Violence, Criminal Behavior, and the EEG
Abstract
The authors studied the relationship of EEG abnormalities and violent criminal behavior in 222 defendants referred for psychiatric evaluation. There was no connection between the number of violent offenses and EEG abnormalities in general. Focal abnormalities, however, especially of the left hemisphere, were related to a significantly higher number of violent offenses. In many cases these abnormalities were accompanied by mental retardation, epilepsy, or earlier brain damage. The findings suggest that impairment of left hemisphere functions may enhance the propensity for violent behavior in a subgroup of offenders.
Electroencephalographic abnormalities in psychopathic personalities and in forensic populations were described in the early days of electroencephalography.1–3 The findings, however, were mainly nonspecific, the studies suffered from methodological problems, and the results could not always be replicated. The significance of these EEG abnormalities is still a matter of debate4,5
In recent years the biological aspect of antisocial personality, crime, and violent behavior has been increasingly recognized.6 Several authors have stressed an association between impulsive violence and cerebral lesions.7,8 In 129 violent offenders, a strong correlation was found between neurological “soft signs”9 and the number of former convictions and early recidivism.10 Wong et al.11 investigated inmates of a high-security special hospital. In the subgroup with the highest degree of violence, they found an increased number of focal abnormalities, located mainly in the temporal region. The same study showed an association of violence and temporally located morphological lesions on cerebral computed tomography. An association between EEG abnormalities and violent behavior was not found by all investigators, however.12,13 It is still unclear whether the abnormalities found in subgroups characterized by high violence and severe psychiatric comorbidity can be generalized to the majority of offenders.
The present investigation is part of a larger project comprising the analysis of 261 consecutive forensic psychiatric pretrial evaluations. Sociobiographical, criminological, psychological, and biological parameters were documented. In the majority of defendants, an electroencephalogram was recorded. We used this extensive, unselected material to examine the postulated association between violent behavior and EEG abnormalities.
METHODS
Our study population consisted of 222 defendants of a state court district who were seen consecutively at Halle Psychiatric University Clinic for pretrial assessment and evaluation of criminal responsibility, including EEG recording. Another 39 subjects did not have EEG recording because of lack of consent or for administrative reasons (evaluation outside the clinic); these cases are not further considered. All subjects were assessed by one of us (A.M.) personally or under his supervision. Sociobiographical, criminological, medical, and psychopathological information was recorded on a standardized score sheet and entered into a computer system for further processing. Participation in the assessment was voluntary; all subjects signed informed consent.
Present and previous offenses were classified as violent or nonviolent. We defined as violent offenses—according to the classification used in official German statistics—murder, manslaughter, rape, robbery, kidnapping, aggravated assault, and battery. All other offenses were rated as nonviolent and were not considered further. Clinical diagnoses were made according to ICD-10 criteria.
A standardized EEG examination was performed on each subject. The recordings were done with a computerized system (Walter Graphtek) with the patient in supine position. The examination took 20 minutes, including 3 minutes of hyperventilation. Nineteen electrodes were placed according to the international 10–20 system. Analysis was done visually by one experienced physician (A.R.), who in most cases did not herself examine the subject and therefore was usually unaware of the clinical data. Rating was conservative; only abnormalities that were unambiguously present were rated. For analysis, EEG abnormalities were coded according to a scheme that includes focal abnormalities and nonfocal abnormalities (diffuse slowing of background rhythm, intermittent theta or delta slowing, paroxysmal activity).
Statistical analysis was performed by using the SPSS software package. Because the number of violent offenses was not normally distributed, group differences were tested with the nonparametric Mann-Whitney U-test for statistical significance.
RESULTS
The majority of subjects were male (n=207; 93.2%). The age range was 15–77 years with a mean of 30.4±10.8 years (values reported as means±SD). Types of offenses showed a broad distribution. Of the index offenses, 71 (32.0%) were nonviolent and 151 (68.0%) were violent. Violent offenses included murder/manslaughter (20.7%), aggravated assault/battery (36.9%), robbery (22.5%), and sexual offenses (18.0%). The mean number of prior convictions was 1.51±2.48 and the mean number of violent offenses was 1.22±1.50. Numbers of offenses did not correlate with age (Spearman's r=–0.057, P=0.4), so correction for age was not necessary.
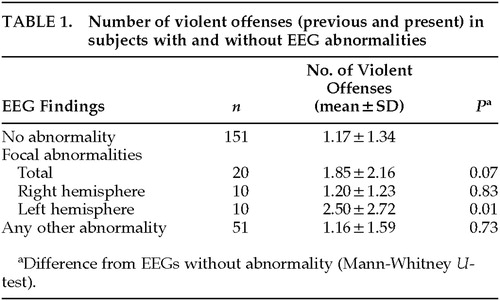
Deviation from the norm was found in one-third of EEG recordings. Most of these alterations consisted of a nonspecific increase of diffuse theta activity or groups of theta or delta waves. Only 1 subject displayed unambiguous spike-wave complexes. A total of 20 subjects showed focal abnormalities, equally divided between the left and the right hemispheres.
Subjects with EEG abnormalities had committed an average of 1.35±1.78 violent offenses. This number is slightly higher than that in subjects without EEG abnormalities, who had committed 1.17±1.34 violent offenses. The difference is not statistically significant (P=0.55).
Further differentiation into focal and nonfocal EEG abnormalities showed that violent offenses in subjects with left-sided focal abnormalities were significantly more frequent (P=0.01) than in subjects without abnormality (Table 1). Localization of abnormalities within the left hemisphere was nonuniform, with the temporal region being involved in most cases.
Of the 20 defendants with focal EEG abnormalities, 17 were right-handed, 2 left-handed; in 1, handedness was not recorded. Four subjects (all with left-sided alterations) were mentally retarded, 3 others (all with right-sided focal abnormalities) had epilepsy, and 2 had previously suffered brain trauma. When analysis was repeated excluding all subjects with mental retardation, epilepsy, or brain trauma, there were still more violent offenses in the group with left focal abnormalities than in the group without EEG abnormalities. This difference, however, was no longer statistically significant (P=0.07).
DISCUSSION
The group of subjects we examined comprised defendants referred for psychiatric evaluation; it is therefore not representative of criminal offenders in general. However, it was a rather large group of consecutively referred subjects showing a wide range of offense types and disorders. The population might therefore be suitable for investigation of the association of violence and cerebral disorder.
Older findings of a general increase in nonspecific EEG abnormalities associated with violent recidivism were not confirmed by our investigation. Several modern studies have failed to replicate these findings.12,14,15 More advanced recording techniques, higher numbers of electrodes placed, and better artifact control may be responsible for the differences from earlier results.
We found, however, a significant excess of violent recidivism in subjects with focal abnormalities, especially left hemispheric foci. In a substantial number of subjects these abnormalities were associated with clinically relevant brain disease, including mental retardation.
Possible limitations of our study are its retrospective character, the limited objectivity of visual EEG scoring, and the fact that the scoring physician was not completely blind to clinical data. However, EEG evaluation was done without knowledge of the present hypothesis of an association of EEG pathology and violent offenses.
Our findings principally confirm those of Wong et al.11 Like us, these authors found no correlation between violence and generalized EEG abnormalities in 372 inmates of a special hospital, but they found a higher incidence of focal abnormalities in a subgroup with the highest violence scores (30.7% focal abnormalities and 20.0% temporal abnormalities in the violent group versus 7.2% and 2.4% in the low-violence group). Although our findings principally agree, the lower prevalence of focal abnormalities in our population (9%) may be explained by the fact that the violent group of Wong et al. represented a population highly selected for the most severe violence. Our findings are also in accord with those of Convit et al.,8 who found a correlation of left hemispheric slow waves and increased propensity for violence in 21 patients of a specialized ward for the violent psychiatrically ill. Another study16 found left temporal focal activity in three of four subjects with repetitive impulsive violence. The electrophysiological data are supported by computed tomographic and PET findings16,17 and by asymmetric neurological soft signs.18
The validity of these findings is further supported by neuropsychological data linking left temporofrontal functional deficit and violent behavior.19 It has been proposed that a diminished faculty to use cognitive strategies depending on left hemispheric or language competence may play a part in the pathogenesis of violent criminal behavior.20 As Miller19 hypothesized, in some criminals a neurodevelopmental maturational deficit affecting the dominant hemisphere may be responsible for a relative inability to use inner speech to modulate affect, thought, and behavior. Under conditions of social frustration these individuals regress to the use of developmentally immature response strategies.
In this context, our findings lend some support to the concept of a connection between left hemispheric cerebral lesion and the propensity for violence. At the same time, they indicate that this association may be caused by a relatively small subgroup of subjects.
 |
1 Hill D, Watterson D: Electroencephalographic studies of psychopathic personalities. J Neurol Psychiatry 1942; 5:47–65Crossref, Medline, Google Scholar
2 Hill D: Cerebral dysrhythmia: its significance in aggressive behavior. Proceedings of the Royal Society of Medicine 1944; 37:317–330Crossref, Medline, Google Scholar
3 Ellingson RJ: The incidence of EEG abnormality among patients with mental disorders of apparently nonorganic origin: a critical review. Am J Psychiatry 1954; 111:263–275Crossref, Medline, Google Scholar
4 Volavka J: Aggression, electroencephalography, and evoked potentials: a critical review. Neuropsychiatry Neuropsychol Behav Neurol 1990; 3:249–259Google Scholar
5 Small J: Psychiatric disorders and EEG, in Electroencephalography: Basic Principles, Clinical Applications, and Related Fields, 3rd edition, edited by Niedermeyer E, Lopez da Silva FH. Baltimore, Williams and Wilkins, 1993Google Scholar
6 Elliott FA: Violence, the neurologic contribution: an overview. Arch Neurol 1992; 49:595–602Crossref, Medline, Google Scholar
7 Lewis DO, Pincus JH, Lovely R, et al: Biopsychosocial characteristics of matched samples of delinquents and nondelinquents. J Am Acad Child Adolesc Psychiatry 1987; 26:744–752Crossref, Medline, Google Scholar
8 Convit A, Czobor P, Volavka J: Lateralized abnormality in the EEG of persistently violent psychiatric inpatients. Biol Psychiatry 1991; 30:363–370Crossref, Medline, Google Scholar
9 Quitkin F, Rifkin A, Klein DF: Neurologic soft signs in schizophrenia and character disorders: organicity in schizophrenia with premorbid asociality and emotionally unstable character disorders. Arch Gen Psychiatry 1976; 33:845–853Crossref, Medline, Google Scholar
10 Kröber HL, Scheurer H, Sass H: Zerebrale Dysfunktion, neurologische Symptome und Rückfalldelinquent, II: Ergebnisse des Heidelberger Delinquenzprojekts. Fortschr Neurol Psychiatrie 1994; 62:223–232Crossref, Medline, Google Scholar
11 Wong MTH, Lumsden J, Fenton GW, et al: Electroencephalography, computed tomography and violence ratings of male patients in a maximum-security mental hospital. Acta Psychiatr Scand 1994; 90:97–101Crossref, Medline, Google Scholar
12 Driver MV, West LR, Faulk M: Clinical and EEG studies of prisoners charged with murder. Br J Psychiatry 1974; 125:583–587Crossref, Medline, Google Scholar
13 Hsu LK, Wisner K, Richey ET, et al: Is juvenile delinquency related to an abnormal EEG? A study of EEG abnormalities in juvenile delinquents and adolescent psychiatric inpatients. J Am Acad Child Psychiatry 1985; 24:310–315Crossref, Medline, Google Scholar
14 Small JG: The organic dimension of crime. Arch Gen Psychiatry 1966; 15:82–89Crossref, Medline, Google Scholar
15 Krakowski MI, Convit A, Jaeger J, et al: Inpatient violence: trait and state. J Psychiatr Res 1989; 23:57–64Crossref, Medline, Google Scholar
16 Volkow ND, Tancredi L: Neural substrates of violent behaviour: a preliminary study with positron emission tomography. Br J Psychiatry 1987; 151:668–673Crossref, Medline, Google Scholar
17 Tonkonogy JM: Violence and temporal lobe lesion: head CT and MRI data. J Neuropsychiatry Clin Neurosci 1991; 3:189–196Link, Google Scholar
18 Stein DJ, Hollander E, Cohen L, et al: Neuropsychiatric impairment in impulsive personality disorders. Psychiatry Res 1993; 48:257–266Crossref, Medline, Google Scholar
19 Miller L: Neuropsychological perspectives on delinquency. Behav Sci Law 1988; 6:409–428Crossref, Google Scholar
20 Dolan M: Psychopathy: a neurobiological perspective. Br J Psychiatry 1994; 165:151–159Crossref, Medline, Google Scholar



