Alteration of Intention and Self-Initiated Action Associated With Bilateral Anterior Cingulotomy
Abstract
Neuropsychological manifestations of bilateral anterior cingulate cortex lesions were studied in patients treated with cingulotomy for chronic intractable pain. Cingulotomy patients more than 1 year postsurgery were contrasted with nonsurgical chronic pain patients. Patients were assessed on a neuropsychological battery, including measures of response intention, initiation, generation, and persistence. Cingulotomy patients were intact across most cognitive domains, but they showed deficits of focused and sustained attention as well as mild executive dysfunction. Self-initiated responding—including spontaneous verbal utterances and unstructured design fluency—was most impaired. Results indicate that the greatest impact of cingulotomy lesions is on response intention and self-initiated behavior, with reduced behavioral spontaneity.
The anterior cingulate cortex (ACC) is a mesocortical brain system long implicated in human affective experience and expression.1–3 Lesions of the ACC from cingulotomy can produce significant alterations in emotional behavior in both humans and laboratory animals.3–6 Blunted ACC activation has been demonstrated in patients with dysphoria and depression,7,8 and lesions of the ACC lead to improvement in certain psychiatric disorders.7,9–17 Cingulotomy has also been shown to have efficacy in the treatment of chronic intractable pain,13,18–21 although it is not clear whether the procedure reduces the actual sensation of pain or reduces the patient's emotional and behavioral reactivity to pain.19,20
The ACC is intricately connected to cortical, limbic, and subcortical structures via afferents from the lateral frontal and parietal regions and efferent pathways to the amygdala, nucleus accumbens, septum, and basal ganglia.22–25 The ACC has reciprocal connections with several thalamic nuclei and the posterior cingulate, along with overlapping projections with the parietal, frontal, and supplementary motor cortex. These functional neuroanatomic relationships provide the foundation for conceptions of the ACC as contributing to certain neuropsychiatric disorders26,27 and as playing a role in the processes of attention, intention, and motoric response selection.28–31
In animal studies, stimulation of the ACC elicits autonomic responses, simple and complex motor behaviors, vocalization, and affective responses such as fear, whereas ACC lesions lead to reduced reward-contingent responding, impaired problem solving and response flexibility, and overreliance on external cues for motor learning.3–5,32–35 Neglect syndromes have been reported in cats, monkeys, and humans following both naturally occurring lesions and controlled ablation studies.36
Relatively few studies have examined how cingulate damage affects neuropsychological functioning in humans, and existing data are largely inconclusive with respect to this question. Clinical case studies of patients with lesions involving the ACC showed that unilateral ACC lesions can result not only in hemineglect,36 but also in instances of behavioral syndromes characterized by hypokinesis, reduced behavioral reactivity, and blunting of emotional response.37 When ACC damage is coupled with damage to subcortical structures, a severe behavioral disturbance may occur that mimics a “frontal lobe syndrome.”38
Early studies of the efficacy of cingulotomy for treatment of psychiatric disorders revealed only mild acute postsurgical problems with visuospatial processing, most of which resolved in the months following surgery, leading to the conclusion that no long-term cognitive impairments result from the procedure.39–44 However, several factors limit these findings. First, the batteries used in most of these studies tended to be limited to tests of broad cognitive domains, and conclusions were based on only a few tests of each domain (e.g., language, memory, visual integration, reasoning). Attention was typically assessed to only a limited degree, and the relationship between changes in emotional experience and neuropsychological functions was largely unexplored.
More recent studies that have involved analysis of the behavioral effects of cingulotomy, as well as physiological activation of the ACC during functional brain imaging, have shown that the ACC plays a significant role in attention. Impaired habituation of the orienting response, a basic attentional phenomenon, has been demonstrated among patients following cingulotomy and seems to involve an alteration in the temporal continuity of the habituation process.44 Deficits of focused attention, in the absence of gross cognitive dysfunction, have also been observed after cingulotomy in studies directed specifically at characterizing attentional performance following this procedure.45,46 Functional imaging studies using positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) suggest that the ACC is one of the most active brain regions when response control is required. ACC activation is greatest when task performance requires the inhibition of competing responses, a switch from overlearned responses to new stimulus-response associations, or the willed generation of responses.47–51 These results not only implicate the ACC as an important structure for attentional processes, but also point to its specific role in the processes of response intention, persistence, and control.
We recently reported longitudinal data from 12 patients who underwent bilateral cingulotomy for the treatment of intractable pain.52 Compared with baseline, postcingulotomy patients showed significant impairment of focused attention, intention, and executive functioning associated with spontaneous response production. In the present study, we compared neurocognitive functioning of a larger sample of postcingulotomy patients with that of chronic pain patients receiving more traditional medical intervention. We hypothesized that compared with control subjects, cingulotomy patients would experience reduced pain, and that this would occur through alteration of attention, intention, and executive response production.
METHODS
Subjects
Eighteen patients who underwent anterior bilateral cingulotomy for treatment of chronic, intractable pain at the University of Massachusetts Medical Center (UMMC) served as subjects. These subjects were obtained from an overall group of 21 consecutive postcingulotomy patients, 3 of whom did not undergo neurocognitive assessment for logistical reasons unrelated to their medical or cognitive status. All patients were informed as to the aims and methods of the study, and consent was obtained.
All cingulotomy patients reported chronic pain secondary to noncerebral traumatic injury. These patients ranged in age from 38 to 63 years (mean=45.2, SD=8.6); 14 were male and 4 were female. All cingulotomy patients had received extensive medical treatment for pain prior to their cingulotomy but had not responded to these other therapies, which included pharmacological intervention, nerve blocks, transcutaneous nerve stimulation, or surgical procedures (e.g., laminectomy) directed at peripheral nerve relief. None of the patients had a history of central nervous system disease or head trauma prior to surgery. Medical and neurological examinations conducted prior to surgery were essentially normal in all patients, except for findings related to the specific peripheral mechanisms responsible for the pain syndrome. Premorbid psychiatric history was also negative for all patients. Prior to surgery, 3 of the 18 cingulotomy patients exhibited major depression, attributed to their pain syndrome.
A group of 20 patients with history of intractable chronic pain served as control subjects for comparison with the cingulotomy patients at baseline. These patients, recruited from the Pain Clinic of the UMMC, chose nonsurgical pain interventions offered by this clinic. This group was composed of consecutive Pain Clinic referrals for neurocognitive testing over a 2-year period, the referrals most often being for assessment of subjective cognitive complaints associated with their pain. No formal effort was made to match patients between groups, except with the inclusion criteria of a history of chronic intractable pain. However, the two groups were quite similar, as they did not differ significantly in gender, age, educational characteristics, or pain severity. Both cingulotomy and control subjects reported severe pain on a 10-point visual analog rating scale, in which 0=no pain, and 10=severe excruciating pain. There was no significant difference in reported pain levels between the two groups (mean±SD: cingulotomy, 8.6±1.2; control, 8.9±1.5). In both groups, patients had pain associated with neuromuscular injury involving the cervical, thoracic, or lumbar regions of the back and neck. For all patients, the chronicity of pain was greater than 2 years (cingulotomy, 3.6±1.5 years; control, 4.2±2.1 years).
All cingulotomy and control subjects had been treated with a variety of medications for pain control, including narcotics, nonsteroidal anti-inflammatory drugs (NSAID), anticonvulsants, tricyclic antidepressants, and other analgesics prior to and at the time of their baseline assessment. Chi-square analyses indicated that the groups did not differ with respect to the proportions of patients on each of these classes of medications at baseline (NSAID: cingulotomy, 88.8%, control, 85.0%; opiates: cingulotomy, 94.4%, control, 90.0%; antidepressants: cingulotomy, 33.3%, control, 45%; anticonvulsants: cingulotomy, 27.7%, control, 35.5%). At the time of the postsurgical evaluation, a majority of cingulotomy patients (88.9%) were still taking some medication for control of pain, but only 44.4% were taking opiates. A majority of cingulotomy patients were still taking NSAID (62.5%), whereas 27.7% were taking anticonvulsants and/or antidepressants. Two cingulotomy patients were taking no pain medications at the time of the postsurgical evaluation.
Surgical Procedure
For all cingulotomy patients, surgery was performed by using sterotactic thermal probes, producing bilateral lesions of the ACC. The lesions were approximately 5 mm in diameter and were slightly lateral of midline. The exact location and size of each lesion was analyzed postoperatively by digitized analysis of CT scan images. These anatomical analyses revealed stable, bilateral lesions consistent with the surgical descriptions of lesion size and sites, as shown in Figure 1.
Neuropsychological Assessment
Patients and control subjects were evaluated with a battery of neuropsychological tests designed to measure the functional domains of language, visual integration, learning and memory, executive control, attention motor control, and reasoning, as well as several experimental measures of response intention, generation, and persistence. The neuropsychological battery consisted largely of standardized tests with well-established reliability and validity.53
The Wechsler Adult Intelligence Scale–Revised (WAIS-R) was used to assess overall intellectual functioning.54 Language measures included subtests from the Boston Diagnostic Aphasia Exam (BDAE), including Auditory Comprehension, Commands, and Repetitions.55 The Boston Naming Test was used to assess confrontation naming.56 Several verbal fluency measures were also administered, including animal-naming fluency.55,57 Visual integration was assessed with the Hooper Visual Organization Test,58 Judgment of Line Orientation (JLO),59 and Rey Complex Figure–Copy (CFT-Copy).60 Learning and memory were assessed with the Wechsler Memory Scale (WMS),61 the Auditory Verbal Learning Test (AVLT),62 and the CFT immediate and delayed recall.60 The WMS was used for all patients because the initial collection of subjects for this study began before the revised form (WMS-R) was available. Motor measures included the Grooved Pegboard and Finger Tapping tests.63 Executive control and attention measures included the Wisconsin Card Sorting Test (WCST),64 Stroop Color-Word Interference Test,65 Trail Making Test,66 Luria Motor Programs and Rampart Figures,53 Adaptive Rate Continuous Performance Test (ARCPT),31 and Controlled Oral Word Association test (COWAT).57 In addition to the battery of standard neuropsychological tests, several experimental measures, described below, were developed for this study.
Spontaneous Utterances:
Given that we hypothesized a reduction in spontaneous verbal output following cingulotomy, a measure was developed for the present study to provide an index of spontaneous verbal response initiation and production. Standard verbal fluency tests have specific task demands and therefore do not measure spontaneous verbalization. Our task employed a behavioral-event recording method and provided information about the frequency of verbal production without prompts or specific instructions. Reductions in the number of spontaneous utterances following surgery would provide additional evidence that the ACC is involved in the initiation and production of behavior. A frequency count was obtained based on the number of times the patient spontaneously produced a verbalization over a 30-minute period during the course of an initial clinical interview. An utterance was counted if it consisted of at least a complete phrase or sentence that had meaning. Multiple sentences produced without break were counted as one utterance, whereas discrete sentences with greater than a 5-second lapse between utterances were counted as two separate utterances. This count was initiated 5 minutes into the interview to allow the patient time to adjust to the session. An utterance was counted only if it was produced spontaneously, and not in response to any question.
Design Fluency:
An unstructured version of Design Fluency was administered.67 The patient was given a blank sheet of paper and instructed to draw or doodle on the paper with a pencil and to produce as many different designs as possible within a 5-minute period. A sample was drawn for the patient by the examiner to provide an example of a design. The number of designs drawn in 5 minutes was recorded.
Object Construction:
A set of 100 Tinker Toy components53 was given to the patient with instructions to construct as many different objects as possible in 5 minutes. Patients were told that the objects did not have to resemble any real-world objects. A sample object was constructed for the patient. The number of objects constructed in 5 minutes was recorded.
Verbal Problem Solving:
Five verbal problems that have more than one correct answer were presented orally to each patient. An example of such a problem is, ”Why are automobiles important in our society?“ Patients were told to give as many different answers as possible for each question. Scores were based only on the number of different solutions generated by the patient, not the quality of any particular solution.
Pain Assessment
A brief pain inventory was administered to all cingulotomy and control patients prior to and 1 year after their cingulotomy. On this inventory, patients rated the severity of their pain on a 10-point Likert scale. They also rated on similar scales the extent to which their pain was disrupting various aspects of their daily activities, including work, socialization, family relationships, recreation, emotional state, and overall quality of life. This inventory also included a series of questions that required yes/no responses regarding whether they felt the surgery had been beneficial, whether they still experienced significant pain, and whether they were bothered as much by their pain.
Assessment Procedure
A neuropsychological assessment was conducted a minimum of 12 months postsurgically (range 12–36 months) for the cingulotomy patients. Assessments were completed during individual test sessions lasting approximately 6 hours per subject. For a subset of the cingulotomy group (n=12), a baseline neuropsychological assessment was also obtained 1 week prior to surgery. This baseline assessment was conducted when possible, but it was not performed on the first 3 patients in the study or on 3 other patients who were not available prior to surgery. Data from the control subjects were obtained during a 6-hour assessment conducted in conjunction with their Pain Clinic workup.
Data Analysis
Descriptive statistics were derived for all demographic, clinical, and neuropsychological measures. Neuropsychological measures were grouped so as to reflect the major domains of cognitive functioning as described above. To validate the grouping of measures across these domains, two independent experts in neuropsychological assessment were asked to assign the test measures to cognitive domains. The two expert raters were found to have 92% agreement with respect to how they grouped the test measures. Two different attentional domains were included in the analyses because previous studies have indicated that these measures can be disassociated into distinct attentional factors.60–61 No more than six measures within a given domain were ever subjected to subsequent analysis.
Next, a series of multivariate analysis of variance (MANOVA) procedures was conducted to compare the cingulotomy patients with control subjects across the nine domains of functioning: Intellectual, Language, Visual Integration, Motor Functioning, Memory, Attention Selection-Span, Attention Focus-Speed, Executive Control, and Intention. Each MANOVA was conceptualized as a separate study. We hypothesized that group differences would be evident with respect to the measures of attention and executive control, but not the analyses of other cognitive domains. Planned univariate comparisons between groups were then conducted for each measure in conjunction with the MANOVA for each domain. Bonferroni corrections were used to adjust for error rates while making multiple comparisons within and across domains.
A discriminant function analysis was conducted to determine which neuropsychological variables accounted for most of the group difference between cingulotomy and control subjects. Six variables were selected a priori from the executive, attentional, and intentional domains and were entered into the analysis. These measures were the Digit Symbol scaled score, Stroop Interference Index, Trail Making part B, ARCPT Inconsistency Index, Spontaneous Verbal Utterances, and Design Fluency. A forward stepwise discriminant function procedure was employed using a jackknife (leave-one-out) approach.
RESULTS
Precingulotomy and Postcingulotomy Neurocognitive Functioning
The 12 cingulotomy patients for whom presurgical baseline data were available and the 20 nonsurgical Pain Clinic control patients did not significantly differ before surgery on any of the neurocognitive domains as indicated by MANOVA. As mentioned above, the groups also did not differ with respect to baseline pain severity.
We previously reported that cingulotomy patients showed significant postsurgical declines on executive functions, including intention, spontaneous response initiation and production, and focused attention compared with their presurgical performance.52 After surgery, other neurocognitive functions did not change significantly relative to baseline. The following results are based on comparisons of neurocognitive functioning between a larger sample (n=18) of postcingulotomy patients and nonsurgical control subjects (n=20).
Executive Functions
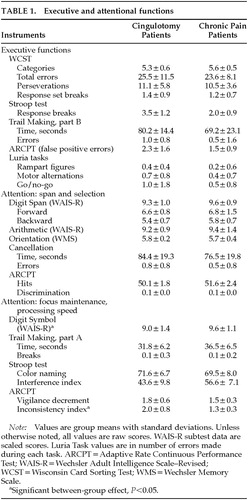
Cingulotomy patients showed weaker executive control performance compared with control subjects (Wilks' lambda=0.45, F=4.52, df=8,29, P<0.01). Groups differed in their performance on the Stroop Interference Index (P<0.01). They also showed differences that approached significance on total time for completion of Trail Making part B (P=0.07) and number of errors on the Go/No-Go task (P=0.08). No between-group differences in WCST performance were found, nor were between-group differences found for other executive indices, including reciprocal motor programs, graphomotor sequencing (Rampart Figures), or other Trail Making indices. Results for executive functioning are given in Table 1.
Attention Functions
Results for attention functions are given in Table 1. Cingulotomy patients showed reduced performance compared with control subjects on measures of attention span and selection (F=1.48, df=6,31, not significant). Groups did not differ on the Digit Span or Arithmetic subtests of the WAIS-R, nor did they show decreased target detection ability on the ARCPT or cancellation tasks.
An overall significant between-group difference was found on measures of focused attention and processing speed by MANOVA (Wilks' lambda=0.49, F=6.81, df=45,32, P<0.001). Groups did not differ on simple timed tasks requiring tracking, response speed, and persistence (Trails A, Stroop Color Naming). However, cingulotomy patients showed weaker performance than control subjects on the Inconsistency index (P<0.01) of the ARCPT.
Intention, Initiation, and Generation
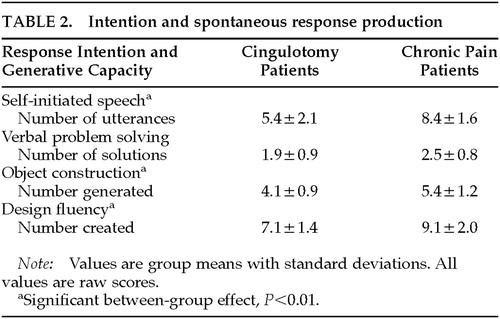
Cingulotomy patients had much weaker performance than control subjects on the measures of spontaneous response initiation and generation (Wilks' lambda=0.51, F=7.86, df=4,33, P<0.001). Significant between-group differences were found for Verbal Utterances (P<0.001), Object Construction (P<0.01), and Design Fluency (P<0.01). Table 2 provides descriptive statistics for these indices.
Overall Neurocognitive Functioning
Cingulotomy and control patients did not differ significantly with respect to Full Scale, Visual, or Performance IQ from the WAIS-R or Memory Quotient from the WMS (MANOVA). Groups did not differ on any of the WAIS-R Verbal or Performance subtests included in this analysis. Overall between-group differences in learning and memory performance were not found for either immediate or delayed memory measures on MANOVA. Groups did not differ with respect to overall language performance, nor were there between-group differences on any of the specific language indices from the Boston Diagnostic Aphasia Battery, Boston Naming Test, or animal naming. Groups also did not differ with respect to overall visual integrative performance (Hooper, JLO, CFT-Copy), or motor functioning on the Grooved Pegboard or Finger Tapping tasks. Table 3 contains descriptive statistics for cingulotomy patients and control subjects across these core neuropsychological functions.
Discriminating Cingulotomy From Control Patients
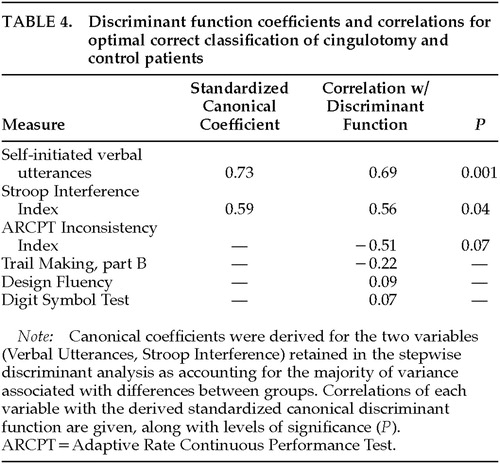
Discriminant function analysis revealed a highly significant difference between cingulotomy patients and control subjects (Wilks' lambda=0.36, P<0.001). This analysis was conducted by using a forward stepwise procedure with six variables entered into the analysis. Two measures, Spontaneous Verbal Utterances and Stroop Interference Index, accounted for a 92.1% correct classification rate between the two groups. The leave-one-out jackknife procedure, which was performed to cross-validate this finding, revealed an 84.2% correct classification rate. Table 4 gives standardized canonical discriminant function coefficients; correlations between each variable and the canonical discriminant function; and significance levels.
Effects on Pain
Relative to baseline, cingulotomy patients reported a statistically significant decrease in pain at follow-up (presurgery, 8.6±1.2; postsurgery, 6.8±2.1; P<0.05). Postcingulotomy patients also reported less severe pain compared with the control subjects (9.2±1.1, P<0.05). Most patients reported mild improvements in pain severity, although none reported a complete absence of pain. Most patients (66.6%) reported overall benefit from cingulotomy and being less bothered by pain (61.1%); none indicated a worsening in pain or functioning relative to baseline. All cingulotomy patients were using opiates for pain relief prior to surgery, but only 8 of 18 (44.4%) required opiates following surgery. Almost all patients were also using NSAID medications prior to surgery, whereas only 62.5% required this medication postsurgically. As a group, cingulotomy patients reported significant reduction following surgery in the extent to which pain interfered with behavioral and social functioning (presurgery, 9.5±0.8; postsurgery, 4.3±2.5; t=4.1, df=17, P<0.01). Although cingulotomy produced only mild improvement in pain severity, patients' functional status and response to pain improved to an even greater extent and were the most clinically significant aspects of their improvement.
DISCUSSION
Although early studies indicated that cingulotomy caused minimal impairment to most neurocognitive functions,39–42 there is mounting evidence that the ACC plays an important role in certain aspects of cognition and behavioral functioning.3,45–52 The present findings demonstrate that although many cognitive functions, such as language, visual perception, motor control, and memory are largely unaffected by cingulotomy, impairments do occur in the domains of attention and executive control. Cingulotomy patients showed weaker performance than control subjects on measures that reflect the ability to focus on and sustain attention to the task at hand. Although mild deficits were evident on the Stroop and Continuous Performance Tests, tasks sensitive to focused and sustained attention,47,68 cingulotomy patients had greatest impairment on attention/executive measures of response intention, generation, and persistence. Following surgery, they showed reduced output on Spontaneous Utterances, Design Fluency, and Object Construction, measures of their ability to spontaneously generate and persist in responding. Other aspects of executive functioning, including abstraction, problem solving, and response inhibition/switching, remained intact following cingulotomy. The pattern of postcingulotomy impairment supports the hypothesis that the ACC plays a fundamental role in the intention to act and in the generation of spontaneous behavior, and this pattern also indicates that not all frontal-executive functions are negatively affected by cingulotomy.
These findings are consistent with data from a growing body of research suggesting that the ACC is important for the control of attention.28–31,36,44–48,52 Data from functional neuroimaging studies using PET and fMRI suggest that magnitude of cingulate activation is a function of demand for attentional focus and intensive processing.47–51 Demanding tasks invariably lead to greater cingulate activation than less demanding tasks. Accordingly, the ACC appears to influence the maintenance and intensity of attentional focus relative to prevailing task demands. The present findings are consistent with previous studies showing impaired focused attention in patients following cingulotomy,45,46,52 and they extend these findings by demonstrating that those attentional/executive processes that are associated with response intention, generation, and persistence are most affected by anterior cingulate damage.
Our previous finding of alterations in the temporal consistency of autonomic habituation following cingulotomy is also supported by the present finding.44 A defining characteristic of the deficits noted in cingulotomy patients was a failure to initiate and/or sustain responding over time. Therefore, the present findings provide additional evidence that the ACC influences the dynamics of attending over time, including the consistency of sustained attention.
Current neuropsychological theories that have considered the ACC to be important for attention and aspects of executive control associated with response initiation28–31 generally view the ACC as part of a larger functional brain network. Within this perspective, attention is the by-product of the interaction of multiple brain systems, each with different functional roles. For instance, the reticular activating system sets attentional tone by effecting and modulating arousal, such that either excessive or low levels of activation lead to diminished attention. In contrast, the ACC seems to influence attention and executive control by modulating the propensity to initiate action51,69–71—making changes in attentional focus in accordance with changes in motivation, incentive, and effortful task demands.47–50,68 Attention appears to be a function of multiple processes that converge to control sensory and response selection and maintenance.
That the ACC is so highly interconnected with other cortical and subcortical areas, most notably the frontal cortex, basal ganglia, thalamus, and limbic nuclei,22–25 probably accounts for its role in both attention and emotional processing.24,26,27 In fact, the ACC may serve to bridge these two important aspects of human behavior. By modulating the intensity of affective and motivational signals relative to task demands, the ACC serves to integrate the salience of environmental information so that attention can be effectively directed to the most important stimuli from moment to moment.
This aspect of cingulate function may have significance for understanding the effect of cingulotomy on chronic pain and neuropsychiatric disorders such as obsessive-compulsive disorder.9–18 Chronic pain may be viewed as the byproduct of feedforward loops, through which pain signals are operantly reinforced and sustained.72 Obsessive-compulsive disorder could involve a similar process.13–17 The ACC may play an essential role in these disorders by either amplifying or attenuating the tendency of people to continuously respond, emotionally and behaviorally, to pain or other salient stimuli.19,20 These findings have implications for potential development of future neuropsychiatric interventions that might effect subtle change in cingulate functioning to treat pain and other behavioral disorders.
The present findings have potential clinical implications. For patients considering cingulotomy for treatment of chronic pain, it seems reasonable to advise them that the procedure is unlikely to cause major changes in intellectual or memory abilities, but that subtle changes in attention and certain aspects of their behavioral responding may occur. Expectations regarding diminished spontaneity and response persistence should be discussed. Furthermore, these attentional manifestations may also correspond with perceived change in personality. Patients and health care providers should weigh these effects in determining the risk/benefit ratio of surgery for each individual. Studies are also needed to further determine how the neurobehavioral manifestations of ACC lesions affect daily functioning, including social interaction, occupational productivity, and quality of life.
Because this was a clinical study, several methodological limitations exist and may have had some impact on the results. Subjects could not be randomly assigned to have cingulotomy or nonsurgical Pain Clinic treatment. Although the groups were quite similar in demographic characteristics, baseline neurocognitive abilities, and the type and severity of their chronic intractable pain, it is possible that some unaccounted-for difference existed between patients opting for one or the other of these treatment approaches. Control subjects typically underwent neurocognitive testing because of subjective cognitive complaints, most often involving concentration difficulties; however, this factor does not appear to be a significant confound, since control patients performed better than postsurgical cingulotomy patients despite having these subjective complaints. In fact, this selection factor may actually have reduced the between-group effect size. Another potential confound is the fact that examiners were not blind to group status, nor were subjects blind to their treatment procedure. This methodological feature was unavoidable given the very different nature of the treatments and the fact that it was obvious which patients had undergone surgery and which had not. There is little reason to believe that results were biased in any meaningful way by the lack of a double-blind design, as data were based on objective neurocognitive measures that were largely free of subjective interpretation. Although the individual patients' regimens of pain control medications were not identical, it seems unlikely that this had a confounding effect, since patients in the two groups used similar medications at baseline, and cingulotomy patients actually showed a reduction in opiate use following cingulotomy. Diminished cognitive performance would not be expected from decreased use of pain medications. Although the aforementioned methodological limitations do not appear to have adversely affected interpretation of the results, future studies that control for these factors would be helpful to validate the present findings.
ACKNOWLEDGMENTS
The efforts of Dr. Ping Hu, biostatistician for the Department of Psychiatry and Human Behavior of Brown University, in review of the data analysis and statistical recommendations are greatly appreciated.
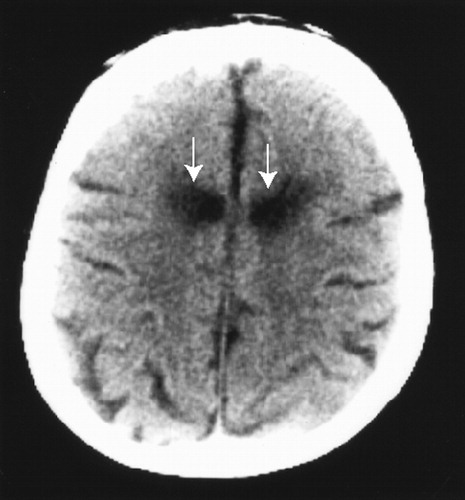
FIGURE 1. Axial computed tomography scan showing bilateral anterior cingulate lesions (arrows) in a typical patient following surgeryLesions were symmetric and well circumscribed to the anterior cingulate cortex (855.4±61.2 mm3) for all patients.
 |
 |
 |
 |
1 Ward A: The cingular cortex: area 24. J Neurophysiol 1948; 11:13–23Crossref, Medline, Google Scholar
2 Maclean PD: Introduction: perspectives on the cingulate cortex in the limbic system, in Neurobiology of Cingulate Cortex and Limbic Thalamus: A Comprehensive Handbook, edited by Vogt BA, Gabriel M. Boston, Birkhauser, 1993, pp 1–19Google Scholar
3 Devinsky O, Morrell MJ, Vogt BA: Contributions of anterior cingulate cortex to behaviour. Brain 1995; 118(pt 1):279–306Google Scholar
4 Woodruff ML, Baisdan RH, Douglas JR: Effect of cingulate and fornix lesions on emotional behavior in rabbits. Exp Neurol 1981; 74:379–395Crossref, Medline, Google Scholar
5 Slotnick BM: Disturbances of maternal behavior in the rat following lesions of the cingulate cortex. Behavior 1967; 29:204–236Crossref, Medline, Google Scholar
6 Talairach J, Bancaud J, Geier S, et al: The cingulate cortex and human behavior. Encephalogr Clin Neurophysiol 1973; 34:45–52Crossref, Google Scholar
7 George MS, Ketter TA, Parekh PI, et al: Blunted left cingulate activation in mood disorder subjects during a response interference task (the Stroop). J Neuropsychiatry Clin Neurosci 1997; 9:55–63Link, Google Scholar
8 Pardo JV, Pardo PJ, Raichle ME: Neural correlates of self-induced dysphoria. Am J Psychiatry 1993; 150:713–719Crossref, Medline, Google Scholar
9 Ballantine HT Jr, Bouckoms AJ, Thomas EK, et al: Treatment of psychiatric illness by stereotactic cingulotomy. Biol Psychiatry 1987; 22:807–819Crossref, Medline, Google Scholar
10 Kullberg G: Differences in effect of capsulotomy and cingulotomy, in Neurosurgical Treatment in Psychiatry, Pain, and Epilepsy, edited by Sweet WH, Obrador S, Martín-Rodríguez JG, et al. Baltimore, University Park Press, 1977, pp 301–308Google Scholar
11 Cassidy WL, Ballantine HT Jr, Flanagan NB: Frontal cingulotomy for affective disorders. Recent Advances in Biological Psychiatry 1965; 8:269–282Medline, Google Scholar
12 Paniagua JL, Jimeno AL, Diaz-Aramendi A: Stereotactic cingulotomy in aggression, in Neurosurgical Treatment in Psychiatry, Pain, and Epilepsy, edited by Sweet WH, Obrador S, Martín-Rodríguez JG, et al. Baltimore, University Park Press, 1977, pp 399–400Google Scholar
13 Kurlan R, Kersun J, Ballantine HT Jr, et al: Neurosurgical treatment of severe obsessive-compulsive disorder associated with Tourette's syndrome. Mov Disord 1990; 5:152–155Crossref, Medline, Google Scholar
14 Fodstad H, Strandman E, Karlsson B, et al: Treatment of chronic obsessive compulsive states with stereotactic anterior capsulotomy or cingulotomy. Acta Neurochir 1982; 62:1–23Crossref, Medline, Google Scholar
15 Jenike MA, Baer L, Ballantine T, et al: Cingulotomy for refractory obsessive-compulsive disorder: a long-term follow-up of 33 patients. Arch Gen Psychiatry 1991; 48:548–555Crossref, Medline, Google Scholar
16 Korzenev AV, Shoustin VA, Anichkov AD, et al: Differential approach to psychosurgery of obsessive disorders. Stereotact Funct Neurosurg 1997; 68:226–230Crossref, Medline, Google Scholar
17 Baer L, Rauch SL, Ballantine HT Jr, et al: Cingulotomy for intractable obsessive-compulsive disorder: prospective long-term follow-up of 18 patients. Arch Gen Psychiatry 1995; 52:384–392Crossref, Medline, Google Scholar
18 Foltz EL, White LE: Pain “relief” by frontal cingulotomy. J Neurosurg 1962; 19:89–100Crossref, Medline, Google Scholar
19 Rainville P, Duncan GH, Price DD, et al: Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science 1997; 277:968–971Crossref, Medline, Google Scholar
20 Lenz FA, Rios M, Zirh A, et al: Painful stimuli evoke potentials recorded over the human anterior cingulate gyrus. J Neurophysiol 1998; 79:2231–2234Google Scholar
21 Hassenbusch SJ, Pillay PK, Barnett GH: Radiofrequency cingulotomy for intractable cancer pain using stereotaxis guided by magnetic resonance imaging. Neurosurgery 1990; 27:220–223Crossref, Medline, Google Scholar
22 Baleydier C, Mauguiere F: The duality of the cingulate gyrus in monkey: neuroanatomical study and functional hypothesis. Brain 1980; 103:525–554Crossref, Medline, Google Scholar
23 Pandya DN, Van Hoesen GW, Mesulam M-M: Efferent connections of the cingulate gyrus in the rhesus monkey. Exp Brain Res 1981; 42:319–330Crossref, Medline, Google Scholar
24 Alexander GE, DeLong MR, Strick PL: Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 1986; 9:357–381Crossref, Medline, Google Scholar
25 Musil SY, Olson CR: Organization of cortical and subcortical projections to anterior cingulate cortex in the cat. J Comp Neurol 1988; 272:203–218Crossref, Medline, Google Scholar
26 Mega MS, Cummings JL: Frontal-subcortical circuits and neuropsychiatric disorders. J Neuropsychiatry Clin Neurosci 1994; 6:358–370Link, Google Scholar
27 Mega MS, Cummings JL, Salloway S, et al: The limbic system: an anatomic, phylogenetic, and clinical perspective. J Neuropsychiatry Clin Neurosci 1997; 9:315–330Link, Google Scholar
28 Morecraft RJ, Geula C, Mesulam M-M: Architecture of connectivity within a cingulo-fronto-parietal neurocognitive network for directed attention. Arch Neurol 1993; 50:279–284Crossref, Medline, Google Scholar
29 Heilman KM, Valenstein E, Watson RT: Localization of neglect, in Localization in Neuropsychology, edited by Kertesz A. New York, Academic Press, 1983, pp 471–492Google Scholar
30 Mesulam M-M: A cortical network for directed attention and unilateral neglect. Ann Neurol 1981; 10:309–325Crossref, Medline, Google Scholar
31 Cohen RA: Neuropsychology of Attention. New York, Plenum, 1993Google Scholar
32 Gabriel M: Functions of anterior and posterior cingulate cortex during avoidance learning in rabbits. Prog Brain Res 1990; 85:467–482Crossref, Medline, Google Scholar
33 Seamans JK, Floresco SB, Phillips AG: Functional differences between prelimbic and anterior cingulate regions of the rat prefrontal cortex. Behav Neurosci 1995; 109:1063–1073Google Scholar
34 Pribram K, Fulton JF: An experimental critique of the effects of anterior cingulate ablation in monkey. Brain 1954; 77:235–246Crossref, Google Scholar
35 Bussey TJ, Everitt BJ, Robbins TW: Dissociable effects of cingulate and medial frontal cortex lesions on stimulus-reward learning using a novel pavlovian autoshaping procedure for the rat: implications for the neurobiology of emotion. Behav Neurosci 1997; 111:908–919Crossref, Medline, Google Scholar
36 Watson RT, Heilman KM, Cauthen JC, et al: Neglect after cingulectomy. Neurology 1973; 23:1003–1007Google Scholar
37 Barris RW, Schuman HR: Bilateral anterior cingulate gyrus: syndrome of the anterior cingulate gyri. Neurology 1953; 3:44–52Crossref, Medline, Google Scholar
38 Degos JD, da Fonseca N, Gray F, et al: Severe frontal syndrome associated with infarcts of the left cingulate gyrus and the head of the right caudate nucleus: a clinical pathological case. Brain 1993; 116:1541–1548Google Scholar
39 Sharma T: Absence of cognitive deficits from bilateral cingulotomy for intractable pain in humans. Tex Med 1973; 69:79–82Medline, Google Scholar
40 Corkin S, Twitchell TE, Sullivan EV: Safety and efficacy of cingulotomy for pain and psychiatric disorders, in Modern Concepts in Psychiatric Surgery, edited by Hitchcock ET, Ballantine HT, Meyerson BA. Amsterdam, Elsevier, 1979Google Scholar
41 Corkin S: Hidden-figures-test performance: lasting effects of unilateral penetrating head injury and transient effects of bilateral cingulotomy. Neuropsychologia 1979; 17:585–605Crossref, Medline, Google Scholar
42 Teuber HL, Corkin SH, Twitchell TE: Study of cingulotomy in man: a summary, in Neurosurgical Treatment in Psychiatry, Pain, and Epilepsy, edited by Sweet WH, Obrador S, Martín-Rodríguez JG, et al. Baltimore, University Park Press, 1977, pp 355–362Google Scholar
43 Ballantine HT Jr, Cassidy WL, Flanagan NB, et al: Stereotaxic anterior cingulotomy for neuropsychiatric illness and intractable pain. J Neurosurg 1967; 26:488–495Crossref, Medline, Google Scholar
44 Cohen RA, Kaplan RF, Meadows M-E, et al: Habituation and sensitization of the orienting response following bilateral anterior cingulotomy. Neuropsychologia 1994; 32:609–617Crossref, Medline, Google Scholar
45 Cohen RA, McCrae V, Phillips K, et al: Neurobehavioral consequences of bilateral medial cingulotomy (abstract). Neurology 1990; 40(suppl 1):A198Google Scholar
46 Janer KW, Pardo JV: Deficits in selective attention following bilateral anterior cingulotomy. Journal of Cognitive Neuroscience 1991; 3:231–241Crossref, Medline, Google Scholar
47 Pardo JV, Pardo PJ, Janer KW, et al: The anterior cingulate cortex mediates processing selection in the Stroop attentional conflict paradigm. Proc Natl Acad Sci USA 1990; 87:256–259Crossref, Medline, Google Scholar
48 Posner MI, Petersen SE, Fox PT, et al: Localization of cognitive operations in the human brain. Science 1988; 240:1627–1631Google Scholar
49 Paus T, Petrides M, Evans AC, et al: Role of the human anterior cingulate cortex in the control of oculomotor, manual, and speech responses: a positron emission tomography study. J Neurophysiol 1993; 70:453–469Crossref, Medline, Google Scholar
50 Paus T, Koski L, Caramanos Z, et al: Regional differences in the effects of task difficulty and motor output on blood flow response in the human anterior cingulate cortex: a review of 107 PET activation studies. Neuroreport 1998; 9:R37–R47Google Scholar
51 Frith CD, Friston K, Liddle PF, et al: Willed action and the prefrontal cortex in man: a study with PET. Proc R Soc Lond B Biol Sci 1991; 244:241–246Crossref, Medline, Google Scholar
52 Cohen RA, Kaplan RF, Moser DJ, et al: Impairments of attention following cingulotomy. Neurology (in press)Google Scholar
53 Lezak M: Neuropsychological Assessment, 3rd edition. New York, Oxford University Press, 1995Google Scholar
54 Wechsler D: Wechsler Adult Intelligence Scale–Revised. New York, Psychological Corporation, 1981Google Scholar
55 Goodglass H, Kaplan E: Assessment of Aphasia and Related Disorders, 2nd edition. Philadelphia, Lea and Febiger, 1983Google Scholar
56 Kaplan E, Goodglass H, Weintraub S: Boston Naming Test. Philadelphia, Lea and Febiger, 1983Google Scholar
57 Spreen O, Benton AL: The Neurosensory Center Comprehensive Examination for Aphasia (NCCEA). Victoria, BC, University of Victoria Neuropsychological Laboratory, 1977Google Scholar
58 Hooper HE: Hooper Visual Organization Test (HVOT). Los Angeles, Western Psychological Services, 1983Google Scholar
59 Benton AL, Hannay HJ, Varney NR: Visual perception of line direction in patients with unilateral brain damage. Neurology 1975; 25:907–910Crossref, Medline, Google Scholar
60 Osterrieth PA: Le test de copie d'une figure complexe. Archives de Psychologie 1993; 30:206. Excerpts translated by Corwin J, Bylsma FW. The Clinical Neuropsychologist 1993; 7:9–15Google Scholar
61 Wechsler D: Wechsler Memory Scale: Manual. San Antonio, TX, The Psychological Corporation, 1974Google Scholar
62 Rey A: L'examen clinique en psychologie. Paris, Presses Universataires de France, 1964Google Scholar
63 Kløve H: Clinical neuropsychology, in The Medical Clinics of North America, edited by Forster FM. New York, WB Saunders, 1963Google Scholar
64 Heaton RK: Wisconsin Card Sorting Test (WCST). Odessa, FL, Psychological Assessment Resources, 1981Google Scholar
65 Stroop JR: Studies of interference in serial verbal reactions. J Exp Psychol 1965; 18:643–662Crossref, Google Scholar
66 Army Individual Test Battery: Manual of Directions and Scoring. Washington, DC, War Department, Adjutant General's Office, 1944Google Scholar
67 Jones-Gotman M, Milner B: Design Fluency: the invention of nonsense drawings after focal cortical lesions. Neuropsychologia 1977; 15;653–674Google Scholar
68 Bench CJ, Frith CD, Grasby PM, et al: Investigations of the functional anatomy of attention using the Stroop test. Neuropsychologia 1993; 31:907–922Crossref, Medline, Google Scholar
69 Badgaiyan RD, Posner MI: Mapping the cingulate cortex in response selection and monitoring. Neuroimage 1998; 7:255–260Crossref, Medline, Google Scholar
70 Passingham RE: Attention to action. Philos Trans R Soc Lond B Biol Sci 1996; 351:1473–1479Google Scholar
71 Blakemore SJ, Rees G, Frith CD: How do we predict the consequences of our actions? A functional imaging study. Neuropsychologia 1998; 36:521–529Crossref, Medline, Google Scholar
72 Melzac R, Wall PD: Pain mechanisms: a new theory. Science 1965; 150:971–979Crossref, Medline, Google Scholar



