the left hemisphere at the expense of right hemisphere skills, pointing to the higher prevalence of left-handedness among many talented groups, including artists, sportspeople, and skilled performers of many kinds.17 Diana Kilshaw noted that consistent right-handers in my samples tended to be slower for peg moving than nonright-handers, especially with the nonpreferred hand.35 When time for each hand is plotted against the R-L difference, the right hand improves slightly but the left hand declines dramatically from left to right,36 an observation that has been independently replicated several times.37–39 Strong left-handers show a similar pattern of weakness of the right hand, but such individuals are much less prevalent, of course, than strong right-handers.40,41 The pattern resembles findings for the planum temporale (PT).42 Symmetry is associated with large plana on both sides, whereas typical asymmetry is associated with a smaller planum on the right. For both handedness and PT, it is as if modal asymmetry is associated with loss of right hemisphere function.
At the “cerebral” level in Figure 3, right hemisphere disadvantage is hypothesized to be absent in RS−− genotypes, moderate in RS+−, and strong in RS++. At the “cognitive” level, risks to phonology fall from left to right but other risks, provisionally labeled “visuospatial,” rise. Risks for the RS++ genotype could be more general, such as for loss of intelligence or motor skill. The raised proportions of left-handers among mathematicians43,44 and among professionals in many sports could be due to absence of RS++ genotypes (strong right-handers) rather than intrinsic advantages for left-handers.45 Heterozygote (RS+−) advantage is expected to depend on efficient speech learning at minimal cost to the right hemisphere. Children with mild and moderate biases to dextrality outperform those to either side for Peabody Picture Vocabulary,46 Progressive Matrices,36 and educational achievement.47 These and other abilities studied in the light of the BP+HA hypothesis, including spatial reasoning,48 reading,49 and phonology,50 were reviewed51 with commentaries. Here, I outline the RS approach to dyslexia and then a new idea for psychosis.
DYSLEXIA WITH AND WITHOUT RS
If RS−− genotypes are at risk for dyslexia because of poor phonology, some dyslexics should lack the typical bias to right-handedness (Orton's “motor-intergrades”52). Controversies about associations between handedness and dyslexia could have two main causes: first, failure to distinguish the direction of predictions between school and clinic samples, and second, the presence of some strongly right-handed (RS++) dyslexics. Differences are not expected when normal samples are classified for handedness and compared for language skills, because the majority of left- and mixed-handers carry the RS+ gene (see Figure 4). Clinical cases, however, are selected for language difficulties, and if these difficulties are associated with reduced or absent RS, there should be excess nonright-handers. Schoolchildren classified for handedness did not differ on tests of ability, but in the same sample those with specific delays in learning to read were more often left- and mixed-handed.53
The presence of strong right-handers in a remedial clinic suggested that some dyslexics might be RS++ genotypes.46 The idea was checked in a new school sample, which found children at both ends of the laterality continuum were poorer readers than those in the center.49 If the two ends are associated with different genotypes and hence different patterns of cerebral dominance, there should also be different cognitive problems. Poor phonology is predicted at the left and some other weakness at the right, as in the distinction of “phonological” versus “dyseidetic” poor spellers54 and “phonological” versus “surface” dyslexics.55 The question for the RS theory was whether a dissociation occurs between type of cognitive weakness and type of handedness.
Thirty-five poor readers were identified among a cohort of some 450 children.56 Almost all were dyslexic by discrepancy criteria, 17 with poor phonology and 18 without. Among the former there were 5 left-writers (29.4%) and among the latter there were none (0%). The findings were consistent with predictions for handedness in RS−− and RS++ genotypes, shown at the “behavior” level of Figure 3. Handedness effects for the two genotypes are likely to have canceled each other in some studies, giving the inconclusive findings typical of the field.57,58
SCHIZOPHRENIA AND AUTISM AS DUE TO AN AGNOSIC RS+ GENE
A new idea occurred59 when I considered Crow's60,61 original and challenging theory that schizophrenia is due to an anomaly of CD. If the RS theory is correct that there is only one systematic influence on CD, Crow's theory implies that schizophrenia is an anomaly of the RS+ gene. The message of the RS+ gene is, in effect, “Impair the growth of one hemisphere, the right, in early life.” Suppose such a gene had evolved recently, and then consider what part of the instruction might be most vulnerable to mutation. If “the right” were lost, the instruction would be, “Impair the growth of one hemisphere (unspecified) in early life.” The gene would be “agnosic” for left versus right and impair either hemisphere with equal probability because chance is a universal default on the RS theory. Impairment of one hemisphere would not be a problem because this occurs in about 80% of normal individuals, but an agnosic gene would give some people an impairment of both hemispheres. When the agnosic gene is paired with normal RS+ (RS++a genotype) the normal gene would impair the right hemisphere while the agnosic gene would give a second impairment in 50% (as in normal RS++) but would impair the left hemisphere in 50%. This would give a subtle but potentially devastating impairment of language-related cortex on both sides.
Although intriguing, the idea would not be worth taking seriously unless it worked quantitatively. Figure 5 sets out the combinations. If the lifetime risk for schizophrenia is about 1%, the frequency of the agnosic gene must be about 0.02. (The frequency of normal RS+ is then 0.55 instead of 0.57 as above, and RS− remains unchanged at 0.43.) The agnosic gene does not cause psychosis directly because when it is paired with RS− one hemisphere is unimpaired (side at random) and when it is paired with RS+ the left hemisphere remains unimpaired in 50% of cases. Among MZ twins only 50% are affected, but the probability of affected offspring is the same for both twins, as observed,62 and is at the level for other affected parents, estimated at 14% to 15%. The children of two schizophrenic parents follow the classic Mendelian pattern for heterozygote matings, 25% entirely normal (RS++), 25% homozygote for the agnosic gene (RS+a+a), and 50% heterozygote. Of the last, 50% are normal. At least half the children of two schizophrenics should be normal and up to one-half should be at risk. Predictions for DZ twins, siblings, and other relatives are consistent with risks estimated by Gottesman.63
If the frequency of the agnosic gene is 0.02, as argued above, then the frequency of homozygotes for the agnosic gene (RS+a+a) must be the square of this proportion (0.0004), about the frequency of autism.64 This raises the question whether autism is due to a double dose of a gene that impairs the language mechanisms during early growth. Two agnosic genes causing impairments to language-related cortex at random (illustrated in Figure 5) would give scope for many different patterns of disability as seen in autism and other severe disorders of childhood, as well as loss of bias to dextrality.65
CONTRASTS, CRITICISMS, AND PREDICTIONS
The popular view is that handedness is discrete, that left-handers are “deviant,” that there are specific causes, and that the causes are likely to be the same as for CD. The RS theory suggests that handedness is continuous, that left-handers are natural variants, that the causes of all types of handedness are accidents of development, and that these causes are independent of CD except insofar as the mechanisms that induce right hemisphere disadvantage increase the probability of dextrality. Contrasts with other theories will be considered for these four issues: continuity, pathology, chance, and CD.
No other theory recognizes a hand preference continuum that needs empirical study. Laterality quotients of various kinds have been proposed to quantify degrees of handedness, most notably Oldfield's66 Edinburgh Handedness Inventory (EHI). However, in the EHI the same scores are given to different actions (writing and opening a box lid) that differ in relative skill and frequency of left preference; hence, the usefulness of the resulting quotients is in doubt.11 Peters and Murphy67 distinguished five handedness subgroups in a cluster analysis of questionnaire responses, but they did not validate these against external criteria. Why is this issue of classifying hand preference important for new research? It is important because cerebral asymmetries vary continuously, not discretely.42,68,69 One reason for the difficulty of drawing clear conclusions about relationships between handedness and other variables70 could be that handedness groups have been selected and classified differently, thus cutting the preference continuum at different thresholds. My prediction is that variables will be more interpretable if they are examined for levels of handedness, like the subgroups in Figure 1. If manual and cerebral asymmetries are both influenced by the RS+ gene, a linear relationship is likely, but if the RS+ influence is absent, then most variables are likely to be independent. For example, PT and parietal operculum (PO) were both larger in the left hemisphere in consistent right-handers, but among nonright-handers the two cerebral asymmetries were independent.71 This finding is consistent with RS+ influence on manual and cerebral asymmetries in the first group but reduced or absent influence in the second group, such that the two asymmetries are then unrelated. The question of whether this applies to other cerebral and behavioral asymmetries needs investigation.
With regard to pathology, the higher frequency of left preference among the learning disabled has long been attributed to the effects of early brain damage,72,73 but the fact that some left-handedness is due to pathology does not imply that all left-handedness is pathological. Theories of defects of personality74,75 and brain damage at birth76 have been influential. There is an issue here for medical philosophy, namely why pathology explanations seem more acceptable than natural individual differences. The terms “alinormal”77 and “anomalous”78 imply deviations from species norms. Geschwind and Galaburda78 sought to explain the supposed anomalous lateralities in terms of hormonal influences on the relative growth of the cerebral hemispheres that lead to gendered differences for handedness and dyslexia, to immune system dysfunctions, and to the talents of special groups such as artists and architects with raised incidences of nonright-handedness. Support for the details of this scenario is hard to find.79 How much simpler to say, with the RS theory, that left-handedness occurs naturally in all primates and that the human biases for hand and brain are influenced by any factors that affect growth in utero.
The idea that handedness is due to chance plus RS was prompted by the two overlapping curves of Figure 2. Layton80 distinguished chance versus directional asymmetry independently when he noticed that a strain of mice had situs inversus in 50% of animals and postulated an iv mutation. The typical arrangement of the viscera is common to vertebrates and atypical situs is infrequent (about 1 per 10,000 humans81), so the directional gene must be well fixed and chance asymmetry due to a rare mutant. For the RS theory of handedness, the situation is very different because chance is universal and RS is a recent innovation that is not fixed; nearly 20% do not carry the gene. However, an agnosic RS+ gene for CD does resemble the iv gene. Both are mutants of a gene for directional bias, which then affects either side at random.
Most current theories of handedness adopt “chance” postulates but vary in the interpretation of chance. Geschwind followed the Layton model in supposing that chance asymmetries are aberrant. Yeo and Gangestad82 suggested that there is a species norm of moderate right-handedness, while left- and strong right-handedness are deviant. The argument is based on a continuum of R–L hand skill, but this is regarded as the product of many genes of small effect (instead of the nongenetic accidents postulated by the RS theory). Genetic accidents of development are considered more probable in homozygotes at both ends of the distribution than in heterozygotes, who are buffered from developmental instabilities. This theory leads to the surprising prediction that people at both extremes of the R-L distribution are likely to have left-handed parents. A quadratic trend for number of nonright-handed parents over a normal distribution of R-L peg moving in students was suggested to support this idea.83 Modern computer statistical packages produce trends on very little evidence, and the weak upward trend at the right tail of the distribution rested on very few cases. Checks in my samples found that the probability of left-handed parents decreased with increasing dextrality.31 Laland et al.84 proposed a universal chance distribution (like the RS theory) but suggested that the association of handedness in families could be due to cultural rather than genetic causes, in spite of good arguments to the contrary.85 In the RS model, cultural influences affect the threshold of expression but not the underlying distributions.
The McManus86 theory of handedness includes several chance postulates. There is a gene for chance (C), which gives 50% left-handers and is expressed by chance in heterozygotes (DC) as well as in CC homozygotes to give a “true” incidence of 7.75% left-handers. The alternative allele (D) is more common and determines right-handedness. Observed incidences rarely match the supposed true value, so these “errors” are corrected by reassigning genotypes to phenotypes in proportion to their frequency—that is, by random shuffle. It is this third chance postulate that allows any observed incidence to be fitted. The superficial similarities between the alleles D versus C and RS+ versus RS− have led many to treat the RS and McManus theories as equivalent,82,84,87 but this view is seriously mistaken. McManus proposes genes for handedness (versus a gene for CD in the RS model) and directional or chance asymmetry like the iv gene (versus chance plus shift in the RS model). Because McManus88 specifically rejected the continuity hypothesis, he must use the shuffle rule instead of matching incidences to thresholds. Predictions for handedness in families fit for incidences in the range for which the model was designed, but above this range, when parents are classified for nonright-handedness, the model breaks down because the genotype shuffle makes all family types very similar.31,89
With regard to cerebral asymmetries, no other theory distinguishes the causes of handedness from the causes of modal cerebral dominance. For McManus's theory, the D allele is for typical CD as well as right-handedness, but atypical cases require further chance postulates. For the RS theory, all asymmetries, including cerebral ones, depend on accidents of development, but a specific genetic instruction induces the typical pattern of anatomical and functional asymmetry in some, but not all, humans. It is a challenge to new research to discover the gene and its mechanisms. The RS theory suggests that the effect of RS is to impair the control of speech systems in the right hemisphere, thus channeling speech learning and other language functions to the left side. No other theory suggests that typical CD carries costs for human abilities. Geschwind's idea that special talents might be due to disorders of brain development that enhance right hemisphere functions is much less probable, in my view, than that these special talents are prevalent in brains that escape deficit to either hemisphere (see top left of Figure 5). The BP+HA hypothesis has been criticized,37–39 but in ways that I believe ill judged.90,91 It is also likely to be misrepresented in future attempts to assimilate the RS theory to current hemisphere specialization and hemisphericity paradigms.
The fundamental puzzle for the BP+HA hypothesis was why the spread of the RS+ gene has been limited. The hypothesis that it may mutate to an agnosic form, with consequent risk of schizophrenia, offers a possible solution. The theory of an agnosic gene has been met with understandable skepticism.92–96 My personal conviction that the idea is worth further examination comes chiefly from the fact that it was the most astonishing of the many “aha!” experiences afforded by the right shift theory. The puzzle pieces simply dropped into place once the idea of an agnosic RS+ gene occurred. This does not mean that the problems are solved, but rather that a new field of inquiry is opened up.
ACKNOWLEDGMENTS
Figures were drawn by John Ashworth.
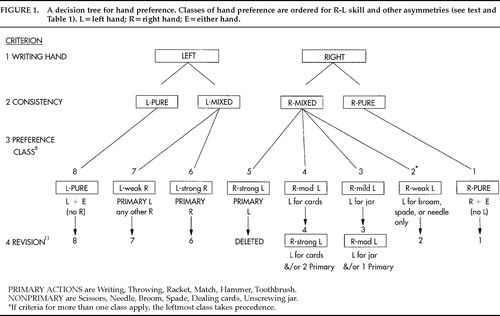
Figure 1. A decision tree for hand preference. Classes of hand preference are ordered for R-L skill and other asymmetries (see text and Table 1). L=left hand; R=right hand; E=either hand.
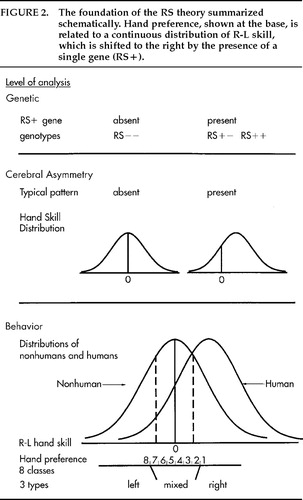
Figure 2. The foundation of the RS theory summarized schematically. Hand preference, shown at the base, is related to a continuous distribution of R-L skill, which is shifted to the right by the presence of a single gene (RS+).
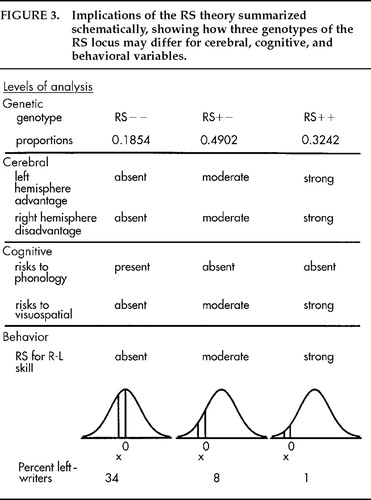
Figure 3. Implications of the RS theory summarized schematically, showing how three genotypes of the RS locus may differ for cerebral, cognitive, and behavioral variables.
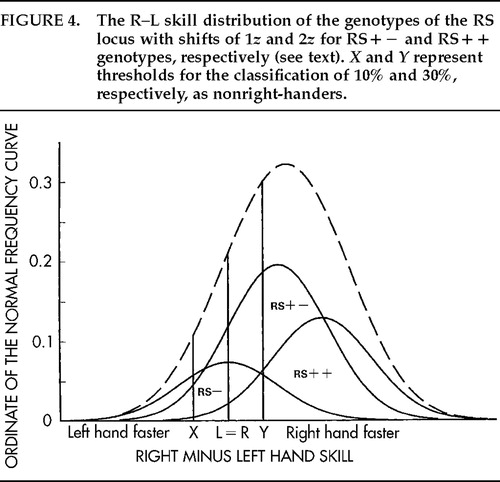
Figure 4. The R–L skill distribution of the genotypes of the RS locus with shifts of 1z and 2z for RS+− and RS++ genotypes, respectively (see text). X and Y represent thresholds for the classification of 10% and 30%, respectively, as nonright-handers.
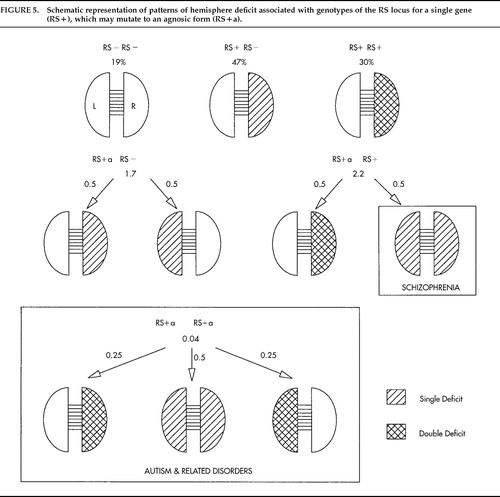
Figure 5. Schematic representation of patterns of hemisphere deficit associated with genotypes of the RS locus for a single gene (RS+), which may mutate to an agnosic form (RS+a).
 |
1. Coren S, Halpern DF: Left-handedness: a marker for decreased survival fitness. Psychol Bull 1991; 109:90–106Crossref, Medline, Google Scholar
2. Ramaley F: Inheritance of left-handedness. The American Naturalist 1913; 47:730–738Crossref, Google Scholar
3. Davis A, Annett M: Handedness as a function of twinning, age and sex. Cortex 1994; 30:105–111Crossref, Medline, Google Scholar
4. Hécaen H, Ajuriaguerra J: Left-handedness: Manual Superiority and Cerebral Dominance. New York, Grune and Stratton, 1964Google Scholar
5. Gloning K, Quatember R: Statistical evidence of neuropsychological syndromes in left-handed and ambidextrous patients. Cortex 1966; 2:484–488Crossref, Google Scholar
6. Annett M: The distribution of manual asymmetry. British Journal of Psychology 1972; 63:343–358Crossref, Medline, Google Scholar
7. Annett M: The binomial distribution of right, mixed and left handedness. Quarterly Journal of Experimental Psychology 1967; 19:327–333Crossref, Medline, Google Scholar
8. Annett M: A classification of hand preference by association analysis. British Journal of Psychology 1970; 61:303–321Crossref, Medline, Google Scholar
9. Falconer DS: The inheritance of liability to certain diseases, estimated from the incidence among relatives. Ann Hum Genet 1965; 29:51–76Crossref, Google Scholar
10. Annett M: A coordination of hand preference and skill replicated. British Journal of Psychology 1976; 67:587–592Crossref, Medline, Google Scholar
11. Annett M: Left, Right, Hand and Brain: The Right Shift Theory. London, Erlbaum Associates, 1985Google Scholar
12. Annett M: Handedness as a continuous variable with dextral shift: sex, generation and family handedness in subgroups of left and right-handers. Behav Genet 1994; 24:51–63Crossref, Medline, Google Scholar
13. Woo TL, Pearson K: Dextrality and sinistrality of hand and eye. Biometrika 1927; 19:165–199Crossref, Google Scholar
14. Peterson GM: Mechanisms of handedness in the rat. Comparative Psychology Monographs no 46, 1934Google Scholar
15. Collins RL: On the inheritance of handedness, II: selection for sinistrality in mice. J Hered 1969; 60:117–119Crossref, Medline, Google Scholar
16. Collins RL: When left-handed mice live in right-handed worlds. Science 1975; 187:181–184Crossref, Medline, Google Scholar
17. Annett M: A Single Gene Explanation of Right and Left Handedness and Brainedness. Coventry, UK, Lanchester Polytechnic, 1978Google Scholar
18. Annett M: Handedness in the children of two left handed parents. British Journal of Psychology 1974; 65:129–131Crossref, Medline, Google Scholar
19. Annett M: Hand preference and skill in 115 children of two left-handed parents. British Journal of Psychology 1983; 74:17–32Crossref, Medline, Google Scholar
20. Conrad K: Uber aphasische sprachstorungen bei hirnverletzten linkshander [On aphasic speech disorders in brain-injured left-handers]. Nervenarzt 1949; 20:148–154Google Scholar
21. Newcombe F, Ratcliff GG: Handedness, speech lateralization and ability. Neuropsychologia 1973; 11:399–407Crossref, Medline, Google Scholar
22. Annett M: Hand preference and the laterality of cerebral speech. Cortex 1975; 11:305–328Crossref, Medline, Google Scholar
23. Bingley T: Mental symptoms in temporal lobe epilepsy and temporal lobe gliomas. Acta Psychiatrica et Neurologica Scandinavica 1958; 33(suppl 120)Google Scholar
24. Pedersen PM, Jorgensen HS, Nakayama H, et al: Aphasia in acute stroke: incidence, determinants and recovery. Ann Neurol 1995; 38:659–666Crossref, Medline, Google Scholar
25. Alexander MP, Annett M: Crossed aphasia and related anomalies of cerebral organization: case reports and a genetic hypothesis. Brain Lang 1996; 55:213–239Crossref, Medline, Google Scholar
26. Annett M, Alexander MP: Atypical cerebral dominance: predictions and tests of the right shift theory. Neuropsychologia 1996; 34:1215–1227Crossref, Medline, Google Scholar
27. Rasmussen T, Milner B: The role of early left brain injury in determining lateralization of cerebral speech functions. Ann NY Acad Sci 1977; 299:355–369Crossref, Medline, Google Scholar
28. Annett M: A model for the inheritance of handedness and cerebral dominance. Nature 1964; 204:59–60Crossref, Medline, Google Scholar
29. Annett M: Handedness in families. Ann Hum Genet 1973; 37:93–105Crossref, Medline, Google Scholar
30. Chamberlain HD: The inheritance of left-handedness. J Hered 1928; 19:557–559Crossref, Google Scholar
31. Annett M: In defense of the right shift theory. Percept Mot Skills 1996; 82:115–137Crossref, Medline, Google Scholar
32. Collins RL: The sound of one paw clapping: an inquiry into the origin of left handedness, in Contribution to Behavior-Genetic Analysis: The Mouse as a Prototype, edited by Lindzey G, Thiessen DD. New York, Appleton-Century-Crofts, 1970, pp 115–136Google Scholar
33. McManus IC: Handedness in twins: a critical review. Neuropsychologia 1980; 18:347–355Crossref, Medline, Google Scholar
34. O'Callaghan MJ, Burn YR, Mohay HA, et al: Handedness in extremely low birth weight infants: aetiology and relationship to intellectual abilities, motor performance and behavior at four and six years. Cortex 1993; 29:629–637Crossref, Medline, Google Scholar
35. Kilshaw D, Annett M: Right and left hand skill, I: effects of age, sex and hand preference showing superior skill in left-handers. British Journal of Psychology 1983; 74:253–268Crossref, Medline, Google Scholar
36. Annett M, Manning M: The disadvantages of dextrality for intelligence. British Journal of Psychology 1989; 80:213–226Crossref, Medline, Google Scholar
37. McManus IC, Shergill S, Bryden MP: Annett's theory that individuals heterozygous for the right shift gene are intellectually advantaged: theoretical and empirical problems. British Journal of Psychology 1993; 84:517–537Crossref, Medline, Google Scholar
38. Palmer RE, Corballis MC: Predicting reading ability from handedness measures. British Journal of Psychology 1996; 87:609–620Crossref, Medline, Google Scholar
39. Resch F, Haffner J, Parzer P, et al: Testing the hypothesis of the relationships between laterality and ability according to Annett's right-shift theory: findings in an epidemiological sample of young adults. British Journal of Psychology 1997; 88:621–635Crossref, Medline, Google Scholar
40. Annett M: Parallels between asymmetries of planum temporale and of hand skill. Neuropsychologia 1992; 30:951–962Crossref, Medline, Google Scholar
41. Leask SJ, Crow TJ: How far does the brain lateralize? An unbiased method for determining the optimum degree of hemispheric specialization. Neuropsychologia 1997; 35:1381–1387Crossref, Medline, Google Scholar
42. Galaburda AM, Corsiglia J, Rosen GD, et al: Planum temporale asymmetry: reappraisal since Geschwind and Levitsky. Neuropsychologia 1987; 25:853–868Crossref, Google Scholar
43. Annett M, Kilshaw D: Mathematical ability and lateral asymmetry. Cortex 1982; 18:547–568Crossref, Medline, Google Scholar
44. Benbow CP: Physiological correlates of extreme intellectual precocity. Neuropsychologia 1986; 24:719–725Crossref, Medline, Google Scholar
45. Annett M: Right hemisphere costs of right-handedness, in Vision and Visual Dyslexia, vol 13: Vision and Visual Dysfunction, edited by Stein JF. London, Macmillan, 1991, pp 84–93Google Scholar
46. Annett M, Kilshaw D: Lateral preference and skill in dyslexics: implications of the right shift theory. J Child Psychol Psychiatry 1984; 25:357–377Crossref, Medline, Google Scholar
47. Annett M: Handedness and educational success: the hypothesis of a genetic balanced polymorphism with heterozygote advantage for laterality and ability. British Journal of Developmental Psychology 1993; 11:359–370Crossref, Google Scholar
48. Annett M: Spatial ability in subgroups of left and right handers. British Journal of Psychology 1992; 83:493–515Crossref, Medline, Google Scholar
49. Annett M, Manning M: Reading and a balanced polymorphism for laterality and ability. J Child Psychol Psychiatry 1990; 31:511–529Crossref, Medline, Google Scholar
50. Annett M: Phonological processing and right minus left hand skill. Quarterly Journal of Experimental Psychology 1992; 44A:33–46Google Scholar
51. Annett M: The right shift theory of a genetic balanced polymorphism for cerebral dominance and cognitive processing. Current Psychology of Cognition 1995; 14:427–480Google Scholar
52. Orton ST: Reading Writing and Speech Problems in Children. London, Chapman Hall, 1937Google Scholar
53. Annett M, Turner A: Laterality and the growth of intellectual abilities. Br J Educ Psychol 1974; 44:37–46Crossref, Medline, Google Scholar
54. Boder E: Developmental dyslexia: a diagnostic approach based on three atypical reading-spelling patterns. Dev Med Child Neurol 1973; 15:663–687Crossref, Medline, Google Scholar
55. Castles A, Coltheart M: Varieties of developmental dyslexia. Cognition 1993; 47:149–180Crossref, Medline, Google Scholar
56. Annett M, Eglinton E, Smythe P: Types of dyslexia and the shift to dextrality. J Child Psychol Psychiatry 1996; 37:167–180Crossref, Medline, Google Scholar
57. Bishop DVM: Handedness and Developmental Disorder. Oxford, Blackwell, 1990Google Scholar
58. Eglinton E, Annett M: Handedness and dyslexia: a meta-analysis. Percept Mot Skills 1994; 79:1611–1616Crossref, Medline, Google Scholar
59. Annett M: Schizophrenia and autism considered as the products of an agnosic right shift gene. Cognitive Neuropsychiatry 1997; 2:195–214Crossref, Medline, Google Scholar
60. Crow TJ, Ball J, Bloom SR, et al: Schizophrenia as an anomaly of development of cerebral asymmetry. Arch Gen Psychiatry 1989; 29:247–253Google Scholar
61. Crow TJ: Schizophrenia as failure of hemispheric dominance for language. Trends Neurosci 1997; 20:339–343Crossref, Medline, Google Scholar
62. Gottesman II, Bertelson A: Confirming unexpressed genotypes of schizophrenia. Arch Gen Psychiatry 1989; 46:867–872Crossref, Medline, Google Scholar
63. Gottesman II: Schizophrenia Genesis: The Origins of Madness. New York, Freeman, 1991Google Scholar
64. Rutter M: Autism as a genetic disorder, in The New Genetics of Mental Illness, edited by McGuffin P, Murray R. London, Butterworth, 1991, pp 223–244Google Scholar
65. McManus IC, Murray B, Doyle K, et al: Handedness in childhood autism shows a dissociation of skill and preference. Cortex 1992; 28:373–381Crossref, Medline, Google Scholar
66. Oldfield RC: The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia 1971; 9:97–113Crossref, Medline, Google Scholar
67. Peters M, Murphy K: Cluster analysis reveals at least three and possibly five distinct handedness groups. Neuropsychologia 1992; 30:373–380Crossref, Medline, Google Scholar
68. Steinmetz H: Structure, function and cerebral asymmetry: in vivo morphometry of the planum temporale. Neuroscience and Behavioral Reviews 1996; 20:587–591Crossref, Medline, Google Scholar
69. Binder JR, Swanson SJ, Hammeke TA: Determination of language dominance using functional MRI: a comparison with the Wada test. Neurology 1996; 46:978–984Crossref, Medline, Google Scholar
70. Beaton A: The relation of planum temporale asymmetry and morphology of the corpus callosum to handedness, gender and dyslexia: a review of the evidence. Brain Lang 1997; 60:255–322Crossref, Medline, Google Scholar
71. Habib M, Robichon F, Levrier O, et al: Diverging asymmetries in temporo-parietal cortical areas: a reappraisal of Geschwind/Galaburda theory. Brain Lang 1995; 48:238–258Crossref, Medline, Google Scholar
72. Gordon H: Left handedness and mirror writing, especially among defective children. Brain 1921; 43:313–368Crossref, Google Scholar
73. Brain WR: Speech and handedness. Lancet 1945; 249:837–841Crossref, Google Scholar
74. Blau A: The Master Hand. Research Monograph no 5. American Orthopsychiatric Association, 1946 Google Scholar
75. Lombroso C: Left-handedness and left-sidedness. North American Review 1903; 177:440–444Google Scholar
76. Bakan P: Handedness and birth order (letter). Nature 1971; 229:195Crossref, Medline, Google Scholar
77. Coren S, Searleman A: Birth stress and left-handedness: the rare trait marker model, in Left-handedness: Behavioral Implications and Anomalies, edited by Coren S. Amsterdam, North-Holland, 1990, pp 3–32Google Scholar
78. Geschwind N, Galaburda AM: Cerebral lateralization: biological mechanisms, associations and pathology, I–III. Arch Neurol 1985; 42:428–458, 521–552, 634–654Crossref, Medline, Google Scholar
79. Bryden MP, McManus IC, Bulman-Fleming MB: Evaluating the empirical support for the Geschwind-Behan-Galaburda model of cerebral lateralization. Brain Cogn 1994; 26:103–167Crossref, Medline, Google Scholar
80. Layton WM Jr: Random determination of a developmental process. J Hered 1976; 67:336–338Crossref, Medline, Google Scholar
81. Torgerson J: Situs inversus, asymmetry and twinning. Am J Hum Genet 1950; 2:361–370Medline, Google Scholar
82. Yeo RA, Gangestad SW: Developmental origins of variation in human hand preference. Genetica 1993; 89:281–296Crossref, Google Scholar
83. Gangestad SW, Yeo RA: Parental handedness and relative hand skill: a test of the developmental instability hypothesis. Neuropsychology 1994; 8:572–578Crossref, Google Scholar
84. Laland KN, Kumm J, Van Horn JD, et al: A gene-culture model of human handedness. Behav Genet 1995; 25:433–445Crossref, Medline, Google Scholar
85. Levy J: A review of evidence for a genetic component in the determination of handedness. Behav Genet 1976; 6:429–453Crossref, Medline, Google Scholar
86. McManus IC: Handedness, language dominance and aphasia: a genetic model. Psychol Med 1985; monograph suppl 8Google Scholar
87. Corballis MC: The genetics and evolution of handedness. Psychol Rev 1997; 104:714–727Crossref, Medline, Google Scholar
88. McManus IC: Right and left hand skill: failure of the right shift model. British Journal of Psychology 1985; 76:1–16Crossref, Medline, Google Scholar
89. Annett M: Eye dominance in families predicted by the right shift theory. Laterality (in press)Google Scholar
90. Annett M: Rejoinder to “Annett's theory” by McManus, Shergill and Bryden. British Journal of Psychology 1993; 84:539–544Crossref, Google Scholar
91. Annett M: Author's response: the fertility of the right shift theory. Current Psychology of Cognition 1995; 14:623–650Google Scholar
92. Corballis MC: A house of cards? Cognitive Neuropsychiatry 1997; 2:214–216Google Scholar
93. Green MF: What is atypical about atypical handedness in schizophrenia? Cognitive Neuropsychiatry 1997; 2:216–218Google Scholar
94. Sham P: Schizophrenia, autism and agnosic right shift gene. Cognitive Neuropsychiatry 1997; 2:219–220Google Scholar
95. Elvevag B, Weinberger DR: Schizophrenia and the right shift gene hypothesis: some comments on the devil in the details. Cognitive Neuropsychiatry 1997; 2:221–225Google Scholar
96. McManus IC: Autism and schizophrenia are not due to a single genetic locus. Cognitive Neuropsychiatry 1997; 2:226–230Google Scholar



